Abstract
The present study investigated existing and potential approaches for hot cracking analysis of cast alloys and showed a promising and very applicable new specimen for high alloyed steels. The reliability of this specimen has been demonstrated by adding niobium to alloy HH (ASTM A297), or alloy 1.4837 (ISO 11973). Niobium shows a very positive impact on hot cracking behavior and can thereby reduce scrap in steel foundries. Amounts above 0.6 wt% niobium can reduce the size and number of hot cracks significantly. The reduction of hot cracking tendency was quantified by the cracking factor CFNb2. The positive effect is associated with the reduction in solidification interval length that was measured by thermal analysis and the observed “healing” of cracks in the microsections. Both effects are promoted by niobium. The promotion of the ferritic solidification seems to have no positive impact on the hot cracking behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Currently, many steel foundries are facing complications with rising raw material costs, which forces them to improve their process, e.g., by producing with lower scrap rates. One of the reasons for scrapping a casting is the occurrence of hot cracks, a shrinkage related casting defect. This defect depends on the solidification mode of the alloy, the stiffness of the molding material, the design of the casting, and other factors. If it is not possible to change the geometry or the sand system, other ways have to be found to reduce hot cracks.1,2
There are several hot cracking tests for cast alloys. The first example is the “dog-bone” sample, mostly used for aluminum alloys.3 Furthermore, there is a cross-like sample,2 and a bar-like4 which are used for steels. Both specimens for steels show a high necessity for material, instrumentalization, and preparation for result estimation. Neither of these samples were found in the literature to be ever used for high alloyed steels. Other samples are concluded as reviewed by Lu et al.5.
Heat resistant materials can be used for high temperature application in, e.g., automotive turbine housings, such as the 1.4837 (GX40CrNiSi25-12). These high alloyed steels have a high stability against oxidation and corrosion as well as a good creep resistance. Niobium is generally known to improve strength and creep properties of these materials at high temperatures.6,7,8 The carbon affine metal niobium leads to the precipitation of the thermodynamically stable, cubic M(C,N)-carbides.9,10 These carbides precipitate interdendritically during the eutectic reaction in the material 1.4837.11 The modification of properties such as the solidification interval and carbide precipitation due to niobium addition can be shown by JMatPro (version 13) simulation (See Figure 1). The solidification interval is reduced from ΔTSOL = 137.8 °C (TL = 1391.7 °C; TS = 1253.9 °C) without niobium addition to ΔTSOL = 124.2 °C (TL = 1372.1 °C; TS = 1247.9 °C) with 1.80 wt% niobium. Furthermore, niobium promotes the ferritic solidification.
Different researchers state that niobium, being added to filler materials for welding, can reduce hot cracking of the produced welds.12,13
Several non-scientific sources, e.g., foundrymen from India, Brazil, and Germany, report that niobium actually decreases the tendency to hot cracking in high alloyed steel castings. The reduction of solidification interval should promote this property, as well as ferritic solidification, which can improve the crack resistance by reduction of the thermal expansion coefficient. For this reason, a sensitive specimen had to be developed for high alloyed steels and the results of the hot cracking tendency should be confirmed by thermal analysis and dilatometric measurements.
Experimental Procedure
The first attempt was to design a “dog-bone” specimen (similar to3) that is easy to cast and evaluate by X-ray, but the samples cast in cold box molds quickly showed that no representative results could be obtained. In the next step, two stages of molds were simulated using a numerical software package, MAGMASOFT version 5.5.1. After promising solidification simulation results (See Figure 2 for the stage 1 sample), the stage 2 was run and the molds were printed.
The printed sand molds were made of furan bonded silica sand (FS001). The cylindric chills added for directional solidification were made of regular steel (grade S235JR). In stage 1, the chill at the end of the bar was surrounded by a cast ring of solidified metal (See Figure 3a). Dovetail endings of the rods were designed to prevent free contraction in the stage 2 sample (Cf. Figure 3b). Thus, both of the stages used a form-fitting fixture to prevent free contraction. The main differences between the two stages are the length of the bars (stage 1: 40, 50, 60, 80, 100, 120 mm and stage 2: 30, 50, 70, 90, 120, 150 mm) and the number of chills placed at the end of the bars (stage 1: 1 and stage 2: 2). Additionally, every mold was fixed in a steel tooling that avoids stress relief by the opening of the mold parting line.
All the HH alloys (See Table 1) were cast at a temperature of 1545–1555 °C in the molds for hot cracking analysis after melting of low alloyed steel scrap, nickel, ferrosilicon, ferrochromium, ferromanganese, and ferroniobium (grade 111) in a 10 kg lifting crucible inductive furnace. Sulfur and phosphorus were kept at a certain level below 0.02 and 0.04 wt%, respectively.
Thermal analysis was done by using type S thermocouples (tolerance class 1) in an optimized Quik-Cup with a cover (See Figure 4a, b).14 An Almemo 710 with a sampling rate of 100 Hz was used for data acquisition. The casting temperature for the thermal analysis was 1575–1585 °C. An exemplary thermal analysis curve and the first derivate can be seen in Figure 4c. For the evaluation of the recorded curves, smoothening with a Savitzky–Golay filter (polynomial function of 2nd degree with a 1000p smoothening) was done.15
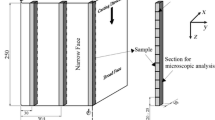
Evaluation of all hot crack samples was conducted in the laboratory of the Foundry Department (Technical University Bergakademie Freiberg) by visual inspection and eddy current testing with the device Norton 600 by Olympus. Afterward, for the quantification and detection of inner cracks, the specimens were analyzed via X-ray on a YXLON YSMART 583 device. The SEM investigations were performed on the VEGA3 W-REM (Tescan) with the EDS-sensor XFlash 610M (Bruker Nano). The microstructure was analyzed on an Olympus BX51M microscope after embedding the samples in resin, wet grinding and polishing with a 1 µm OPU final polishing step. The dilatometric measurements were conducted on the push-rod dilatometer DIL 402 C (Netzsch).
Results and Discussion
Direct Observation
The results showed that the analyzed cracks where hot cracks, having a dendritic structure (See Figure 5).
After visual inspection, due to the sensitivity of the sample, it can be seen that niobium has a tremendously positive impact on hot cracking, which was the first time possible to confirm with a scientifically applied sample. By the number of broken rods, shown in Figure 6, and the evaluation of their lengths (l2) (Table 2), it can be seen, that the tendency of hot cracking is lower with higher niobium additions. Overall, the cracks appear, as expected, in the simulated mushy zones (Cf. Figure 2).
After alloying niobium, the hot cracks appeared to be lower in quantity and smaller in size. By adding 1.85 wt% niobium (alloy Nb18) no hot cracks were detectable in the stage 1 geometry (See Figure 6b).
To improve the resolution of the results, the lengths of the bars in stage 2 were adjusted. The results after X-ray examination of the stage 2 sample are shown in Figure 7. The improved geometry allowed to rate a wider range of the hot cracking tendency.
The analysis of the microstructure of the as-cast samples showed, that the ferrite content increases with niobium (See Figure 8). Confirmation of the ferrite stabilization was gathered by magnetic measurements, e.g., Feritscope. This goes along with the JMatPro simulation (See Figure 1).
The interdendritic carbides changed from being chromium-rich M23C6 to the Chinese script niobium-rich M(C,N)-type. While in alloy Nb00 (Cf. Figure 8a) only M23C6 was present, in the alloys Nb12 and Nb18 (See Figure 8b) only M(C,N) could be detected. Analyzing alloy Nb06 it could be shown that both carbide types coexist in the microstructure. This was confirmed in the second trial series, analyzing the stage 2 geometry. The increased Cr-content in the M23C6 was confirmed by EDS-measurements, the definition of M23C6 was done by the evaluation of the simulation results in Figure 1. M(C,N) were detected by EDS measurement and confirmed to be niobium-rich.
Thermal Analysis, Thermal Expansion and Cracked Length
Niobium exhibits two significant changes in the solidification behavior of the selected HH alloy. On the one hand, it decreases the liquidus temperature, and on the other hand, it significantly increases the solidus temperature (See Table 2). By bonding with carbon (and nitrogen), niobium can raise the solidification temperature of the alloy.
The expansion coefficient α, shown in Table 2, increases with increasing niobium content and does not go along with the reduction in hot cracking tendency. Since ferrite is known to have a lower thermal expansion coefficient than austenite, the induced tensions should be reduced. Whenever a change of carbide type and solid solution of Nb increases the thermal expansion slightly at the temperature of 1100 °C. The impact of higher ferrite contents during solidification, like it is shown in Figure 1, shall be investigated by future dilatometric measurements within the solidification interval.
Table 3 shows the same trend of reduction in liquidus temperature and increase in solidus temperature. Aligning both trial series for the 1.4837, the trend of a slight decrease in liquidus temperature can be seen in Figure 9. In the range from 0.01 to 1.85 wt% niobium the liquidus temperature is reduced by 9.6°C/wt% Nb, whenever the scattering is relatively high (R2 = 0.22) due to small variations in the base alloy (e.g., chromium content).
The solidus temperature shows a local minimum at 0.4 wt% Nb (alloy Nb04a), which can be addressed to the solid solution of niobium in the austenite (0.11 wt% in alloy Nb06a) and residual interdendritic melt (Cr rich carbides: 0.27 wt% in alloy Nb06a). Above 0.4 wt% Nb the solidus temperature is continuously increased by 49.8 °C/wt% Nb (R2 = 0.89).
The calculation of the solidification interval after the following equation (Eqn. 1):
revealed a maximum at 0.4 wt% Nb (alloy Nb04a). Above this content the solidification interval was reduced by 51.8 °C/wt% Nb (R2 = 0.87) (See Figure 10). Taking the full range of variation in niobium content from 0.01 to 1.85 wt% Nb into account, the solidification interval was reduced by 38.8°C/wt% Nb (R2 = 0.79). Comparing the practical results of the thermal analysis with the simulation using JMatPro software, a significant difference can be observed (Cf. Figure 1). The reduction of the solidification interval length in the practical tests is significantly higher than in the simulation. It can be especially shown that solidus temperature has a completely different trend by comparing simulation and practical results. This could be addressed to the differences in cooling conditions (infinite cooling time in the simulation and comparingly fast cooling in the thermal analysis).
Cracking Factor
To have the possibility of a quantification of the hot cracking tendency, the cracking factor for the new developed sample of stage 2 for the alloying element niobium CFNb2 was introduced (Eqn. 2):
where CFbar, i is the crack factor of each of the 12 bars. It is evaluated depending on its length and the size of the crack (Eqn. 3):
where lF is the length factor of the cracked bar, ranging from 1 to 7. Whereby the longest bar of 150 mm will be evaluated by the smallest factor of 1. The shorter the fractured bar, the higher the factor lF. Furthermore, it is considered whether the bar is completely ruptured. In this case, the critical factor cF will receive a value of 2, in the case of a smaller crack or microcrack, the value will be 1. The indication factor x is rated by 0 (uncracked) or 1 (cracked).
The calculated cracking factor (Cf. Table 4 and Figure 11) is consistent with the visual inspection and the X-ray results (See Figure 7), depending on the niobium content. The cracking factor and the visual appearance of cracks increase slightly from no niobium addition (trace element in alloy Nb00a) to a niobium content of 0.4–0.6 wt% (in alloy Nb04a/Nb06a).
Higher additions of niobium lead to a significant reduction of the visual cracking appearance and the calculated cracking factor. This result goes along with the qualitative feedback from foundries, which report a significant reduction of casting scrap when adding more than 0.6 wt% niobium to high alloyed steels.
Even though the hot cracking tendency can be significantly reduced from 0.6 wt% Nb (alloy Nb06a) to 0.93 wt% Nb (alloy Nb09a), a further increase of niobium can decrease the hot cracking tendency even more successfully. This effect is important, especially for complex geometries.
The correlation of the cracking factor with the solidification interval is shown in Figure 12. It can be seen that the crack factor is dependent on the solidification interval (R2 = 0.86). The reduction of the solidification interval is one of the main influencing factors to prevent hot cracking.
“Healing” of Hot Cracks
Significantly cracked areas of the specimen with different niobium contents were analyzed in microscopic cross sections. In case of the alloy that was not alloyed with niobium, significant interdendritic cracks appeared in the mushy zone (Cf. Figure 2). These cracks show no indication of any crack “healing” (See Figure 13a). The dendrites and interdendritic spaces are homogeneously distributed.
In comparison, Figure 13b reveals strong agglomerations of niobium-rich interdendritic areas with precipitated Chinese script M(C,N)-carbides. With higher niobium contents, it can be seen that the interdendritic areas that were ruptured during early solidification are filled with the niobium-rich residual melt while the proceeding solidification. The process can be explained by ruptured areas that create a decreased pressure within the cracks. The moment, when crack opening appears, the liquid niobium-rich melt seeps into the crack, filling the void, solidifying, and ultimately preventing the hot crack casting defect from appearing.
Conclusions
With the newly developed specimen of stage 2, the possibility for an accurate, comparative quantification of the tendency for hot tearing of high alloyed steels is given.
-
1.
The specimen is easy to cast and does not require big melting furnaces or any expensive and time-consuming evaluation methods.
-
2.
The sample is flexible by varying the length of the used cracking bars.
Furthermore, niobium has a production relevant impact on the steel 1.4837 to decrease the scrap rate by reducing the occurrence of hot cracks.
-
3.
The length of the solidification interval of the high alloyed steel 1.4837 can be reduced by the addition of niobium. This reduction can be directly correlated with the hot cracking tendency.
-
4.
Niobium alloying in this heat resistant steel leads to a crack “healing” within the mushy zones of the cast specimens.
The simulation with JMatPro shows an increased content of ferrite during the solidification, which can be confirmed by higher residual ferrite contents in the samples. Whenever the differences between the simulation results of liquidus and solidus temperature and the results measured by thermal analysis are high, the significant shortening of the solidification interval after niobium addition above 0.4 wt% is not shown in detail by JMatPro simulation.
Outlook
A correlation of the measured cracking lengths with serial production parts has to be established. The transferability of this specimen to other alloys like 1.4826 and 1.4848 and for aluminum alloys has to be analyzed. Additionally, the impact of grain refinement on the hot cracking resistance has to be investigated. Also, the investigation of other influencing elements on hot cracking, such as, e.g., nitrogen and sulfur shall be included in future studies.
The ongoing studies should include investigating the influence of healed cracks filled with niobium-rich interdendritic carbides on the mechanical properties. Also, the impact of ferritic phase content during solidification on the expansion coefficient shall be evaluated.
References
W.D. Röpke, A. Sabé, Das Fließvermögen und die Warmrißneigung von Stahl mit besonderer Berücksichtigung des Einflusses von hohen Molybdängehalten (Springer Fachmedien, Wiesbaden, 1962). (in German)
A. Bührig-Polaczek, W. Michaeli, G. Spur, Handbuch Urformen (Carl Hanser Verlag, München, 2014). (in German)
G.K. Sigworth, F. DeHart, Recent developments in the high strength aluminum–copper casting alloy 206. AFS Trans. 111, 341–354 (2003)
C.W. Briggs, Elements of Design for Prevention of Hot Tearing in Steel Castings, Steel Founders’, (Vol. 38). Society of America Research Report (1957)
Y. Lu, L.N. Bartlett, R.J. O’Malley, A review on hot tearing of steels. Int. Metalcast. 16, 45–61 (2022). https://doi.org/10.1007/s40962-021-00599-3
A.J. DeArdo, Niobium in modern steels. Int. Mater. Rev. 48(6), 371–402 (2003)
E. Fritsche, Vorhersage von Gefügebestandteilen und mechanischen Eigenschaften von austenitischem Stahlguss über die Thermische Analyse (Dissertation, Universität Duisburg-Essen, 2016) (in German)
P. Matteis, Niobium-Alloyed Stainless Steel for Automotive Exhaust Systems (Master Thesis, Politecnico di Torino, 2021)
R. Bürgel, H.J. Maier, T. Niendorf, Handbuch Hochtemperatur-Werkstofftechnik, 4th Edn. (Vieweg+Teubner, Springer Fachmedien Wiesbaden, 2011) (in German)
K.G. Buchanan, M.V. Kral, Crystallography and morphology of niobium carbide in as-cast HP-niobium reformer tubes. Metall. Mater. Trans. A 43A, 1760–1769 (2012)
Y. Zhang, J. Yang, Formation of Nb(C, N) carbonitride in cast austenitic heat-resistant steel during directional solidification under different withdraw rates. Mater. 11, 1–17 (2018)
G. Posch, R. Vallant, W. Klagges, H.- H. Cerjak, Influence of Niobium on Mechanical Properties and Hot Crack Susceptibility of Nickel-Base Cored-Wire Weld Metal Type 70/20 and 70/15, Tantalum-Niobium International Study Center General Assembly (2006)
T. Ogawa, E. Tsunetomi, Hot cracking susceptibility of austenitic stainless steels. Weld. J. 61(3), 82–93 (1982)
N. Scheidhauer, Schädigungsmechanismen in PKW-Turbinengehäusen und Anpassung des Gussgefüges im GX40NiCrSiNb38-19 zu deren Vermeidung (unreleased Dissertation, TU Bergakademie Freiberg, 2022) (in German)
A. Savitzky, M.J.E. Golay, Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 36(8), 1627–1639 (1964)
Acknowledgements
The authors would like to acknowledge the project partner CBMM (Companhia Brasileira de Metalurgia e Mineração) from Brazil for the support and supply of the ferroniobium (grade 111). Furthermore, special thanks to all accompanied persons from the Foundry Department at the Technical University Bergakademie Freiberg, Nb Consulting, and Metaprol from Brazil.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper is an invited submission to IJMC selected from presentations at the 74th World Foundry Congress, held October 16 to 20, 2022, in Busan, Korea, and has been expanded from the original presentation.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Scheidhauer, N., Brambila, J.A., Wolf, G. et al. New Evaluation Method for Hot Cracking Tendency of High Alloyed Steels Containing Niobium. Inter Metalcast 17, 2484–2492 (2023). https://doi.org/10.1007/s40962-023-00996-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40962-023-00996-w