Abstract
Purpose
Medical technology has gone a long way in diagnosis and characterization of breast tumors. Diffusion-weighted MR imaging is the state of the art for breast screening and diagnosing. The aim of this meta-analysis is to evaluate the diagnostic performances of diffusion-weighted MR imaging in characterization of breast lesions with different b value in 1.5 T MRI.
Method
An extensive search on Scopus, Embase, and PubMed databases were performed on studies published between January 2000 and 2020. The systematic seek initially yielded 2467 studies, out of which 27 research were covered on this meta-evaluation. The included studies for meta-analysis utilized different b value and noted that the ADC value was highly influenced by the b value, for differential diagnosis of breast tumors.
Results
The current meta-analysis has shown the ADC values was lower for malignant breast lesions as compared with benign lesions. The recommended mean threshold ADC was 1.25 ± 0.17 × 10–3 mm2/s range from 0.93 to 1.60 × 10–3 mm2/s for differential diagnosis of breast tumors. Sub-group analysis on the bases of b value showed statistically significant differences in the ADC value of benign and malignant breast tumors.
Conclusion
In conclusion, we noted that b value has a significant effect in calculating the ADC value of the breast lesions as well as ADC threshold value but lacks standardization. The ADC value measurement has a potential for differentiation between benign and malignant breast lesions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Advanced medical technology has gone a long way in diagnosis and characterization of breast tumors. Though mammography is considered the gold standard for breast screening, however differential diagnosis of breast lesions still remains a challenge especially with dense breast. In addition, the survival rates and prognosis for breast cancer patients varies depending on the tumor stage at diagnosis, type of cancer, and also the choice of treatment [1]. Mammography along with breast MRI has been playing a significant role in the diagnosis of breast cancer at an earlier phase [2]. Diffusion-Weighted MR Imaging is an evolving technique that assess the micro-structural characteristics of water diffusion in biological tissues and characterizes the dimensional mobility of water in vivo and allows indirect analysis of tissue microstructure [3].
In the field of oncology, Diffusion-Weighted MR breast imaging has been associated to lesion aggressiveness and tumor response. Breast lesions that illustrate high cellular density and restricted diffusion of water molecules on Diffusion-weighted imaging (DWI) are usually malignant, which aids to discriminate them from benign lesions [4]. Diffusion-weighted (DW) echo planar imaging (EPI) is predominantly the frequent technique due to its high signal-to noise ratio (SNR) and also its lack to susceptibility to motion. Basically, the diffusion sensitivity is highly influenced by a gradient factor known as the “b value” which is proportional to the gradient amplitude, the duration of the gradient and the time interval between the paired gradients [5]. In Diffusion-weighted imaging, the apparent diffusion coefficient (ADC) values enable quantitative analysis of diffusion signal which assist in differential diagnosing of breast tumors [6].
DW-MRI is an appealing technique for breast imaging since it is non-invasive, does not entail exogenous contrast agents, and yields both qualitative and quantitative diagnosis and evaluation. However, these appealing characteristics are offset by many challenges that face the standardization of breast imaging DWI parameter and techniques such as divergence among and between vendors on data measurements, image acquisition technique, multi-exponential decay components which affect the ADC values, incomplete validation and documentation of reproducibility, lack of multicentre working methodologies [3, 7,8,9,10]. The aim of this meta-analysis is to evaluate the diagnostic performances of Diffusion-Weighted MR Imaging in characterization of breast lesions with different b value in 1.5 T MRI.
2 Materials and Methods
2.1 Literature Search
An extensive search on Scopus, Embase, and PubMed databases were performed on studies published between January 2000 and 2020. Comprehensive cross-checking of the reference lists of all retrieved articles were performed manually to identify additional potential relevant of research papers. The database searches used the subsequent as clinical concern heading phrases and medical text words: “Diffusion Weighted Imaging” or DWI or “Magnetic Resonance Imaging” or MRI “Diffusion Weighted MR Imaging” or “Diffusion‑Weighted MRI” or “Apparent Diffusion Coefficient” or ADC or “b value” and “breast‑tissue cancer” or “breast cancer” or” breast lesions” or “Breast neoplasm.”
2.2 Selection Criteria’s
The inclusion criteria’s considered for analyses in this study were (a) 1.5 T (b) studies done only in female gender (c) MR imaging sequence should be Diffusion-weighted sequences (d) breast lesions which includes both benign and malignant (e) ADC values of both benign and malignant lesions should be pronounced which had been confirmed with the aid of pathology or at least a diagnostic follow with-up (f) mean ADCs of malignant and benign lesions (g) ADC threshold (h) Sensitivity and Specificity value should be reported (i) Published research with authentic facts in peer-reviewed journals (j) Studies published in English language only.
The selection of the articles were conducted with accordance to the guidelines of the systematic reviews of the diagnostic test [11]. The articles underwent screening of the title and abstract fulfilling all the exclusion and inclusion standards. Then the next step involves determining of the final studies to be included in the meta-analysis after employing the same inclusion and exclusion criterions to the complete content of the articles. The excluded articles were those that involves MRI scanner of 3 Tesla, neo-adjuvant, no b value reported, no mean ADC values of benign or malignant lesion separately, no threshold ADC cut off value reported along with the sensitivity and specificity, studies not related to diagnostic performances and pre-clinical studies. Case report, letters, review articles, unpublished articles, and comments were excluded.
2.3 Quality Assessment and Data Extraction
All studies were reviewed and scrutinized meticulously. Selection of the studies and collection of the data were executed independently by two readers. The quality assessment and eligibility of the studies were accomplished using (QUADAS instrument a fine assessment tool particularly evolved for systematic opinions of diagnostic accuracy research) [12]. The three wide viewpoints were determined: (1) Bias: 0–9; (2) viability: 0–2; and (3) reporting: 0–3. QUADAS ranged from 0 to 16; and a score ≥ 10 revealed a good methodological quality. In order to remedy the disagreements among the reviewers, a third reviewer assessed all the items and a majority opinion had been considered for evaluation via dialogue or session. This evidence-based tool was developed specifically to assess the quality of diagnostic accuracy studies and includes 14 quality items. The 14 items were scored as “Yes,” “No,” or “Unclear.” The total score ranges from 0 to 14, in which 14 was the maximum score. The following descriptive were extracted—the author, country, year of publication, study design, MR scanner company (vendor), b values, mean age of the subjects, total number of subjects, lesions (benign and malignant), contrast status, mean ADC value of normal fibro-glandular tissue, mean ADC values of the lesions, threshold of the ADC value for differential diagnosis with sensitivity and specificity were extracted from each study.
3 Results
3.1 Study Selection and Quality Assessment
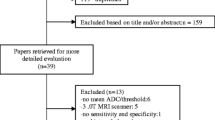
The systematic seek initially yielded 2467 studies, out of which 27 research were covered on this meta-evaluation (Fig. 1). All research studies have been published between 2002 and 2018. According to the QUADAS tool, the score ranged from 11 to 14. The queries that many researches have an uncertain answer had been Item 9 (the outline of the execution of the reference standard) and item no 10 and 11 (The translation effects of the reference standard without information of the index test consequences and the translation of the index test outcomes without information of the reference standard, respectively).
3.2 Study Characteristics
The 27 included studies involved 2642 patients who underwent MRI with diffusion-weighted sequence in which 2686 lesions were diagnosed and ADC values were calculated. The total number of benign lesions were 1088 and malignant lesion were 1598. In 22 studies, prospective study designs were used, whereas in 5 studies, retrospective study designs were used. All studies have been executed in 1.5 Tesla MR scanner. Detailed information on the subjects with breast lesions and their characteristics of the qualified studies are summarized in Tables 1 and 2
3.3 Publication Biases
The funnel plot suggests that the research blanketed for the meta-analysis had been disbursed asymmetrically on a scatter plot Deeks’ funnel plot (Fig. 3) indicates that there was no evidence of publication bias discovered. No evidence of publication bias was observed [p = 0.6783] based on the Egger’s test.
3.4 Homogeneity Test and Meta-regression Analysis
Inconsistency index (I2) was maneuver to estimate the heterogeneity of character research contributing to the pooled estimate. The homogeneity has to be assessed if the differences across the research studies were greater than expected by chance. P < 0.05 suggests presence of heterogeneity beyond what could be expected by chance alone. I-squared (I2) expresses the percentage of the variation across various studies due to heterogeneity and was also used as a mode to quantify the amount of heterogeneity. Heterogeneity is suggested by I2 > 50%. We conducted a subgroup analyses among one of a kind observe characteristics to assess their quantitative consequences on heterogeneity. Although the articles which are only in English language are included in this systematic review, the funnel plot analysis indicated that there was no notable publication bias in the present meta-analysis. There is a high probability that publication biases might overpitch the clinical diagnosis. Hence, in systematic reviews or meta-analysis, a radical literature search is crucial to identify all applicable and relevant studies.
3.5 Statistical Analysis
Estimation of the ADC value was done by the standard mean difference (SMD) with 95% confidence interval (95% CI). Cochran’s Q-statistic (p value < 0.05) and I2 tests was used to quantify heterogeneity among the included studies. Fixed/random-effects model were used, in order to calculate the pool SMD. In case of significant heterogeneity (p value < 0.05 or I2 test exhibited > 50%) random-effects model was employed, otherwise the fixed-effects model was applied. If significant heterogeneity was noted, then subgroup analysis was performed to detect the potential explanatory cause. We also adopted a sensitivity analyses to determine whether one single study had the weight to influence the overall estimate. Furthermore, to assess the effect of publication bias, Egger’s linear regression test (p value < 0.05 was considered significant) was used and funnel plot generated for the asymmetry, in which the asymmetric plot revealed viable publication bias.
3.6 Meta-analysis
3.6.1 Overall Effect
Meta-analysis with Random effect model was performed to pool the mean difference as there was a high level of heterogeneity (I2 = 86.98%). The pooled estimate was found to be 0.57 [0.52, 0.62] which indicates that average value of ADC is 0.57 unit more in the benign lesions compared to malignant lesions, which is found to be statistically significant (p value < 0.001). The forest plot is represented in Fig. 2. According to Fig. 2, there are 31 items in the study column and 4 studies [13,14,15,16] have been cited twice. These studies used 2 different b values, hence the ADC value for each b value was calculated separately, to find out the optimal b value for differential diagnosis of benign and malignant breast lesions.
3.7 Effect of b Value on ADC and Threshold
In the subgroup of six maximum b values the average pooled estimate of ADC was found to be more in the benign lesions compared to malignant lesions. The highest ADC value mean difference of the benign and malignant lesions for differential diagnosis was obtained with the b value of 0, 500 and lowest with 0, 1500, however all the mean differences was found to be statistically significant (Table 3), but there was no statistically significant difference observed in the pooled estimate of ADC mean difference among six different b values (p = 0.14). The highest mean value for benign and malignant lesion as well as threshold was observed in b value of 0, 600 and lowest was observed for the b value of 0, 1500. The effect of maximum b values on the ADC of benign (p = 0.015) and malignant (0.005) was found to be significant and no significant effect of maximum b values on ADC threshold cut off (p = 0.056).
In the subgroup of b value categories (b value ≤ 600 and > 600) the average pooled estimate of ADC is found to be more in the benign lesions compared to malignant lesions. The highest mean difference was obtained with the b value group of ≤ 600 and lowest with the b value group of > 600 and the mean differences was found to be statistically significant, but there was no statistically significant difference observed in the pooled estimate of ADC mean difference value for two b value categories (p = 0.53). The effect of b values group of ≤ 600 and > 600 on the ADC of benign (p = 0.002) and malignant lesions (p = 0.003) and ADC threshold cut off (p = 0.035) was found to be significant.
3.8 Effect of Contrast Media on ADC and Threshold
In the subgroup of contrast media, the average pooled estimate of ADC for pre- and post-contrast was found to be more in benign lesions compared to malignant lesions and the mean difference was statistically significant. However, there was no statistically significant difference observed in the pooled estimate of ADC mean difference value for pre- and post-contrast sub-group (p = 0.75). There was no significant effect of contrast media on the ADC of benign (p = 0.720) and malignant lesions (p = 0.543), and ADC threshold cut off (p = 0.418).
3.9 Effect of Manufacturers on ADC and Threshold
In the subgroup of manufacturers, the average pooled estimate of ADC was found to be more in benign lesions compared to malignant lesions. The highest mean difference was obtained with Toshiba and lowest with GE and all the mean differences was found to be statistically significant. There was also statistically significant difference observed in the pooled estimate of ADC mean difference among manufacturer subgroups (p = 0.03). The highest mean value for benign and malignant lesion was obtained with Philips and GE, respectively, and lowest mean value for benign and malignant lesion was obtained with Siemens and Toshiba, respectively. The highest threshold value was obtained in GE, whereas the lowest was obtained with Toshiba. There was no significant effect of manufacturer on mean ADC of benign (p = 0.941) and malignant lesion (p = 0.159) and ADC threshold cut off (p = 0.156).
3.10 Effect of Country (Where the Study was Conducted) on ADC and Threshold
In the subgroup of 10 countries, the average pooled estimate of ADC was found to be more in the benign lesions as compared to malignant lesions. The highest mean difference was obtained in Turkey and the lowest in Hong Kong and all the mean differences was found to be statistically significant except for USA (p = 0.90), China (p = 0.11), and Japan (p = 0.75). There was also statistically significant difference observed in the pooled estimate of ADC mean difference among 10 different countries (p = 0.03). The highest mean value for benign lesion was obtained in India and malignant lesion was obtained in Italy. The lowest mean value for benign and malignant lesion was obtained in Hong Kong (Table 4). The highest threshold value was obtained in Japan, whereas the lowest was obtained in Hong Kong. There was no significant effect of various countries on mean ADC of benign (p = 0.159) and malignant lesion (p = 0.682) and ADC threshold cut off (p = 0.615).
3.11 Publication Bias
Egger’s test indicated that there was no evidence of publication bias is observed (p = 0.6783) and Funnel plot showed that there was no evidence of publication bias observed based on funnel plot (Fig. 3).
4 Discussion
Accurate detection and differential diagnosing of breast tumor is highly essential to assist focal treatment planning. Breast MRI is usually performed with T2-weighted, T1-weighted, STIR, DWI, and dynamic contrast enhanced pulse sequences, but unfortunately, in DWI sequence, the ADC value for differential diagnosis of breast tissues is not standardized. ADC value can add remarkable information of the tissue at the cellular level [17]. Hence, DWI offers a crucial quantitative biophysical parameter that may be used to distinguish benign from malignant breast lesions [6]
To begin with, the meta-analysis included 27 articles with 32 data, and the reported mean ADC for benign was 1.60 ± 0.17 × 10–3 mm2/s range from 1.27 to 2.0 × 10–3 mm2/s, malignant was 1.03 ± 0.13 × 10–3 mm2/s range from 0.82 to 1.33 × 10–3 mm2/s. All studies included in the current analysis have shown the ADC values was lower for malignant breast lesions as compared with benign lesions (MD = 0.57, 95% CI 0.52–0.62, p < 0.001). The recommended mean threshold ADC was 1.25 ± 0.17 × 10–3 mm2/s range from 0.93 to 1.60 × 10–3 mm2/s. Xin chan et al., [9] reported similar findings with the mean ADC values for benign ranged from 1.00 to 1.82 × 10–3 mm2/s and malignant ranged from 0.87 to 1.36 × 10–3 mm2/s and the threshold ADC value for differential diagnosis ranges from 0.90 to 1.76 × 10–3 mm2/s. The mean ADC value of benign lesions was significantly higher as compared to malignant lesions.
The included studies for meta-analysis utilized different b values and noted that the ADC value was highly influenced by the b value, for differential diagnosis of breast tumors. Increase in the b value decreases the ADC value for both benign and malignant lesions due to the effect of micro-perfusion. Subgroup meta-analysis was performed for each b value of 500, 600, 750, 800, 1000, and 1500 and b value ≤ 600 and > 600. The ADC value of both benign and malignant obtained with b value of 0,600 was higher as compared to the b value of 0, 1500, and ADC value of both benign and malignant for b value ≤ 600 was lower than b value > 600. Increasing the b value, decreases the ADC value of benign and malignant lesions and the changes were statistically significant. We also noted that the b value influences the ADC threshold value for differential diagnosis. Additionally, numerous b value combinations were exercised in various studies and it significantly affects the ADC value of the breast tissues. In maximum studies the analyses of the DWI examinations were primarily based on the combination of two b values, 5 studies employed than two b values combination. Theoretically, ADC calculation error is probably reduced by using the use of extra b values, but this hypothesis has now not been proved through any of the research [18]. This meta-evaluation suggests that the huge variety of b value combinations applied in various studies notably influences the ADC of breast lesions and therefore confounds quantitative DWI. Each published ADC threshold value, reflects optimal differential diagnosis of breast lesions based on the ADC outcomes. Therefore, it is very important to identify the optimum b value in DWI sequence since the b value plays a significant role in characterization of breast lesions with ADC value.
Apart from the b value, sub-group analysis was also performed on the pre- and post-contrast imaging to check the influenced of contrast media on ADC value. The report suggested that there was no significant difference in the mean ADC value of benign, malignant lesions. We also noted that the ADC threshold value obtained before or after contrast administration has no statistically significant differences. However, the significant difference of ADC value between benign and malignant lesions does exist for pre- and post-contrast. In Monique et al., report there was a significant difference between ADC value of the pre- and post-contrast, where they reported that there was an increase in the ADC value of benign lesions (p = 0.08) in post-contrast but no statistical differences in malignant breast lesions. There are number of studies conducted to verify the influences of the contrast media on the ADC value, and according to our analysis we noted that the contrast media does have any significant effect on the ADC value of the breast lesions. Therefore, DWI can easily be recommended for screening sequence for high risk breast cancer population while avoiding contrast media sequence for patients who have contrast media contraindications. Additionally, the use of contrast media can be avoided for differential diagnosis of breast lesions since the contrast media has high probability of leaving deposits in the brain, according to the report of EUSOBI [19].
Furthermore, the subgroup assessment was also carried out on the basis of the other factors such as manufacturing company and also country in which the study was conducted in order to check its effect on the ADC value of the breast tissues. In the subgroup analysis of manufacturing company, we noted a statistically significant difference in the pooled estimate of ADC mean difference among manufacturing company subgroups, but the mean ADC value of benign, malignant lesions and the ADC threshold value was not statistically significant across various manufacturing company. Which states that the mean ADC of breast lesions as well as the threshold were similar across various manufacturing company. Similarly, for sub-group of countries in which the studies were conducted the reported mean ADCs and threshold differences were not statistically significant. However, we noted a significant difference in the mean ADC value of the benign and malignant lesions across all countries except for the mean difference of USA, China, and Japan. Though there were no statistically significant differences noted in both the mean and also threshold of ADC value, which may be due to lack of sample size and studies conducted from the same country. However, the small wave for the differences noted in ADC value across various countries may be due to the culture, regional, diet, family history, and environmental influences on the types of breast cancer.
The present meta-analysis showed that there is heterogeneity amidst the various included studies. Hence, it was expository to analyze the origin of heterogeneity, in order to ascertain the potential factors and to assess the pertinent of the accuracy estimates of statistical pooling. The resources of heterogeneity noted in the present analysis may be defined by the vast variation of data, data acquisition and data analysis in all the 27 covered research studies. We also noted the number of lesions, size of the lesions, varied across the studies. There was an extensive variation in DWI sequence parameter among the research which includes the use of different b values and undesirable variations in the magnitude of TR, TE, technique of acquisition (EPI/parallel imaging), and time of acquisition. Furthermore, there have been systematic discrepancies within the willpower of region of interest (ROI) for the strategies of calculation of the ADCs among studies. Therefore, these were few of the issues that causes the heterogeneity, thus defile our comparisons. Therefore, we employed a random-effects model that explicate these results with caution.
Our study has some limitations, in which we limited our search only to 1.5 T, higher tesla was not included. We did not include in our inclusion criteria’s such as the shape, size of the lesions and also the method and techniques of calculation, which may also have an effect in ADC value calculation. Although QUADAS was adopted to affirm the quality of studies included in the analysis, still in the majority of the studies, the MRI scans interpretation was performed qualitatively, and in many studies, blinding was either absent or unclear. Thus, there has been a risk of subjective interpretation which may additionally outcomes in decrease diagnostic accuracy. However, potential publication bias may still prevail, because studies with positive and effective outcome may have the probability of getting published more easily than microscopic studies with negative results. Moreover, we blanketed studies published in English, which may revolt to the so-called “Tower of Babel” bias, which refers that the researchers functioning in other languages than English could be sending positive research work to international journals. Therefore, studies with negative outcomes could have been overlooked. Such variation would possibly have an effect on the overall estimates of the sensitivity and specificity.
5 Conclusion
In conclusion, we noted that b value has a significant effect in calculating the ADC value of the breast lesions as well as ADC threshold value in DWI and lacks standardization. The ADC value measurement has a potential for differentiation between benign and malignant breast lesions. However, the results must be interpreted with caution, since a number of prognostic and confounding factors may influence the ADC value. Hence the mean ADCs and threshold cut off for differential diagnosis of breast lesions could not be made based on this study. In order to have a finer and comprehensive knowledge of the role of DWI in clinical MR breast imaging, future study should be conducted and also large-scale randomized control trials (RCTs) to optimize the DWI parameters especially in terms of choosing appropriate b value for scanning.
Availability of Data and Material
Not applicable.
References
Wu, L. M., Chen, J., Hu, J., Gu, H. Y., Xu, J. R., & Hua, J. (2014). Diffusion-weighted magnetic resonance imaging combined with T2-weighted images in the detection of small breast cancer: a single-center multi-observer study. Acta Radiologica, 55(1), 24–31.
Warner, E., Messersmith, H., Causer, P., Eisen, A., Shumak, R., & Plewes, D. (2008). Systematic review: using magnetic resonance imaging to screen women at high risk for breast cancer. Annals of Internal Medicine, 148(9), 671–679.
Sung, H. K., Eun, S. C., Hyeon, S. K., Bong, J. K., Jae, J. C., Ji, H. J., et al. (2009). Diffusion-weighted imaging of breast cancer: Correlation of the apparent diffusion coefficient value with prognostic factors. Journal of Magnetic Resonance Imaging, 30(3), 615–620.
Wilmes, L. J., McLaughlin, R. L., Newitt, D. C., Singer, L., Sinha, S. P., Proctor, E., et al. (2013). High-resolution diffusion-weighted imaging for monitoring breast cancer treatment response. Academic Radiology [Internet]., 20(5), 581–589. https://doi.org/10.1016/j.acra.2013.01.009
Luypaert, R., Boujraf, S., Sourbron, S., & Osteaux, M. (2001). Diffusion and perfusion MRI: basic physics. European Journal of Radiology, 38, 19–27.
Partridge, S. C., Nissan, N., Rahbar, H., Kitsch, A. E., & Sigmund, E. E. (2017). Diffusion-weighted breast MRI: clinical applications and emerging techniques. Journal of Magnetic Resonance Imaging, 45, 337.
Kuroki, Y., Nasu, K., Kuroki, S., Murakami, K., Hayashi, T., Sekiguchi, R., et al. (2004). Diffusion-weighted imaging of breast cancer with the sensitivity encoding technique: Analysis of the apparent diffusion coefficient value. Magnetic Resonance in Medical Sciences, 3(2), 79–85.
Matsuoka, A., Minato, M., Harada, M., Kubo, H., Bandou, Y., Tangoku, A., et al. (2008). Comparison of 3.0-and 1.5-tesla diffusion-weighted imaging in the visibility of breast cancer. Radiation Medicine—Medical Imaging and Radiation Oncology, 26(1), 15–20.
Chen, X., He, X.-J., Jin, R., Guo, Y.-M., Zhao, X., Kang, H.-F., et al. (2012). Conspicuity of breast lesions at different b-values on diffusion-weighted imaging. BMC Cancer, 12, 1.
Baron, P., Dorrius, M. D., Kappert, P., Oudkerk, M., & Sijens, P. E. (2010). Diffusion-weighted imaging of normal fibroglandular breast tissue: Influence of microperfusion and fat suppression technique on the apparent diffusion coefficient. NMR in Biomedicine, 23(4), 399–405.
Campbell, J. M., Klugar, M., Ding, S., Carmody, D. P., Hakonsen, S. J., Jadotte, Y. T., et al. (2015). Diagnostic test accuracy: methods for systematic review and meta-analysis. International Journal of Evidence-Based Healthcare, 13(3), 154–162.
Whiting, P. F., Weswood, M. E., Rutjes, A. W. S., Reitsma, J. B., Bossuyt, P. N. M., & Kleijnen, J. (2006). Evaluation of QUADAS, a tool for the quality assessment of diagnostic accuracy studies. BMC Medical Research Methodology, 6, 1–13.
Yili, Z., Xiaoyan, H., Hongwen, D., Yun, Z., Xin, C., Peng, W., et al. (2009). The value of diffusion-weighted imaging in assessing the ADC changes of tissues adjacent to breast carcinoma. BMC Cancer, 9, 1–10.
Jin, G., An, N., Jacobs, M. A., & Li, K. (2010). The role of parallel diffusion-weighted imaging and apparent diffusion coefficient (ADC) map values for evaluating breast lesions. Preliminary results. Academic Radiology [Internet], 17(4), 456–463. https://doi.org/10.1016/j.acra.2009.12.004
Moschetta, M., Telegrafo, M., Rella, L., Capolongo, A., Stabile Ianora, A. A., & Angelelli, G. (2014). MR evaluation of breast lesions obtained by diffusion-weighted imaging with background body signal suppression (DWIBS) and correlations with histological findings. Magnetic Resonance Imaging [Internet], 32(6), 605–609. https://doi.org/10.1016/j.mri.2014.03.009
Eghtedari, M., Ma, J., Fox, P., Guvenc, I., Yang, W. T., Dogan, B. E., et al. (2016). Effects of magnetic field strength and b-value on the sensitivity and specificity of quantitative breast diffusion-weighted MRI. Quantitative Imaging in Medicine and Surgery [Internet]., 6(4), 374–380. https://doi.org/10.21037/qims.2016.07.06
Goto, M., le Bihan, D., Yoshida, M., Sakai, K., & Yamada, K. (2019). Adding a model-free diffusion MRI marker to BI-RADS assessment improves specificity for diagnosing breast lesions. Radiology, 292(1), 84–93.
Pereira, F. P. A., Martins, G., Figueiredo, E., Domingues, M. N. A., Domingues, R. C., Da Fonseca, L. M. B., et al. (2009). Assessment of breast lesions with diffusion-weighted MRI: comparing the use of different b-values. American Journal of Roentgenology, 193(4), 1030–1035.
Baltzer, P., Mann, R. M., Iima, M., Sigmund, E. E., Clauser, P., Gilbert, F. J., et al. (2020). Diffusion-weighted imaging of the breast—a consensus and mission statement from the EUSOBI International Breast Diffusion-Weighted Imaging working group. European Radiology, 30(3), 1436–1450.
Acknowledgements
We have to express our appreciation to Dr. Samir Paruthikunnan Mustaffa and (L) Dr. Smiti Sripathi for sharing their pearls of wisdom with us during the course of this work.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization RK and WD; methodology RK, WD, SK, AP and SS; Data extraction: WD, AP and SS; and SK; Analysis RK, WD, SK, AP and SS; writing original draft— WD, SS and AP, supervision RK and WD. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
Not Applicable.
Consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dkhar, W., Kadavigere, R., Sukumar, S. et al. Diagnostic Performances of ADC Value in Diffusion-Weighted MR Imaging for Differential Diagnosis of Breast Lesions in 1.5 T: A Systematic Review and Meta-analysis. J. Med. Biol. Eng. 43, 497–507 (2023). https://doi.org/10.1007/s40846-023-00825-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40846-023-00825-9