Abstract
This work aims to propose earth-abundant materials for CO2 photoreduction to generate renewable solar fuels to provide practical solutions to global warming. The selected material in this case is cuprous oxide (Cu2O), one of the most promising photocatalysts for CO2 photoreduction due to its high affinity to solar radiation and electronic properties. Cu2O nanoparticles (NPs) were synthesized using Psidium guajava residue for the photocatalytic CO2 reduction. The aqueous residue of the Psidium guajava fruit proved to be suitable for stabilizing and acting as a reducing agent for the synthesis of Cu2O NPs. The XRD analysis confirmed the formation of the cubic structure of Cu2O. The nanoparticles absorb light from 430 nm with a direct bandgap value of around 1.8 eV. Cu2O NPs exhibited activity for CO2 photoreduction, whose efficiency was optimized by an orthogonal Taguchi L9 design. The factors studied were catalyst loading, air flow, and temperature. During the use of Cu2O NPs in the CO2 photoreduction HCOOH was identified as the main product, with an optimized production of 103.4 µmol h− 1 under visible light. Also, it was demonstrated the photocatalytic activity of the Cu2O NPs for H2 evolution by water splitting.
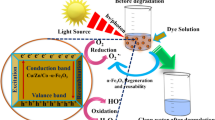
Graphical Abstract

Highlights
Cu2O NPs were synthesized using aqueous extract of Psidium guajava.
Cu2O NPs were used as photocatalyst for CO2 photoreduction.
The products obtained from the photoreduction process were formic acid, methanol and formaldehyde.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
One of the major concerns and challenges worldwide is the increase in greenhouse gas emissions, especially carbon dioxide (CO2), causing the gradual increase in the temperature of the planet, bringing frequent and prolonged droughts, devastating fires, tsunamis, floods, and cyclones (Valls et al. 2022). As a result, a gradual increase in CO2 emissions of 2.7% has been recorded during the years 2018 and 2019 (Syed and Ullah 2021). The main sectors that release the most greenhouse gases are the cement industry, power generation, public, private, and cargo transport. Additionally, residential, commercial, and housing sectors (Alemán et al. 2022). That is why one of the most critical tasks facing governments and the scientific community worldwide is to reduce the release of CO2. For this, existing and well-known technologies have been implemented. Those are: carbon capture, utilization, and storage (CCUS) (Monteiro and Roussanaly 2022; NganDo et al. 2022), catalytic conversion, thermochemical energization, electrochemical reduction (NganDo et al. 2022), and geological carbon sequestration (GCS) in which CO2 is injected and stored in geological caverns (Punnam et al. 2023). However, there are alternative technologies that have been proposed to mitigate the high CO2 concentration in the atmosphere and at the same time its use in obtaining high value-added products (Apriandanu and Yulizar 2019; Kusumah et al. 2024). One of them is the CO2 photoreduction for the production of synthetic fuels and chemicals such as methane (CH4), methanol (CH3OH), acetone (CH3COCH3), acetic acid (CH3COOH), isopropanol (CH3CH(OH)CH3), formaldehyde (HCHO), and formic acid (HCOOH) (Faria et al. 2021). The simple oxides, MxOy (M = Ti, Zn, Cu, Mg, Fe, V) represent a good option for this process due to their easy synthesis and good photocatalytic activity (Cai et al. 2022; Lu et al. 2021). One family of simple oxides that have shown remarkable activity is cupper oxides, CuxOy (x = 1,2, y = 1,2) (Aguirre et al. 2017; Yao et al. 2022). Also, these materials are abundant and of low-cost. Particularly, Cu2O is a p-type semiconductor with a band gap of approximately 2.0 eV with a suitable negative conduction band edge (-1 V vs. NHE) to reduce CO2 to a wide variety of hydrocarbons, e.g., formic acid (HCOOH) (Akrami et al. 2022; Peixoto et al. 2022). The main drawback of these type of materials is that they are synthesized mainly using expensive chemical precursors. Most of the time, methods such as hydrothermal, solvothermal, sonochemical, chemical precipitation and sol-gel are used (Apriandanu and Yulizar 2019). However, they are characterized by being slow and requiring too much time to obtain, they also require strict control of the variables (for example, time, temperature, etc.) (Halomoan et al. 2022). As an alternative, green synthesis aims to reduce the use of chemical products and energy expenditure to produce these compounds by employing low-cost and environmentally friendly techniques. In the following section some wastes and alternative additives to synthesize semiconductor oxides is discussed.
1.1 Literature review about green synthesis
Recently, the use of extracts from plants, flowers, fruit pulp, fungi, and leaves represents one of the most promising alternatives to synthesize different nanoparticles in order to provide cleaner and more energy-efficient technologies (Rahman et al. 2022). One of the advantages of these raw materials is its high content of secondary metabolites such as alkaloids, flavonoids and saponins (Indriyani et al. 2021; Yulizar et al. 2020). In addition, the properties of the extracts, such as metabolic concentration, pH, etc. could benefit the physical and chemical characteristics of the materials (Marcony et al. 2022). It exhibited superior characteristics compared to commercial materials with large surfaces and novel morphologies, exhibiting superior photocatalytic properties (Fadhila et al. 2024; Yulizar et al. 2020). So far, these extract have been used as reducing agents, templates, surfactants, and solvents for the formation of NPs with unique characteristics and sizes within the range of 1–100 nm (Aguirre et al. 2017; Liu et al. 2019). One example of these bio-templates is the Psidium guajava or guava of the Myrtaceae family. Its flowers are white and the fruit contains pulp and small hard seeds inside (Amadike et al. 2022). This fruit is usually very popular among the population due to its characteristic sweet flavor and its peculiar presence of small seeds inside the pulp. The Psidium guajava is made up of various nutrients, minerals and bioactive compounds such as; ascorbic acid (vitamin C), phenols, carotenoids, tannins, alkaloids, triterpenes, saponins, lectins, dietary fiber (pectin), unsaturated fatty acids (linoleic acid) and carbohydrates which are located mainly in the pulp and in the peel (Cui et al. 2022; Jiang et al. 2021). This composition could serve as weak bases and stabilizing agents for the formation of Cu2O NPs (Elviera et al. 2022). Among the main minerals include iron, phosphorus and calcium (Amadike et al. 2022). This fruit is grown mainly in India, China, Pakistan, Thailand, Bangladesh, Mexico, and Indonesia, cultivated in tropical and subtropical regions with an estimated world production around 55.85 million tons per year (Biswal et al. 2020).
During the entire production chain of this fruit agro-industrial waste is generated, which does not have an economic value for the industry. For example, Psidium guajava skin is very soft and vulnerable to bruising during shipping. In addition, during its storage it requires low temperatures, which causes superficial damage to the fruit (burns) and microbial propagation, decreasing its market value and generating a large amount of waste made up of peel, seed, and pulp. It is estimated that the processing of one metric ton of Psidium guajava fruit generates approximately 80 kg of waste (Sathiyavimal et al. 2021). They are disposed in open-air landfills causing bad odors, propagation of vectors after their degradation, in addition to generating a negative impact on the environment. Recently, researchers have used these Psidium guajava residues to provide them a new added value for the synthesis of different nanoparticles through green synthesis. For example; (Biswal et al. 2020) used the green synthesis to elaborate α-Fe2O3/Ag NPs from Psidium guajava leaf extract, as an adsorbent for the decontamination of chromium (VI) ions in aqueous media with an adsorption capacity of 71.34 mg/g. Also, (Santhoshkumar et al. 2014) synthesized TiO2 NPs to evaluate the antibacterial and antioxidant activity through the Psidium guajava extract. On the other hand, (Sathiyavimal et al. 2021) used the phytocompounds and bio-compounds present in the extract of the Psidium guajava leaf to act as a stabilizing, protective and reducing agent to be able to synthesize nanoparticles of cupric oxide (CuO) with the aim of carrying out the industrial removal of red dyes of Congo and methylene blue through sunlight irradiation.
For this reason, the research objective of this work is to use the extract of the agro-industrial residue of Psidium guajava to synthesize Cu2O nanoparticles by a rapid and clean methodology. This method avoids the use of highly pollutant and toxic raw materials that are commonly used during the nanoparticle synthesis. The nanoparticles will be used as photocatalyst in the CO2 photoreduction to obtain solar fuels. The solar fuels production was optimized by means of an orthogonal L9 design by modifying three operational factors during the CO2 reduction to achieve higher efficiencies.
2 Methodology
2.1 Materials
The agro-industrial residue of guajava (Psidium guajava) was acquired from a plantation for domestic use in the municipality of Temixco, Morelos. The reagents used for the synthesis of the Cu2O nanoparticles were sodium hydroxide (NaOH), copper II acetate (Cu(CH3COO)2), isopropyl alcohol (C3H8O, 99%) supplied by the J.T. Baker, Fermont and Appcrom México, respectively. These reagents were used as supplied and no further purification was performed. For the preparation of the extract and solutions, deionized water (DI) with a resistivity of 18.2 MΩ.cm (Elix Technology Inside) was used.
2.2 Preparation of the Psidium guajava extract
The fruit was washed with plenty of tap water to remove traces of dirt and dust that might have adhered to the fruit peel. Then, it was washed with a solution of 1 M sodium hypochlorite (NaClO) to disinfect and inhibit the growth of pathogenic microorganisms. Subsequently, the Psidium guajava was cut into slices of approximately 2.5 cm x 2.5 cm, completing 1 kg of the guajava fruit. The Psidium guajava slices underwent a dehydration process in order to extend their shelf life, preserve and increase the main constituents of the fruit (sugars) (Aleman et al. 2020) responsible for carrying out the formation and stability of Cu2O NPs. For this, the slices were placed in metal trays and heated in a forced convection drying oven (RedLine by BLINDER) at a constant temperature of 55 °C for 24 h. At the end of this process, 3 g of crushed and dehydrated guava was taken; it was transferred to a 250 mL beaker and 100 mL of deionized water was added. The mixture was kept under constant stirring at 350 rpm for 30 min at a temperature of 60 ºC. The mixture was then filtered using a vacuum filtration kit using Whatman filter No. 1 with a diameter of 90 mm. Finally, the extract was stored in hermetic flasks and kept under refrigeration at 4 °C until it was used in the development of copper oxide (I) NPs.
2.3 Green synthesis of Cu2O nanoparticles
The synthesis of Cu2O nanoparticles was based on a 1:1 ratio, for which 20 mL of 2 M Cu(CH3COO)2 solution and 20 mL of the aqueous extract of the guajava were used, the latter acted as the reducing agent and stabilizer for Cu2O NPs. Afterwards, 20 mL of 5 M NaOH was added to the initial mixture, which acts as a precipitating and oxidizing agent for the formation of Cu2O NPs. The synthesis was carried out under mild conditions at a set temperature of 75 ºC for 10 min under constant magnetic stirring of 100 rpm. At the end of the reaction, the synthesized NPs were subjected to several washes with deionized water by means of centrifugation (Solbat/C-40) at 2800 rpm for 15 min to remove any carboxylic or phenolic compound from the supernatant that could form during the development of the NPs. Then, a final wash was performed with isopropyl alcohol to remove the internal moisture from the copper (I) oxide NPs. Finally, the synthesized material was heated in a forced convection drying oven (redLine by BINDER) at a constant temperature of 70 °C for 7 h. The powder obtained was collected and stored for later characterization and use in the CO2 photoreduction process.
2.4 Characterization of copper oxide NPs
For the structural, chemical, and optical characterization of the Cu2O NPs different techniques and characterization methods were used. The surface morphology of the Cu2O NPs was observed using a Scanning Electron Microscope (SEM, JEOL 6490 LV) operated at an accelerating voltage of 20 kV. Likewise, the elemental composition was performed through mapping in different areas of the NPs using energy dispersive spectroscopy (EDS) coupled to the SEM. Meanwhile, the optical properties of the NPs were recorded in the range from 200 to 800 nm through a spectrophotometer (UV-Vis, Cart 5000). The crystallinity of the synthesized material was studied through X-ray diffraction (XRD, SmartLab9KW, Rigaku, Japan) using CuKα radiation of λ = 0.15406 nm at a scanning speed of 20° min− 1. The average size of the crystallite was calculated using the Debye-Scherrer formula (Eq. 1)
where: D is the crystallite size (mm), λ is the X-ray wavelength (radiation) (λ = 0.154 nm), β is the width at the average height of the diffraction peak or by its initials in English FWHM (full width at half maximum) and θ is the angle of the peak in the XRD pattern, values of β and θ are in radians (Torres et al. 2023. On the other hand, the functional groups were identified using Fourier transform infrared spectroscopy analysis (FTIR, Perkin Elmer FTIR/FIR Frontier) equipped with an ATR accessory, measurements were made in a range of 500–3500 cm− 1.
2.5 CO2 reduction photocatalytic test
The photocatalytic activity of the Cu2O NPs in the reduction of CO2 was carried out using a photocatalytic reactor. For these experiments, 0.1 g of Cu2O was dispersed in 100 mL of deionized water and placed in a Pyrex batch reactor. The reactor was saturated with CO2 to remove the dissolved oxygen inside the reactor. The reduction reaction was carried out at room temperature with constant stirring of 150 rpm, which was kept under permanent irradiation with a 20 W LED lamp for 60 min. At the end of the process, the products generated during the reduction process were filtered and stored for their respective quantification and identification of the products using liquid chromatography (HPLC Prominence LC-2030 C 3D Plus) and gas chromatography (Thermo Scientific, Trace 1310) equipped with a flame ionization detector (FID).
The production of the solar fuels was optimized by means of a Taguchi L9 orthogonal design of experiments by modifying the factors shown in Table 1. These factors were selected from previous reports. For example, Chen et al. (2010) found that higher photocatalyst content increased the number of photons and pollutant molecules adsorbed. Therefore, it is supposed that the efficiency could be enhanced with the increase of the photocatalyst concentration; however, an excess of its concentration led to opacity of the suspension, which prevents the catalyst being illuminated due to the scattering and screening effects. Also, high concentration of the photocatalyst promotes its agglomeration and reduce the activity. So, it is important to optimize this parameter. Regarding the second factor, (Wu et al. 2019) it was found that the CO2 from the air can be reduced to CH3OH with high selectivity (98.35%), which represents a practical application of the artificial photosynthesis. Also, Xie et al. (2023) reported that the addition of O2 did not suppress CO2 reduction; rather, a fivefold increase was found when compared to the lack of aerobic environment. On the other hand, recently our research group found that the temperature is a critical parameter for the CO2 photoreduction (Xie et al. 2023); thus, this research proposed the optimization of this factor.
In order to optimize the production of the solar fuels, the signal to noise (S/N) ratio: Larger is better was selected to maximize the CO2 conversion under visible light (Eq. 2).
where n refers to the number of experiments and yi is the self-cleaning efficiency of the ith experiment (Mitra 2011). The analysis of the data was performed using the Minitab®18 software.
3 Results and discussion
3.1 XRD analysis
To verify the crystal phase and the average crystallite size, the powder obtained from the green synthesis was subjected to XRD analysis. These results can be seen in Fig. 1. The diffraction peaks at 2θ = 29.5°, 36.4°, 42.2° and 61.3° correspond to the crystal faces of (1 1 0), (1 1 1), (2 0 0) and (2 2 0) of the cubic structure of Cu2O, confirmed by PDF card 01-078-2017. No additional reflections corresponding to CuO, Cu or Cu(OH)2 were detected, which means that the synthesized NPs are in a pure state free of impurities. On the other hand, according to the Debye-Scherrer calculation, the crystallite size was 50 nm for Cu2O NPs.
3.2 SEM and EDS analysis
The surface morphology of the Cu2O NPs synthesized by the green pathway using the aqueous extract of Psidium guajava is shown in Fig. 2. The Cu2O NPs developed an octahedral morphology, without the need to implement any template or surfactants for the development and obtention of this type of morphology. This morphology was more homogeneous with smaller particle size than other Cu2O NPs synthesized also with a green approach from the extract of the agro-industrial residue of the banana pulp (Kumari et al. 2020).
In the literature, it has been reported that Cu2O NPs with an octahedral morphology can be prepared by a chemical precipitation process, using Na3PO4 as a precipitating agent (Dou et al. 2021). The SEM images of the Cu2O NPs show perfect octahedrons formed by 8 isosceles triangles with equal sides (1 1 1), with smooth edges and facets. This was possible through a sample precipitation-reduction-stabilization process. This can be attributed to the evolution of morphology mainly due to the various bioorganic compounds and carbohydrates present in the fruit of Psidium guajava as a stabilizing agent adsorbed on the planes (1 1 1) thus preventing growth in perpendicular directions or deformation. This interpretation can be represented as follows, (Eqs. 3 and 4):
However, it is not yet clear which of these biocompounds is responsible for obtaining the octahedral morphology in the synthesized NPs (Mallik et al. 2020). Other morphologies have also been obtained with green methods. For example; (Kumari et al. 2020) used the green synthesis for the synthesis of Cu2O NPs from grape juice; they obtained an almost spherical morphology with a high surface area, which was attributed to the complex composition of primary and secondary metabolites present in the fruit juice. To mention a few, those are flavonoids, quercetin, flavonols, tannins, peonidin, quercetin, phenolic acids, ellagic acid, and anthocyanins, etc. (Dou et al. 2021) synthesized Cu2O nanoparticles from the aqueous extract of Camellia sinensis leaves using NaOH as the precipitating agent. They obtained a morphology and texture like wheat with an agglomeration similar to that of a lump. Camellia sinensis is a plant that is made up of purine alkaloids (xanthines), terpenoids, unsaturated fatty acids, amino acids, phenolic compounds (phenolic acids and proanthocyanidins and their derivatives, catechins, O-glycosylated flavonols and C-glycosylated flavones) 3.
The EDS analysis confirms the homogeneous distribution of the Cu and O elements on the surface of the octahedrons that constitute the Cu2O NPs (Table 2). No traces of impurities were observed, this is mainly due to the washing process, which was effective enough to remove excess precursor salt or other types of agents that had been formed during the synthesis .
3.3 UV-Vis spectroscopy
The optical properties are usually determined by the type of morphology, distribution, and size of the nanoparticles (Mallik et al. 2020). The Cu2O NPs obtained showed an absorbance spectrum of approximately 430 nm, as shown in Fig. 4a. This value obtained can be directly associated with the structural properties of the synthesized Cu2O NPs. Namely; the slight separation that exists between the NPs and the octahedral morphology that they present. In addition, the large amount of NPs formed from the copper ion reduction process, which may be due to the complete binding of copper ions by the aqueous extract of the Psidium guajava fruit (Chinnaiah et al. 2022). This can be seen through SEM analysis. (Sampaio and Viana 2021) reported that the difference between the different values that are reported in the determination of the UV-Vis analysis of the metallic NPs of Cu2O are associated with the different quantities and size distributions, formation of the particles, and the different synthesis parameters used during their production. Thus, the peak width with the size distribution and peak height are correlated with the number of particles produced. UV-Vis diffuse reflectance spectra were used to calculate the band gap energy of the sample using the Kubelka-Munk function (F(R∞). A direct transition band was considered for the calculation of cuprous oxide band gap (Torres et al. 2021). In this work, a band gap value of around 1.8 eV was obtained when the Psidium guajava extract was used (Fig. 4b). Other researchers have reported similar bandgap values for Cu2O NPs synthesized from different green agents, e.g., 2.13 eV and 2.35 eV from banana extract and fresh carrot root, respectively (Celaya et al. 2021; Torres et al. 2021).
3.4 FTIR analysis
Figure 5 shows the FTIR spectrum of the Cu2O NPs. The band located at wavenumbers higher than 600 cm− 1 is associated with Cu-O in Cu2O (Tian et al. 2019). The 1093 cm− 1 is attributed to the presence of oxygen bonding in copper (Cheng et al. 2017). Also, other vibrations were identified from the spectrum of Cu2O; for example, the 1014 cm− 1 peak indicates the presence of carboxylate ions (-COO−). This band could be originated from the biosynthesis because, during the process the aldehyde group of glucose is oxidized to carboxylic acid as it was corroborated it the bands at 1404 and 1599 cm− 1 (Kazemi et al. 2020). Finally, the peaks 2976 and 2160 cm− 1 are related to the stretching vibrational mode of the C-H group (Mou et al. 2021).
3.5 BET analysis
BET method was used to investigate the textural properties of the Cu2O NPs synthesized using Psidium guajava extract. The surface area exhibited by the Cu2O NPs was 34 m2 g− 1. Meanwhile, the pore volume and pore diameter were 0.099 cm1 g− 1 and 1.01 nm, respectively. According to the classification of the International Union of Pure and Applied Chemistry (IUPAC), the synthesized NPs present a mesoporous characteristic, which describes the size of the pores that results in a type IV isotherm. Therefore, it could be successfully used in photocatalytic processes Attia and Abdel 2022). Figure 6 shows the adsorption and desorption isotherms of the Cu2O NPs synthesized using the Psidium guajava extract. The red line is the adsorption isotherm and the blue line represents the desorption isotherm.
3.6 CO2 reduction test
The phototocatalytic activity of the Cu2O nanoparticles synthesized by the green pathway was evaluated by quantifying the products from the CO2 photocatalytic reduction using H2O as an electron donor. All reactions were kept under visible light irradiation (λ > 400 nm). The main product detected was HCOOH. It is worth mentioning that reference experiments were performed, and no product was detected in the absence of the Cu2O photocatalyst.
Table 3 shows the formic acid production obtained from CO2 photoreduction using the green Cu2O NPs. As seen, higher Cu2O amounts favored better efficiencies for CO2 reduction since the formic acid production tended to increase. Also, higher airflow favors the CO2 conversion to HCOOH under visible light. However, a statistical analysis was performed to analyze the effect of each factor on the CO2 conversion to HCOOH.
3.7 Analysis of the design of experiments
Taguchi’s larger the better option was selected to analyze the effect of the three different factors in the HCOOH production. The analysis of variance (ANOVA) was calculated by using Minitab®19 software, whose results are given in Table 4. In this case, ANOVA was used to prioritize the parameters and predict the optimal experimental conditions for maximum CO2 reduction. The ANOVA for means evidence that the three factors studied were significant to explain the response since their p-values were lower than the significance fixed value (α = 0.05) for means and S/N ratios. The catalyst load had the highest contribution to explain the response (47.6%) followed by the airflow (42.1%) and the temperature (10.3%).
The main effect plots of each factor for S/N ratios are shown in Fig. 7, where the HCOOH produced is plotted on the y-axis while the input parameters and their levels are plotted on the x-axis. It can be observed from the trends depicted in Fig. 6a, b that the catalyst load and the temperature are the most influential parameters for HCOOH production. Considering this data, the optimal production of HCOOH using the Cu2O NPs is obtained with: 0.2 g of catalyst, 0.7 L min− 1 of air flow, and 50 °C. This combination of the studied factors that produced the highest HCOOH production (103.4 µmol h− 1) is shown in Table 4.
Previous research demonstrated that as temperature increases, the CO2 photoreduction enhanced since the kinetic energy of the molecules will collide (Ávila et al. 2022). Also, photocatalytic reactors with Cu2O NPs could be a promising option to be installed in CO2 capture stations since these units can be operated at 50 °C (Alexanda and Masoudi 2019). Additionally, this temperature seems to be the adequate to favor the desorption of the products from the photocatalyst surface, preventing the poisoning of the photocatalyst.
Under the optimal experimental conditions, other products were analyzed using gas and liquid chromatography. HCOH, CH3OH, and CH3CH2OH were identified in low amounts (< 10 µmol), which confirms the selectivity of the reaction for HCOOH production.
The origin of the enhanced activity of Cu2O NPs under optimal conditions is discussed below. The Cu2O loading generates more active species that reduce the CO2 molecule to HCOOH. Even at higher loads, the turbidity of the dispersion seems not be affected significantly, which could be related to the low particle size promoted by the green synthesis method that supports a good dispersion of the photocatalyst. On the other hand, the addition of O2 (~ 20%) of the air enhanced the conversion. Previous work with MOF (metal organic framework) materials suggested that under aerobic CO2 reduction, they tended to be hydroxylated. These groups could interact with the holes by two actions: (i) preventing the photocorrosion and (ii) avoiding the charge recombination.
Compared to previous reports, the HCOOH production obtained in this study is higher than other cases where Cu2O NPs were prepared by the traditional methods (see Table 5). Thus, it is important to modify the operational parameters to assure better efficiencies in the CO2 photoreduction and to scale up the process. As a note, these results are only valid for the selected experimental region and for Cu2O. It is difficult to extrapolate this information with other photocatalysts, however, this information could serve as a guide for future research.
3.8 Photocatalytic water splitting for H2 evolution
The photocatalytic activity of the Cu2O NPs was also evaluated for H2O splitting. Figure 8 shows the time-dependent H2 production under visible-light using the Cu2O NPs as photocatalyst. As is shown, within the first 10 min the reaction reaches the steady state, reaching a H2 production of 1.3 mmol H2/g without the use of sacrificial agents. This efficiency is up to 16 times higher than the production obtained with Cu2O synthesized with commercial glucose as reducing agent in the same photocatalytic reactor (Luévano et al. 2017). At the beginning the reaction is fast probably due to the conversion of Cu2O to CuO that provides additional electrons to reduce the protons (H+) (Luévano et al. 2022). However, the behavior of the sample was stable during the irradiation, indicating a good stability for this sample.
In general, this work evidenced that the use of agro-industrial waste can be useful in synthesizing semiconductor nanoparticles with photocatalytic activity to generate renewable and clean fuels, such as HCOOH and H2, from CO2 and H2O under artificial illumination. However, more research is needed to continue increasing the efficiency of the reactions, and to demonstrate its ability at large scale in bigger (continuous) reactors that operate with direct sunlight. Also, more research should be done to provide strategies to immobilize the catalysts.S
4 Conclusions
The aqueous agro-industrial residue of the Psidium guajava fruit and its constituents proved to be suitable to stabilize and act as reducing agents for the preparation of Cu2O NPs synthesized by the green route. The raw material and NPs obtained are characterized for being easily accessible, renewable and of low cost. The results revealed that these green NPs have a surface area of 34 m2 g− 1 with mesoporous characteristics. The bandgap value of these NPs was calculated as around 1.8 eV. It should be noted that using the agro-industrial residue of Psidium guajava, an octahedral morphology is obtained without the need to implement any surfactant to obtain it. Cu2O NPs exhibited activity for CO2 photoreduction, whose efficiency was optimized by an orthogonal Taguchi L9 design. The factors studied were catalyst loading, air flow, and temperature. In the case of CO2 photoreduction, Cu2O NPs can produce high added value products such as HCOOH, using artificial light and mild reaction conditions. Furthermore, the results indicated that Cu2O NPs may be the ideal nanomaterial to carry out hydrogen production, reaching a value of 1.3 mmol H2/g during the first 10 min of reaction, without sacrificial agents. The synthesized NPs may have other applications, such as in solar cells, dye degradation, and biological applications. It is expected that with the use of these agro-industrial wastes, their polluting effect will be minimized and their added value will increase in the development of biomaterials for the bioenergy area.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- CO2 :
-
Carbon dioxide
- CCUS:
-
Carbon capture, utilization and storage
- GCS:
-
Geological carbon sequestration
- CH4 :
-
Methane
- CH3OH:
-
Methanol
- CH3COCH3 :
-
Acetone
- CH3COOH:
-
Acetic acid
- CH3CH(OH)CH3 :
-
Isopropanol
- HCHO:
-
Formaldehyde
- HCOOH:
-
Formic acid
- NHE:
-
Normal hydrogen electrode
- NPs:
-
Nanoparticles
- DI:
-
Deionized water
- NaClO:
-
Sodium hypochlorite
References
Aguirre ME, Zhou R, Eugene AJ, Guzman MI, Grela MA (2017) Cu2O/TiO2 heterostructures for CO2 reduction through a direct Z-scheme: protecting Cu2O from photocorrosion. Appl Catal B 217:485–493. https://doi.org/10.1016/j.apcatb.2017.05.058
Akrami S, Edalati P, Shundo Y, Watanabe M, Ishihara T, Fuji M, Edalati K (2022) Significant CO2 photoreduction on a high-entropy oxynitride. Chem Eng J 449:137800. https://doi.org/10.1016/j.cej.2022.137800
Aleman JL, Pérez BY, Torres S, Saldaña S, Longoria A, Sebastian PJ (2020) Bioethanol production from ataulfo mango supplemented with vermicompost leachate. Catal Today 353:173–179. https://doi.org/10.1016/j.cattod.2019.07.028
Alemán JL, Okoye PU, Torres S, Mejía M, Sebastian PJ (2022) A review on bioenergetic applications of Leucaena leucocephala. Ind Crops Prod 182:114847. https://doi.org/10.1016/j.indcrop.2022.114847
Alexanda B, Masoudi S (2019) Optimization of Post Combustion CO2 Capture from a Combined-Cycle Gas Turbine Power Plant via Taguchi Design of Experiment. Processes 7(6): 364. https://doi.org/10.3390/pr7060364
Amadike E, Emmanuel O, Ebubechi M, Dike E, Chukwuebuka B, Ibe C, Chibueze V, Nwabu C, Chinyere O (2022) The ethnobotanical, phytochemistry and pharmacological activities of Psidium guajava L. Arab J Chem 15(5):103759. https://doi.org/10.1016/j.arabjc.2022.103759
Apriandanu DOB, Yulizar Y (2019) Tinospora crispa leaves extract for the simple preparation method of CuO nanoparticles and its characterization. Nano-Structures Nano-Objects 20:100401. https://doi.org/10.1016/j.nanoso.2019.100401
Attia Y, Abdel SH (2022) Nano Cu2O catalyzed ultrasonic-assisted green synthesis of some seleno[2,3-b] quinoline derivatives. J Organomet Chem 960:122245. https://doi.org/10.1016/j.jorganchem.2021.122245
Ávila MA, Luévano E, Torres LM (2022) Optimizing the CO2 reduction to produce CH3OH using flexible NiMoO4 coatings as a photocatalyst. J Alloys Compd 918:165549. https://doi.org/10.1016/j.jallcom.2022.165549
Biswal SK, Panigrahi GK, Sahoo SK (2020) Green synthesis of Fe2O3-Ag nanocomposite using Psidium guajava leaf extract: an eco-friendly and recyclable adsorbent for remediation of cr(VI) from aqueous media. Biophys Chem 263:106392. https://doi.org/10.1016/j.bpc.2020.106392
Cai W, Yu X, Cao Y, Hu C, Wang Y, Zhao Y, Bu Y (2022) Electron-coupled enhanced interfacial interaction of Ce-MOF/Bi2MoO6 heterostructure for boosted photoreduction CO2. J Environ Chem Eng 10(3):107461. https://doi.org/10.1016/j.jece.2022.107461
Celaya CA, Delesma C, Torres S, Sebastian PJ, Muñiz J (2021) Understanding CO 2 conversion into hydrocarbons via a photoreductive process supported on the Cu2 O(1 0 0), (1 1 0) and (1 1 1) surface facets: a first principles study. Fuel 306:121643. https://doi.org/10.1016/j.fuel.2021.121643
Chen S, Zhang H, Yu X, Liu W (2010) Photocatalytic reduction of Nitrobenzene by Titanium Dioxide Powder. Chin J Chem 28(1):21–26. https://doi.org/10.1002/cjoc.201090030
Cheng X, Dong P, Huang Z, Zhang Y, Chen Y, Nie X, Zhang X (2017) Green synthesis of plasmonic ag nanoparticles anchored TiO 2 nanorod arrays using cold plasma for visible-light-driven photocatalytic reduction of CO2. J CO2 Utilization 20:200–207. https://doi.org/10.1016/j.jcou.2017.04.009
Chinnaiah K, Maik V, Kannan K, Potemkin V, Grishina M, Gohulkumar M, Tiwari R, Gurushankar K (2022) Experimental and theoretical studies of Green synthesized Cu2O nanoparticles using Datura Metel L. J Fluoresc 32(2):559–568. https://doi.org/10.1007/s10895-021-02880-4
Cui L, Hu L, Shen Q, Liu X, Jia H, Xue J (2022) Three-dimensional porous Cu2O with dendrite for efficient photocatalytic reduction of CO2 under visible light. Appl Surf Sci 581:152343. https://doi.org/10.1016/j.apsusc.2021.152343
Dedong Z, Maimaiti H, Awati A, Yisilamu G, Fengchang S, Ming W (2018) Synthesis and photocatalytic CO2 reduction performance of Cu2O/Coal-based carbon nanoparticle composites. Chem Phys Lett 700:27–35. https://doi.org/10.1016/j.cplett.2018.04.007
Dou L, Zhang X, Zangeneh MM, Zhang Y (2021) Efficient biogenesis of Cu2O nanoparticles using extract of Camellia sinensis leaf: evaluation of catalytic, cytotoxicity, antioxidant, and anti-human ovarian cancer properties. Bioorg Chem 106:104468. https://doi.org/10.1016/j.bioorg.2020.104468
Elviera Y, Apriandanu DO, Marcony R (2022) Fabrication of novel SnWO4/ZnO using Muntingia calabura L. leaf extract with enhanced photocatalytic methylene blue degradation under visible light irradiation. Ceram Int 48(3):3564–3577. https://doi.org/10.1016/j.ceramint.2021.10.135
Fadhila FR, Umar A, Chandren S, Apriandanu DO, Yulizar Y (2024) Biosynthesis of CoCr2O4/ZnO nanocomposites using Basella alba L. leaves extracts with enhanced photocatalytic degradation of malachite green in aqueous media. Chemosphere 352:141215. https://doi.org/10.1016/j.chemosphere.2024.141215
Faria AL, Centurion HA, Torres JA, Gonçalves RV, Ribeiro LS, Riberio C, Da Cruz JC, Nogueira FG (2021) Enhancing Nb2O5 activity for CO2 photoreduction through Cu nanoparticles cocatalyst deposited by DC-magnetron sputtering. J CO2 Utilization 53:101739. https://doi.org/10.1016/j.jcou.2021.101739
Halomoan I, Yulizar Y, Surya RM, Apriandanu DO (2022) Facile preparation of CuO-Gd2Ti2O7 using Acmella uliginosa leaf extract for photocatalytic degradation of malachite green. Mater Res Bull 150:111726. https://doi.org/10.1016/j.materresbull.2021.111726
Indriyani A, Yulizar Y, Tri R, Oky Bagus, Marcony R (2021) One-pot green fabrication of BiFeO3 nanoparticles via Abelmoschus esculentus L leaves extracts for photocatalytic dye degradation. Appl Surf Sci 563:150113. https://doi.org/10.1016/j.apsusc.2021.150113
Jiang H, Katsumata K, Hong J, Yamaguchi A, Nakata K, Terashima C, Matsushita N, Miyauchi M, Fujishima A (2018) Photocatalytic reduction of CO2 on Cu2O-loaded Zn-Cr layered double hydroxides. Appl Catal B 224:783–790. https://doi.org/10.1016/j.apcatb.2017.11.011
Jiang Y, Xia Shen L, Ma, Ma H, Sun T, Lv F, Zhu N (2021) Facet-dependent Cu2O electrocatalysis for wearable enzyme-free Smart Sensing. ACS Catal 11(5):2949–2955. https://doi.org/10.1021/acscatal.0c04797
Kazemi S, Najinasab A, Nikbakht R, Dabiri M (2020) Visible light assisted photocatalytic reduction of CO2 to methanol using Fe3O4@N-C/Cu2O nanostructure photocatalyst. J Photochem Photobiol A 401:112763. https://doi.org/10.1016/j.jphotochem.2020.112763
Kumari N, Kumari P, Jha AK, Prasad K (2020) Green synthesis of Cu2O nanoparticles using grape juice and its antimicrobial activity. AIP Conference Proceedings 2220: 020042. https://doi.org/10.1063/5.0002290
Kusumah AD, Yulizar Y, Apriandanu DO, Surya RM (2024) Fabrication of ZnO and ZnO/CuMoO4 for the improvement of photocatalytic performance. Vacuum 222:113034. https://doi.org/10.1016/j.vacuum.2024.113034
Liu SH, Lu JS, Pu YC, Fan HC (2019) Enhanced photoreduction of CO2 into methanol by facet-dependent Cu2O/reduce graphene oxide. J CO2 Utilization 33:171–178. https://doi.org/10.1016/j.jcou.2019.05.020
Lu X, Luo X, Tan JZ, Maroto MM (2021) Simulation of CO2 photoreduction in a twin reactor by multiphysics models. Chem Eng Res Des 171:125–138. https://doi.org/10.1016/j.cherd.2021.04.011
Luévano E, Torres LM (2020) Dolomite-supported Cu2O as heterogeneous photocatalysts for solar fuels production. Mater Sci Semiconduct Process 116:105119. https://doi.org/10.1016/j.mssp.2020.105119
Luévano E, Torres LM, Sánchez D, Alfaro MR (2017) Cu2O precipitation-assisted with ultrasound and microwave radiation for photocatalytic hydrogen production. Int J Hydrog Energy 42(18):12997–13010. https://doi.org/10.1016/j.ijhydene.2017.03.192
Luévano E, Torres LM, Ávila MA (2022) Visible-light-driven CO2 reduction and H2 evolution boosted by 1D Cu2O/CuO heterostructures. J Phys Chem Solids 170:110924. https://doi.org/10.1016/j.jpcs.2022.110924
Mallik M, Monia S, Gupta M, Ghosh A, Toppo MP, Roy H (2020) Synthesis and characterization of Cu2O nanoparticles. J Alloys Compd 829:154623. https://doi.org/10.1016/j.jallcom.2020.154623
Marcony R, Mauliddiyah S, Bagus DO, Sudirman, Yulizar Y (2022) SmMnO3-decorated ZnO in a hexane-water interface for enhancing visible light-driven photocatalytic degradation of malachite green. Chemosphere 304:135125. https://doi.org/10.1016/j.chemosphere.2022.135125
Mitra A (2011) The Taguchi method. WIRE Comput Stat 3(5):472–480. https://doi.org/10.1002/wics.169
Monteiro J, Roussanaly S (2022) CCUS scenarios for the cement industry: is CO2 utilization feasible? J CO2 Utilization 61:102015. https://doi.org/10.1016/j.jcou.2022.102015
Mou Q, Guo Z, Chai Y, Liu B, Liu C (2021) Visible-light assisted photoreduction of CO2 using CdS-decorated Bi24O31Br10. Mater Sci Semiconduct Process 134:106011. https://doi.org/10.1016/j.mssp.2021.106011
NganDo T, You C, Park M, Kim C, Lee S, Kim J (2022) Optimization-based assessment framework for CO2 utilization to fuels strategies. Comput Aided Chem Eng 51:763–768. https://doi.org/10.1016/B978-0-323-95879-0.50128-4
Ovcharov ML, Mishura AM, Shcherban ND, Filonenko SM, Granchak VM (2016) Photocatalytic reduction of CO2 using nanostructured Cu2O with foam-like structure. Sol Energy 139:452–457. https://doi.org/10.1016/j.solener.2016.10.010
Peixoto JC, Nogueira AE, Dias A, Torres JA, Da Cruz JC, Ribeiro C, Siqueira (2022) Experimental evaluation of the activity and selectivity of pure MnWO4 and doped with rare earth ions in the CO2 photoreduction process. Mater Res Bull 153:111912. https://doi.org/10.1016/j.materresbull.2022.111912
Punnam PR, Dutta A, Krishnamurthy B, Surasani VK (2023) Study on utilization of machine learning techniques for geological CO2 sequestration simulations. Materials Today: Proceedings 72: 378–385. https://doi.org/10.1016/j.matpr.2022.08.109
Rahman A, Chowdhury MA, Hossain (2022) Green synthesis of hybrid nanoparticles for biomedical applications: a review. Appl Surf Sci Adv 11:100296. https://doi.org/10.1016/j.apsadv.2022.100296
Sampaio S, Viana JC (2021) Optimisation of the green synthesis of Cu/Cu2O particles for maximum yield production and reduced oxidation for electronic applications. Mater Sci Engineering: B 263:114807. https://doi.org/10.1016/j.mseb.2020.114807
Santhoshkumar T, Rahuman AA, Jayaseelan C, Rajakumar G, Marimuthu S, Kirthi AV, Velayutham K, Thomas J, Venkatesan J, Kim SK (2014) Green synthesis of titanium dioxide nanoparticles using Psidium guajava extract and its antibacterial and antioxidant properties. Asian Pac J Trop Med 7(12):968–976. https://doi.org/10.1016/S1995-7645(14)60171-1
Sathiyavimal S, Vasantharaj S, Veeramani V, Saravanan M, Rajalakshmi G, Kaliannan T, Al-Misned, Pugazhendhi A (2021) Green chemistry route of biosynthesized copper oxide nanoparticles using Psidium guajava leaf extract and their antibacterial activity and effective removal of industrial dyes. J Environ Chem Eng 9(2):105033. https://doi.org/10.1016/j.jece.2021.105033
Syed F, Ullah A (2021) Estimation of economic benefits associated with the reduction in the CO2 emission due to COVID-19. Environ Challenges 3:100069. https://doi.org/10.1016/j.envc.2021.100069
Tian X, Wen J, Chen Z, Liu X, Peng H, Ji C, Li J, Peng Y, He H (2019) One-pot green hydrothermal synthesis and visible-light photocatalytic properties of Cu2O/Cu hybrid composites using egg albumin as structure modifier. Solid State Sci 93:70–78. https://doi.org/10.1016/j.solidstatesciences.2019.04.013
Torres S, Reyes O, Pantoja J, Aleman JL, Huerta A, Moreira J, Muñiz J, Vargas L, Sebastian PJ (2021) Biosynthesis of cuprous oxide using banana pulp waste extract as reducing agent. Fuel 285:119152. https://doi.org/10.1016/j.fuel.2020.119152
Torres S, Torres LM, Luévano E, Aleman JL, Sebastian PJ (2023) Biologically mediated synthesis of CuO nanoparticles using corn COB (Zea mays) ash for photocatalytic hydrogen production. Mater Chem Phys 301:127640. https://doi.org/10.1016/j.matchemphys.2023.127640
Valls Martínez S, Jaén S, Román Martín Cervantes (2022) Are gender and cultural diversities on board related to corporate CO2 emissions? J Clean Prod 363:132638. https://doi.org/10.1016/j.jclepro.2022.132638
Wang H, Cheng S, Cai X, Cheng L, Zhou R, Hou T, Li Y (2022) Photocatalytic CO2 reduction to HCOOH over core-shell Cu@Cu2O catalysts. Catal Commun 162:106372. https://doi.org/10.1016/j.catcom.2021.106372
Wu X, Li Y, Zhang G, Chen H, Li J, Wang K, Pan Y, Zhao Y, Sun Y, Xie Y (2019) Photocatalytic CO 2 Conversion of M 0.33 WO 3 directly from the air with high selectivity: insight into full Spectrum-Induced reaction mechanism. J Am Chem Soc 141(13):5267–5274. https://doi.org/10.1021/jacs.8b12928
Xie S, Deng C, Huang Q, Zhang C, Chen C, Zhao J, Sheng H (2023) Facilitated Photocatalytic CO 2 reduction in aerobic environment on a copper-porphyrin metal–Organic Framework. Angew Chem Int Ed 62(10):e202216717. https://doi.org/10.1002/anie.202216717
Yao S, Sun BQ, Zhang P, Tian Z-Y, Yin H-Q, Zhang ZM (2022) Anchoring ultrafine Cu2O nanocluster on PCN for CO2 photoreduction in water vapor with much improved stability. Appl Catal B 317:121702. https://doi.org/10.1016/j.apcatb.2022.121702
Yulizar Y, Gunlazuardi J, Apriandanu S (2020) CuO-modified CoTiO3 via Catharanthus roseus extract: a novel nanocomposite with high photocatalytic activity. Mater Lett 277:128349. https://doi.org/10.1016/j.matlet.2020.128349
Zhu Q, Cao Y, Tao Y, Li T, Zhang Y, Shang H, Song J, Li G (2021) CO2 reduction to formic acid via NH2-C@Cu2O photocatalyst in situ derived from amino modified Cu-MOF. J CO2 Utilization 54:101781. https://doi.org/10.1016/j.jcou.2021.101781
Acknowledgements
The authors are grateful to Miss. Maria Luisa Ramón Garcia for the XRD analysis. P.J. Sebastian is grateful to DGAPA-UNAM for the economic support through the project IN108922 and Edith Luévano-Hipólito thanks CONAHCYT for the economic support of the projects: Catedras CONAHCYT 1060 and Paradigmas and Fronteras de la Ciencia 320379. Torres Arellano (619727) and Aleman Ramirez (715276) thank CONAHCYT for the post-doctoral fellowship.
Author information
Authors and Affiliations
Contributions
S. T.: Methodology, Investigation and Data curation. E. L. : Reviewing, writing, and methodology. M. F. : Methodology. J.A. : Investigation, Methodology and Manuscript writing. L.T. : Reviewing. and P.S. : Reviewing and Editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict
of competing interests:
The authors have no financial or personal conflict of interest with this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Torres-Arellano, S., Luevano-Hipolito, E., Fabela-Cedillo, M.G. et al. Optimized CO2 photoreduction using cuprous oxide (Cu2O) nanoparticles synthesized using Psidium guajava extract. Energ. Ecol. Environ. (2024). https://doi.org/10.1007/s40974-024-00331-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40974-024-00331-x