Abstract
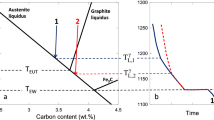
The so-called carbon equivalent of austenite liquidus (CEL) and eutectic carbon equivalent (CE) that are used in cast iron metallurgy are known as linear functions of the composition. This paper first reminds how CEL and CE expressions are obtained from the knowledge of the relevant equilibrium phase diagram, emphasizing that they are not the same quantities. To account for the observed differences between these expressions and the experimental ones, it has been proposed in the literature to add kinetic terms to the equilibrium expressions. This is first discussed by considering austenite liquidus data for cast irons with 1–3 wt% silicon, from which the appropriateness of the CEL is confirmed while also evidencing the role of austenite growth undercooling. Then, experimental results on the solidification onset of near eutectic (slightly hypo- and mildly hyper-eutectic) and strongly hyper-eutectic cast irons are considered for discussing the significance of CE. For this second part, alloys that solidify in the stable system were considered whose results show again the role of austenite growth undercooling, but also that of graphite growth undercooling. The effects of addition of magnesium and of inoculation are discussed.







Similar content being viewed by others
References
M.D. Chaudhari, R.W. Heine, C.R. Loper, Potential applications of cooling curves in ductile iron process control. AFS Trans. 82, 379–386 (1974)
A. Moore, Measurement of carbon equivalent liquidus values in hyper-eutectic flake-graphite irons. Foundry Trade J. 30, 885–890 (1971)
A. Moore, Carbon equivalent of white cast irons. AFS Cast Met. Res. J. 8, 15–19 (1972)
S. Lekakh, Technical Discussion Appendix, Comments on A. Regordosa, J. Sertucha, J. Ramon Olaizola, J. Lacaze article ‘‘When is a cast iron eutectic?’’. Inter. Metalcast. 16, 119–131 (2021). https://doi.org/10.1007/s40962-021-00587-7
D.M. Stefanescu, Analysis of the rationale and accuracy of the use of carbon equivalent and thermal analysis in the quality control of cast iron. Inter. Metalcast. 16, 1057–1078 (2022). https://doi.org/10.1007/s40962-021-00685-6
M.J. Castro-Román, J. Lacaze, A. Regordosa, J. Sertucha, R. del Campo-Castro, Revisiting thermal analysis of hypereutectic spheroidal graphite cast irons. Metall. Mater. Trans. A 51A, 6373–6386 (2020). https://doi.org/10.1007/s11661-020-06005-7
M. Castro, M. Herrera, M.M. Cisneros, G. Lesoult, J. Lacaze, Simulation of thermal analysis applied to the description of the solidification of hypereutectic SG cast irons. Int. J. Cast Met. Res. 11, 369–374 (1999)
A. Regordosa, U. de la Torre, J. Sertucha, J. Lacaze, Quantitative analysis of the effect of inoculation and magnesium content on compacted graphite irons—experimental approach. J. Mater. Process. Technol. 9, 11332–11343 (2020). https://doi.org/10.1016/j.jmrt.2020.08.008
Thermocalc softwares and databases. https://thermocalc.com/products/.
J. Lacaze, J. Sertucha, M.J. Castro-Román, From atom scale to casting: a contemporary monograph on silicon cast irons microstructure. https://oatao.univ-toulouse.fr/26869/
R.W. Heine, Liquidus and eutectic temperatures and solidification of white cast irons. AFS Trans. 85, 537–544 (1977)
M.D. Chaudhari, R.W. Heine, C.R. Loper, Principles involved in the use of cooling curves in ductile iron process control. AFS Trans. 82, 431–440 (1974)
G. Alonso, D.M. Stefanescu, R. Suarez et al., Kinetics of graphite expansion during the solidification of lamellar and spheroidal graphite iron. AFS Trans. 122, 237–248 (2014)
V. Anjos, R. Deike, C.S. Ribeiro, The use of thermal analysis to predict the dendritic coherency point on nodular cast iron melts. Cienca & Tecnologia dos Materiais 29, e27–e33 (2017)
S. Ai, Z. Xu, Z. Liu et al., Evolution of inoculation thermal analysis and solidification morphology of compacted graphite iron. Kovove Mater. 59, 51–57 (2021). https://doi.org/10.4149/km2021151
A. Regordosa, J. Sertucha, J.R. Olaizola, J. Lacaze, When is a cast iron eutectic? Int. J. Metalcast. 16, 119–131 (2022). https://doi.org/10.1007/s40962-021-00587-7
A. Alagarsamy, F.W. Jacobs, G.R. Strong, R.W. Heine, Carbon equivalent vs. austenite liquidus: what is the correct relationship for cast irons? AFS Trans. 92, 871–880 (1984)
A. Regordosa, J. Lacaze, J. Sertucha, M.J. Castro-Roman, U. de la Torre, O. Dezellus, Is thermal analysis able to provide carbon and silicon contents of cast irons? Int. J. Metalcast. (2022). https://doi.org/10.1007/s40962-022-00799-5
F. Mampaey, Modeling and experimental validation of austenite dendrite growth. AFS Trans. 106, 469–476 (1998)
F. Mampaey, Application of austenite dendrite growth model to analyse liquidus temperature measurements in cups. AFS Trans. 106, 461–467 (1998)
J.M. Frost, D.M. Stefanescu, Melt quality assessment of SG iron through computer-aided cooling curve analysis. AFS Trans. 100, 189–200 (1992)
P.K. Basutkar, R.W. Heine, C.R. Loper, Effect of magnesium and cerium additions on the Fe–C–Si diagram. AFS Trans. 81, 336–340 (1973)
K. Yamane, H. Yasuda, A. Sugiyama et al., Influence of Mg on solidification of hypereutectic cast iron: x-ray radiography study. Metall. Mater. Trans. A 46A, 4937–4946 (2015)
W.T. Bourke, S. Harris, T.C. Muff, Method of producing an initial thermal arrest in the cooling curve of hypereutectic cast iron. US Patent 3,546,921 (1970)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1
Tables 1 and 2 list the data from Chaudhari et al. on base alloys (Table 1) and on Ni–Mg spheroidized alloys (Table 2).12 This is the same data as selected by Stefanescu5 with a couple of more results.
Appendix 2: Effect of Magnesium on the Equilibrium Liquidus
Yamane et al.23 investigated in situ using synchrotron X-ray tomography the solidification of two hyper-eutectic alloys, one with 0.002 wt% residual Mg (3.69 C, 2.71 Si, 0.45 Mn, wt%) and one spheroidized with 0.05 wt% Mg (3.73 C, 2.57 Si, 0.45 Mn, wt%). The CE99 value of both alloys is 4.45 wt%. After melting of the alloys, the authors observed during cooling at 0.17 °C/s that nucleation and growth of primary graphite proceeded in a temperature interval of about 20 °C for the alloy without Mg while this temperature interval was limited to 3 °C for the spheroidized alloy. The author mentioned that there could be two explanations to this effect of adding magnesium: 1. It inhibits graphite nucleation (or rather growth); 2. It affects phase equilibria. Yamane et al. disregarded the first possibility and investigated further the second one, concluding that 0.05 wt% Mg depresses the graphite liquidus by 45 °C. Such an effect means that the graphite liquidus depression is about 900 °C/wt% Mg which is more than significant and rather doubtful. In fact, the effect of Mg on the graphite liquidus may be estimated on the C-rich side of the C–Mg phase diagram and the slope is slightly lower than 30 °C/wt% Mg, i.e., 30 times lower than the value implied by Yamane’s assumption. This conclusion was further substantiated by calculation performed with the TCFE-8 database of Thermocalc illustrated in Figure
Isopleth Fe–C section at 2.5 wt% Si calculated with no Mg and with 0.06 wt% Mg using the TCFE-8 databank.9
8. This figure shows isopleth Fe-C sections at 2.5 wt% Si with 0 and 0.06 wt% Mg, and it is seen that they are superimposed (only the second digits in temperature are modified with the addition of Mg). On the contrary, the 45 °C difference mentioned above is of the order of the shift between LG and SG lines in Figures 2 or 3, suggesting that the effect of Mg on growth of graphite was in fact the reason for the observations by Yamane et al.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lacaze, J. Kinetic Effects on the Austenite Carbon Equivalent and Eutectic Carbon Equivalent of Silicon Cast Irons. Inter Metalcast 17, 2062–2071 (2023). https://doi.org/10.1007/s40962-022-00919-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40962-022-00919-1