Abstract
The effect of hydrogen charging duration on the transformation behavior, microstructural evolution, and dynamic microstructural changes associated with thermoelastic martensitic transformation in Ti–Ni shape memory alloy was investigated. Compared with the uncharged specimen, the martensitic transformation start (Ms) and reverse transformation finish (Af) temperatures increased with charging time, whereas the martensitic transformation finish and reverse transformation start temperatures remained almost unchanged. In situ SEM results were consistent with these behaviors. Upon cooling, the transformation progressed from the center to the surface in charged specimens, indicating a higher transformation temperature in the center than the surface. The latent heat of transformation decreased with increasing charging time, quantitatively attributed to an untransformed region consisting of hydrogen-induced martensite and a hydrogen-affected layer. The hydrostatic effect from those layers on the interior B2 phase was proposed as the origin of the increased Ms and Af temperatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Near-equiatomic Ti–Ni alloys are the most commercially available shape memory and superelastic alloys and are widely used in engineering, aeronautics, dentistry, and medicine [1,2,3,4,5,6]. These functional properties are associated with thermoelastic martensitic transformation from the B2 to B19’ structures [1, 2]. In many applications, especially in dentistry and medicine, Ti-Ni alloys are exposed to corrosive environments, including hydrogen [7]. There have been many studies on the effect of hydrogen on the mechanical properties and the transformation behaviors of Ti–Ni alloys [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. In the studies on mechanical properties, hydrogen embrittlement and its mechanism have attracted much interest [7, 10,11,12,13,14,15,16,17,18,19,20, 23, 28]. There have been several reports on the effect of hydrogen on transformation behaviors, such as transformation temperature and latent heat [7, 22, 24, 26, 27]. However, only a few studies have focused on hydrogen-induced modifications of the microstructure and dynamic microstructural development during the thermoelastic martensitic transformation [20, 25, 29]. Although it has been reported that hydride is formed by hydrogen charging [8, 9, 20, 29], we recently have found that a hydrogen-induced phase with an orthorhombic structure, which differs from hydride, is formed on the top surface of the hydrogen-charged specimen [30]. This phase had martensitic properties induced by cooling and stress loading and was very hard with a Vickers hardness of about 700 HV, and thus the phase was defined as hydrogen-induced martensite (HIM). We also revealed with in situ SEM observations that there is an untransformed layer between the HIM layer and the interior region, the latter of which transformed into B19’ martensite upon cooling [30]. Here, we refer to this untransformed layer as the hydrogen-affected layer (HAL). The HAL corresponds to the region where the B19’ martensitic transformation is suppressed in the hydrogen-charged specimen. It has been reported that hydrogen suppresses the thermoelastic martensitic transformation from the B2 structure to the B19' structure experimentally [7,8,9, 20, 24, 26, 27, 30] and theoretically with first-principles calculations [31]. However, the hydrogen-induced microstructure modifications, including the HIM and the HAL, and their interaction with thermoelastic transformation behaviors remain unclear.
In the present work, we investigate experimentally the effect of the hydrogen charging period on the transformation behaviors, microstructural changes, and dynamic microstructural development associated with thermoelastic martensitic transformation in Ti-Ni shape memory alloy. We also discuss quantitatively the correspondence between the differential scanning calorimetry (DSC) analysis of transformation behaviors and in situ SEM observations.
Materials and Methods
Specimens about 2.2 × 2.2 × 1.3 mm in size were cut from a cold rolled plate of Ti-50.7 at% Ni alloy and solution-treated under vacuum at 900 °C for 3.6 ks, and then quenched into ice water. The oxide layers were removed from all surfaces by mechanical polishing and fabricated to a final size of about 2.0 × 2.0 × 1.0 mm. A potentiostat (Model HA-151B, Hokuto Denko Co.) was used for the hydrogen charging. The platinum wire anode and the specimen as the cathode were immersed in aqueous 1 N NaOH solution. The specimens were hydrogen-charged with a current density of 60 A/m2 for 4, 10, and 20 h at room temperature (RT).
The hydrogen desorption behavior was analyzed 0.5 h after hydrogen charging using thermal desorption spectroscopy (TDS; HTDS-000-2, R-DEC Co., Ltd.). The specimens were heated from RT to 600 °C at a heating rate of 100 °C/h. The diffusible hydrogen content was defined as the total amount of desorbed hydrogen in the temperature range from ambient temperature to 600 °C. The transformation temperatures were also measured 0.5 h after hydrogen charging using DSC (DSC 3500 Sirius, NETZSCH) upon cooling from RT to − 100 °C and heating from − 100 to 50 °C. The weight of the plate specimen described above was approximately 25 mg, which was sufficient for DSC measurements. The cooling and heating rate was 0.17 °C/s. In addition, because RT aging effects have been reported [7, 16, 21,22,23,24], DSC measurements were also performed again 6 h after hydrogen charging. This duration was set to allow the transformation behavior in the DSC to be consistent with the results of the following experiments, for which a specimen preparation time of about 6 h was required.
The hardness was measured using a micro-Vickers hardness tester (HMV-G, Shimadzu Co.) at the topmost surface and the cross-section area from the surface to the center in the specimen. The topmost surface measurement was performed with a test force of 1.96 N for 10 s 0.5 and 6 h after hydrogen charging, as in the DSC measurements. The cross-section area measurement was performed 6 h after hydrogen charging with a test force of 0.49 N for 10 s applied at 10 µm intervals. The test force for the cross-section was lower than that for the topmost surface to avoid work strengthening due to the interaction between neighboring indentations. The starting indentation position of the cross-section area was 10 µm from the surface and measurements were performed five times. The distribution of hydrogen was visualized by time-of-flight-secondary ion mass spectroscopy (TOF–SIMS) implemented in a scanning electron microscope (SEM) with a plasma-focused ion beam (Helios 5 Hydra, Thermo Fisher Scientific Inc.). Xenon gas was used for the ion beam source and the beam was operated at 30 kV. The specimens for the TOF-SIMS measurements were prepared without electropolishing to keep a sharp edge. The SEM specimens were prepared by twin jet electropolishing (Model 110, Fischione Instruments) with H2SO4/CH3OH (1:4 v/v) around 0 °C to obtain a smooth surface without the residual strain from the initial mechanical polishing. A field-emission SEM (Ultra 55, Carl Zeiss) system was used for metallographic observations. For in situ observation, a Peltier cooling stage (Coolstage, DEBEN) with a working temperature of − 50 to + 50 °C was used [30, 32,33,34,35,36,37]. The specimens were cooled or heated continuously at 0.025 °C/s.
Results
Hydrogen Contents and Transformation Behaviors
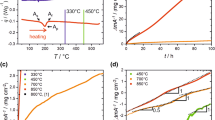
Figure 1 shows TDS curves for the specimens hydrogen-charged for 4, 10, and 20 h. The amounts of desorbed hydrogen are 758, 1453, and 2015 mass ppm for the specimens charged for 4, 10, and 20 h, respectively. Because the hydrogen desorption behavior of Ti–Ni alloys depends on hydrogen charging procedures [9, 11,12,13,14,15,16,17, 19], a quantitative comparison of the current result with previous studies is difficult. Nevertheless, a similar hydrogen desorption curve was reported in Ti–Ni alloy cathodic hydrogen-charged at 10 A/m2 for 120 h in 0.9 mass% NaCl solution [11].
Figure 2a shows the DSC curves of the uncharged specimen and the hydrogen-charged specimens measured 0.5 h after charging. The transformation temperatures and latent heat of transformation in the uncharged and charged specimens were evaluated from each curve in Fig. 2a and those values are summarized in Supplementary Table 1. Figure 2b shows the deviation of transformation temperatures from those of the uncharged specimens. The Ms temperature detected during cooling increases with increasing charging time, but the Mf temperature does not change substantially. In contrast, during heating, the As temperature does not change greatly, but the Af temperature increases. These results indicate that the martensites formed in the initial and final stages of forward transformation during cooling disappeared in the final and initial stages of reverse transformation during heating, respectively. These behaviors are consistent with the nature of the thermoelastic martensitic transformation [38]. Notably, it has been reported that the Ms temperature is lowered, and the latent heat of transformation is decreased in hydrogen-charged Ti-Ni alloys [7, 21, 24]. However, contrary to those previous reports, the Ms and Af temperatures increased unexpectedly in the present work. These temperature increases in Fig. 2a, b were reproduced well for all charging periods. In contrast, the latent heat of transformation decreased with the hydrogen charging as the charging duration increased, as reported previously (Fig. 2c) [7, 21, 24]. Figure 3a shows the DSC curves measured after leaving the specimens at RT for 6 h. This duration corresponds to the preparation time for the rest experiments described in "Materials and Methods" Section. The transformation temperatures and the latent heat were evaluated from each curve in Fig. 3a, and their deviations from the uncharged specimen are plotted in Fig. 3b and c, respectively. The measured values are listed in Supplementary Table 1. The trend for the transformation temperatures over the charging period is the same as in Fig. 2a and b. That is, compared with the uncharged specimen, Ms and Af increase monotonically with the charging period, whereas Mf and As are unchanged or only show small changes. Next, we compare the transformation temperatures of the specimens aged at RT for 0.5 and 6 h after charging. While 5.5 h of aging decreases Ms and Af, they are still higher in the specimens charged for 10 and 20 h than in the uncharged one. There are also large drops from approximately 1 to 2 J/g in the latent heat of the forward and reverse transformations caused by RT aging.
a DSC curves of the uncharged specimen and the hydrogen-charged specimens measured 0.5 h after the hydrogen charging. b Deviations of transformation temperatures from the uncharged specimen estimated from (a). c Deviations of the latent heat of forward and reverse transformations from the uncharged specimen estimated from (a)
a DSC curves of the uncharged specimen and the hydrogen-charged specimens measured 6 h after hydrogen charging. b Deviation of transformation temperatures from the uncharged specimen estimated from (a). c Deviation of the latent heat of the forward and reverse transformations from the uncharged specimen estimated from (a)
To confirm whether the decrease of Ms and Af in hydrogen-charged specimens is caused by RT aging, the effect of thermal cycling on transformation behavior should also be considered [39,40,41,42,43,44,45,46,47]. In the present study, the identical specimens were used for the measurements in Fig. 2a and in Fig. 3a for the corresponding charging period to avoid individual differences between specimens. Thus, the curves in Fig. 2a correspond to the first thermal cycle, and those in Fig. 3a correspond to the second cycle. The effect of the thermal cycle is estimated by comparing the first and second cycles of the uncharged (0 h) specimen in Figs. 2a and 3a. The decreases in the Ms and Af temperatures between the first and the second cycles are only 1.4 and 0.8 °C in the uncharged specimen, respectively, whereas those in the charged specimens decrease remarkably. For example, in the specimen charged for 10 h, the Ms and Af temperatures decrease by 6.9 °C, from − 8.4 to − 15.3 °C, and by 11.5 °C, from 19.9 to 8.4 °C, respectively, after being left at RT for 5.5 h. This result suggests that the contribution of RT aging to the decrease of the Ms and Af temperatures in the hydrogen-charged specimens is greater than that of thermal cycling. It should be noted that the amount of hydrogen desorption for the 20 h hydrogen-charged specimen aged at RT for 5 days is 1814 mass ppm (see Supplementary Fig. 1). In other words, the amount of hydrogen dissipated by RT aging for 5 days is within 10%. Therefore, it is reasonable to assume that hydrogen diffuses into the specimen interior and lowers the Ms temperature due to RT aging. Further details of the aging effect will be reported soon in a separate paper.
The latent heat of transformation decreases with increasing hydrogen charging and RT aging periods (Fig. 3c). These results suggest that the hydrogen charging and the subsequent RT aging induce the HAL and/or the HIM. The formation of the HAL directly contributes to the decrease in the latent heat, because the B2 parent phase is stabilized by hydrogen [30, 31] and the transformation to B19’ martensite is sufficiently suppressed in the HAL [30]. The HIM is formed in the surface layer during the charging, where hydrogen is enriched. In addition, the HIM is a relatively stable phase with respect to temperature changes [30]. The origin of the rise of Ms and Af temperatures and the decrease of latent heat in hydrogen-charged specimens compared with those in uncharged specimen are discussed in detail in "Discussion" Section.
Figure 4a shows the micro-Vickers hardness of the top surface of the uncharged and hydrogen-charged specimens. The average surface hardness of the uncharged samples is 295 HV, whereas that of the charged samples increases as the charging time increases, reaching 550, 705, and 754 HV for the 4, 10, and 20 h charged specimens, respectively, when measured 0.5 h after the charging. The surface hardness of the specimen charged for 20 h shows reasonable agreement with that converted from the nanoindentation test reported in the previous paper [30]. After 6 h of aging at RT, the surface hardness decreases by around 50 HV for all specimens (Fig. 4a). Further details will be reported in a separate paper, but here we briefly mention that the surface hardness decreases continuously as the RT aging time increases, for example, to about 560 HV after 5 days for the 20 h charged specimen. The hydrogen content of the specimen aged at RT for 5 days is 1814 ppm, and only about 200 ppm of hydrogen is released outside the specimen. From the TOF-SIMS results (Fig. 5), hydrogen is concentrated in the HIM, suggesting that hydrogen diffuses from the HIM to the interior of the sample due to RT aging. Figure 4b shows the micro-Vickers hardness values in the cross-section measured at 10 µm intervals from the surface in the uncharged and charged specimens. The hardness of all specimens decreases rapidly from the surface slightly toward the interior and settles to the same constant value as that in the uncharged specimen. The layer harder than the average hardness of 288 HV in the uncharged specimens (black dashed lines) extends as the charging period increases. The thicknesses of the hardened layer extend to approximately 40 µm at 4 h, 60 µm at 10 h, and 100 µm at 20 h. These distances correspond to the HIM and HAL, that is, untransformed regions, observed in the in situ SEM cooling experiments described in "Microstructure" Section.
Figure 5a and b show the net intensity maps of the hydrogen peak in the TOF-SIMS mass spectrum taken from the cross-section of the specimens charged for 4 and 20 h, respectively. Figure 5c shows the profile of hydrogen intensity along the distance from the surface, provided by integrating the intensity maps along the vertical direction in Fig. 5a and b. The intensity is normalized by that of the inner part, defined by the distance range of 200–250 µm from the top surface. Hydrogen is substantially enriched in the top surface regions, in which the HIM is observed, as shown in "Microstructure" Section. The profiles in Fig. 5c indicate that the hydrogen diffusion distance increases with increasing charging period. The diffusion distances reach approximately 110 µm for 4 h and 200 µm for 20 h, which is larger than expected from the results of the micro-Vickers test in Fig. 4b. As discussed later in "Microstructure" Section for in situ SEM observations, the diffusion distance in Fig. 5c corresponds to the distance to which the Ms temperature decreases compared with the center.
Microstructure
SEM in situ observations were performed to investigate dynamic microstructure changes associated with phase transformation. Figure 6a–e and f–j show a series of the secondary electron images upon cooling in the uncharged and the 20 h hydrogen-charged specimens observed 6 h after charging, respectively. The bottom of each micrograph is approximately the center of the cross-section about 0.5 mm from the top surface of the specimen. The single arrow in each micrograph indicates a non-metallic inclusion [48] that serves as a marker to indicate that the in situ observations were performed in the same region in each specimen. Similarly, the double arrows in Fig. 6b–e and g–j indicate the same region, respectively. The double arrows indicate that the surface relief of the B19’ martensite, which appears in the early stage of the transformation, maintains its shape until the end of the transformation. In the uncharged specimen, the surface relief of B19’ martensite appears at − 20.4 °C, which is about 2 °C lower than the Ms temperature of − 18.3 °C (Fig. 6b). The nucleation of B19’ martensite occurs everywhere in the uncharged specimen (Fig. 6b–d) [35]. In Fig. 6d, the transformation to B19’ martensite has not progressed slightly in the region near the top surface of the specimen, but when the specimen is cooled to below the Mf temperature, the transformation is completed over almost the entire region (Fig. 6e). In contrast, in the charged specimen, the transformation initiates from the center and gradually progresses toward the edge (Fig. 6g–j). In the charged specimen, the surface relief of B19’ martensite appears at − 11.9 °C which is about 2 °C lower than the Ms temperature of − 9.6 °C (Fig. 6g). This temperature difference is the same as that of the uncharged specimen and is a characteristic of the cooling stage used. The gradual propagation of the transformation from the center to the surface (Fig. 6h and i) indicates that the transformation temperatures depend on the distance from the surface due to the hydrogen concentration gradient (Fig. 5). To describe this transformation temperature gradient, we consider the location-specific transformation start temperature, Msloc, instead of the general Ms. The general Ms corresponds to the temperature at the onset of the exothermic reaction, as defined in the DSC measurements. The Msloc temperatures are functions of the distance from the top surface in the present study. At the center, the Msloc temperature is identical to the general Ms temperature due to the absence of hydrogen, whereas toward the surface, the Msloc temperature gradually decreases with increasing hydrogen concentration. The width of Msloc < Ms area from the top surface (double-ended arrow in Fig. 6i) is roughly consistent with the hydrogen diffusion distance estimated as 200 µm from the edge in Fig. 5c. Even after cooling below the Mf temperature measured by DSC, there remains an untransformed region, namely, the HAL, up to a distance of 90 µm from the top surface of the specimen (double-ended arrow in Fig. 6j). These results reconfirm that this distance agrees well with that of the hardened layer observed in the micro-Vickers hardness in Fig. 4b, rather than the diffusion distance in Fig. 5c.
Figure 7a–c show the low-magnification SEM-back scattered electron images (BSEIs) of the cross-section in the specimens that were hydrogen-charged for 4, 10, and 20 h and cooled below the Mf temperature for in situ observation. The dotted line at the upper right of each image indicates the position of the top edge. The HIM is not observed at the top surface in the specimen charged for 4 h, whereas the hydrogen-rich layer is clearly observed in the TOF–SIMS measurement in Fig. 5 because the HIM layer is too thin to observe and the edge is rounded due to electropolishing. In the specimens charged for 10 and 20 h, a needle-like morphology with bright contrast is observed (Fig. 7b and c). The range of the HAL expands from the top edge of the specimen and/or the bottom of the HIM to the interior as the charging period increases (double-ended arrows on the right-hand side in Fig. 7a–c). The total thicknesses of the HIM and HAL from the surface are approximately 32, 60, and 85 µm for 4, 10, and 20 h charging, respectively. These values agree well with the distances from the surface where the hardened layer is present (Fig. 4b). The increase in the thickness of the HAL directly leads to the decrease in the latent heat of transformation with increasing charging period. Figure 7d shows the enlarged SEM-BSEI of the region framed with the dashed line in Fig. 7b. The HIM phase is observed over a range of about 10 µm from the top surface with a plate-like surface relief similar to that reported previously [30]. Figure 7e and f show the enlarged SEM-BSEIs of the region framed with the dashed line in Fig. 7c before and after cooling, respectively. The HIM phase with the surface relief is visible in Fig. 7e. The growth of the HIM phase upon cooling is observed (single arrows in Fig. 7f), which shows the athermal nature of the HIM [30]. Similarly, the needle-like products growing toward the interior (single arrows in Fig. 7d) also exhibit the athermal transformation of the HIM. The results in Fig. 7 show that the development of the HIM upon cooling between the Ms and Mf temperatures of the B19’ martensite is limited to a small amount of athermal growth. Thus, the contribution of the HIM to the latent heat is negligible in the present DSC measurements.
a–c Low–magnification SEM-BSEIs showing untransformed area to B19’ martensite in specimens hydrogen-charged for 4, 10, and 20 h, respectively, below the Mf temperatures in in situ observations. d Enlarged SEM-BSEI of the area framed with the dashed line in (b). e and f Enlarged SEM-BSEI of the area framed with the dashed line c before and after cooling showing the athermal nature of HIM
Discussion
Change of Transformation Heat
Figure 8 shows schematics of the hydrogen concentration and constituent phases in cross sections of the specimens hydrogen-charged for 10 h or more. Illustrations of the constituent phases are given for above the Af, between the Ms and Mf near the Ms, and below the Mf temperatures, based on the in situ observations in Fig. 6. The hydrogen concentration in Fig. 8a is depicted along the color scheme on the left, which has been estimated from the TOF–SIMS results in Fig. 5. The green line on the right-hand side shows the variation of the Msloc temperature along the distance from the top and bottom surfaces around the center. Above the Af temperature, the cross section consists of HIM at the top, bottom and both side surfaces and the B2 parent phase in the interior. The green area in the central schematic is the region where the B19' martensitic phase nucleates near the Ms temperature measured by DSC in Fig. 3, as shown in Fig. 6g. That is, Msloc is equal to the general Ms temperature, as shown in the right-hand schematic. Nucleation and growth of the B19' phase progresses toward the upper and lower surfaces upon cooling. In this region, Msloc decreases toward the surface. In other words, the transformation is delayed by the diffused hydrogen, as observed from Fig. 5. The HAL consists of the completely stabilized B2 parent phase, which does not transform even below the Mf temperature measured by DSC. Figure 7 shows that the volume of the region that transforms to B19’ below the Mf temperature, V19’, is reduced by increasing the charging period due to the increase of the volumes of the HIM, VHIM, and the HAL, VHAL. Thus, it is intuitive that the decrease in the latent heat of transformation in the hydrogen-charged specimen is attributed to the increase of the untransformed regions, the volume of which is VHIM + VHAL. Because the latent heat is proportional to the volume of the transformation area, the following relationship can be derived.
Schematics of a hydrogen concentration and the constituent phases in the cross sections of the hydrogen-charged specimen b above the Af, c between the Ms and Mf and d below the Mf temperatures, with the variation of the Msloc temperature along the distance from the top and bottom surfaces, around the center of specimen
Here, VA is the whole volume of the specimen, LHf and L0f are the latent heat of the forward transformation for the charged and uncharged specimens, and LHr and L0r are the latent heat of the reverse transformation for the charged and uncharged specimens, respectively. Although VB19’/VA is slightly larger than the other two values, there is agreement and decreasing trend among the three values for all charging conditions (Table 1). Therefore, the decrease in the latent heat of transformation due to hydrogen charging can be attributed to the fact that the HIM and HAL regions did not transform to B19’. It should be noted that the amount of HIM growth observed during cooling (Fig. 7f) is far smaller than that of the B2 to B19’ transformation. Additionally, HIM did not disappear during heating to 50 °C, while B19' underwent a reverse transformation to B2 [30]. Therefore, the contributions of the HIM growth and the reverse transformation are negligible for the latent heats of the exothermic reaction during cooling and the endothermic reaction during heating, respectively.
Origin of M s and A f Temperature Elevation
The Ms and Af temperatures of specimens hydrogen-charged for 10 and 20 h increased in the DSC measurements 0.5 and even 6 h after charging (Figs. 2 and 3). These changes are presumably related to the presence of the HIM and the HAL as well as those in the latent heat. To confirm the origin of the increase in the Ms and Af temperatures, the results of the additional DSC measurements and their procedures are shown in Fig. 9. The schematics on the right-hand side show the state of the specimen before transformation (above the Af temperature), and those on the left-hand side show the state of the specimen after the end of the transformation (below the Mf temperature). The measured Ms temperatures and latent heat at each step are listed in Supplementary Table 2. First, DSC curves were obtained for two uncharged specimens to check the individual differences. The orange and red lines in Fig. 9 denote the specimens served for a series of DSC experiments with hydrogen charging for 10 and 20 h, respectively. The DSC measurements in Fig. 9b were performed 0.5 h after charging. The results shown in Fig. 9a and b indicate that the two specimens have almost the same Ms and Mf temperatures and that the Ms temperature increases with the hydrogen charging, as described in Fig. 2. After the DSC measurement in Fig. 9b, the surface layers including the HIM and the HAL were removed from all six surfaces in these specimens. The removal was completed with an emery paper of the finest grade (#4000 with an abrasive grain size of 5 µm, Japanese Industrial Standards), and careful buffing with 0.5 µm diamond paste. The DSC measurements in Fig. 9c were performed 6 h after charging. Notably, the Ms temperatures of both specimens in Fig. 9c were lower than those in Fig. 9b. Since the DSC curves in Fig. 9c correspond to the third thermal cycles of transformation, the effect of thermal cycles needs to be incorporated. Figure 9d shows the DSC measurements of the uncharged specimen at the third thermal cycle, performed to confirm the decrease of the Ms temperature due to the thermal cycles. The Ms temperatures in Fig. 9c were close to that in Fig. 9d. The slight decrease in the Ms temperature in Fig. 9d from the initial temperature in Fig. 9a suggests that the thermal cycles contribute to the decrease in the Ms temperature [39,40,41,42,43,44,45,46,47]. Note that possible individual difference in transformation temperatures between the charged (Fig. 9c) and uncharged specimens (Fig. 9d) are expected to be small as observed in Fig. 9a. It is found that the decrease in the Ms temperatures from Fig. 9b to c was larger than that from Fig. 9a to d. Since the former includes the additional contribution from the removal of the surface layer, it is suggested that the increase in the Ms temperature after hydrogen charging (Fig. 9b) was induced by the surface layer that consisted of the HIM and the HAL. It is also confirmed that the Ms temperature and latent heat returned to values similar to those of the uncharged specimen after the removal of the HIM and HAL as listed in Supplementary Table 2.
The hardness of the HIM increased with increasing hydrogen charging period (Fig. 4), and the HAL is the B2 matrix phase with the expanded lattice volume due to hydrogen intrusion [9]. Since all sides of the specimens are covered with the HIM and HAL, those layers act hydrostatically on the internal B2 matrix, which reduces the lattice volume of the internal B2 phase. It is known that the transformation from B2 to B19' in Ti-Ni alloys decreases the volume [49, 50]. Therefore, the compressive hydrostatic pressure from the surface layers accelerates the transformation and the Ms temperature increases, and the impact has been reported quantitatively as 10 K/GPa [50]. Assuming that the HIM and HAL produce similar compressive hydrostatic pressure, we can estimate from the Ms temperature changes that the HIM and HAL in the specimens charged for 10 and 20 h exerted hydrostatic pressures of about 0.9 and 1.2 GPa, respectively, 0.5 h after the hydrogen charging. The decrease in the Ms temperature after 6 h aging at RT was probably caused by the reduction of hydrostatic effects due to the decrease in surface hardness (Fig. 4a) and the stabilization of the B2 phase due to the diffusion of hydrogen toward the specimen interior. The latter is supported by the monotonic expansion of the HAL region under the prolonged aging conditions at RT, which will be reported soon in a separate paper.
Conclusion
The effect of hydrogen charging period on the transformation behavior, microstructural changes, and dynamic microstructural developments associated with martensitic transformation in Ti-Ni alloy was investigated. We focused on the quantitative correspondence between the DSC analysis of the transformation behavior and in situ SEM observations.
-
1.
The Ms temperature detected during cooling increased with increasing charging time, but the Mf temperature did not change substantially. During heating, the As temperature did not change substantially, but the Af temperature increased. The latent heat of transformation decreased with increasing charging time. The Ms temperature of hydrogen-charged specimens decreased as the aging time at RT was increased. The effect of RT aging on the decrease of the Ms temperature in the hydrogen-charged specimens was greater than that of thermal cycling in the uncharged specimen.
-
2.
The Ms and Mf temperatures measured by DSC upon cooling agreed well with those estimated from in situ SEM cooling observations. The hardened layer revealed by hardness measurements corresponded to the HIM and HAL, whereas the diffusion distance of hydrogen detected by TOF–SIMS was consistent with the region where the Msloc temperature was decreased. It was shown quantitatively that the decrease in the latent heat of transformation was due to the presence of the HIM and HAL.
-
3.
The increase in Ms and Af temperatures can be related to the hydrostatic effect from the HIM and HAL on the interior B2 phase of the specimen.
Data availability
Experimental data can be available by contacting the corresponding author.
References
Saburi T (1998) Ti–Ni shape memory alloys. In: Otsuka K, Wayman CM (eds) Shape memory materials. Cambridge University Press, Cambridge, pp 49–96
Otsuka K, Ren X (2005) Physical metallurgy of Ti–Ni-based shape memory alloys. Prog Mater Sci 50:511–678. https://doi.org/10.1016/j.pmatsci.2004.10.001
El Feninat F, Laroche G, Fiset M, Mantovani D (2002) Shape memory materials for biomedical applications. Adv Eng Mater 4:91–104. https://doi.org/10.1002/1527-2648(200203)4:3%3c91::AID-ADEM91%3e3.0.CO;2-B
Mohd Jani J, Leary M, Subic A, Gibson MA (2014) A review of shape memory alloy research, applications and opportunities. Mater Des 56:1078–1113. https://doi.org/10.1016/j.matdes.2013.11.084
Benafan O, Brown J, Calkins FT, Kumar P, Stebner AP, Turner TL, Vaidyanathan R, Webster J, Young ML (2014) Shape memory alloy actuator design: CASMART collaborative best practices and case studies. Int J Mech Mater Des 10:1–42. https://doi.org/10.1007/s10999-013-9227-9
Elahinia M, Shayesteh Moghaddam N, Taheri Andani M, Amerinatanzi A, Bimber BA, Hamilton RF (2016) Fabrication of NiTi through additive manufacturing: a review. Prog Mater Sci 83:630–663. https://doi.org/10.1016/j.pmatsci.2016.08.001
Duerig T, Shelley O, Madamba D, Vien L (2019) A practitioner’s perspective of hydrogen in Ni–Ti alloys. Shape Mem Superelasticity 5:235–248. https://doi.org/10.1007/s40830-019-00225-6
Adachi Y, Wade N, Hosoi Y (1990) Ti–Ni合金の形状記憶効果と変態挙動に及ぼす水素の影響 (Effect of hydrogen on the shape memory effect and transformation behavior of Ti–Ni alloy). J Jpn Inst Met 54:525–531. https://doi.org/10.2320/jinstmet1952.54.5_525
Hoshiya T, Den S, Katsuta H, Ando H (1992) Ti–Ni系形状記憶合金の変態特性および変形挙動に及ぼす水素の影響 (effect of hydrogen on transformation characteristics and deformation behavior in a TiNi shape memory alloy). J Jpn Inst Met 56:747–756. https://doi.org/10.2320/jinstmet1952.56.7_747
Yokoyama K, Hamada K, Moriyama K, Asaoka K (2001) Degradation and fracture of Ni–Ti superelastic wire in an oral cavity. Biomaterials 22:2257–2262. https://doi.org/10.1016/S0142-9612(00)00414-2
Asaoka K, Yokoyama K, Nagumo M (2002) Hydrogen embrittlement of nickel–titanium alloy in biological environment. Metall Mater Trans A 33:495–501. https://doi.org/10.1007/s11661-002-0111-8
Yokoyama K, Ogawa T, Asaoka K, Sakai J, Nagumo M (2003) Degradation of tensile strength of Ni–Ti superelastic alloy due to hydrogen absorption in methanol solution containing hydrochloric acid. Mater Sci Eng A 360:153–159. https://doi.org/10.1016/S0921-5093(03)00403-9
Yokoyama K, Kaneko K, Moriyama K, Asaoka K, Sakai J, Nagumo M (2003) Hydrogen embrittlement of Ni–Ti superelastic alloy in fluoride solution. J Biomed Mater Res 65A:182–187. https://doi.org/10.1002/jbm.a.10457
Ogawa T, Yokoyama K, Asaoka K, Sakai J (2005) Hydrogen embrittlement of Ni–Ti superelastic alloy in ethanol solution containing hydrochloric acid. Mater Sci Eng A 393:239–246. https://doi.org/10.1016/j.msea.2004.10.020
Yokoyama K, Kaneko K, Ogawa T, Moriyama K, Asaoka K, Sakai J (2005) Hydrogen embrittlement of work-hardened Ni–Ti alloy in fluoride solutions. Biomaterials 26:101–108. https://doi.org/10.1016/j.biomaterials.2004.02.009
Yokoyama K, Ogawa T, Takashima K, Asaoka K, Sakai J (2007) Hydrogen embrittlement of Ni–Ti superelastic alloy aged at room temperature after hydrogen charging. Mater Sci Eng A 466:106–113. https://doi.org/10.1016/j.msea.2007.02.102
Tomita M, Yokoyama K, Asaoka K, Sakai J (2008) Hydrogen thermal desorption behavior of Ni–Ti superelastic alloy subjected to tensile deformation after hydrogen charging. Mater Sci Eng A 476:308–315. https://doi.org/10.1016/j.msea.2007.04.104
Miyabe N, Tanaka H, Nakai Y, Kawanishi T (2012) 水素チャージしたTiNi形状記憶合金細線の力学特性 (Effect of hydrogen absorption on mechanical properties of tini shape memory alloy thin wire). J Soc Mater Sci Jpn 61:905–911. https://doi.org/10.2472/jsms.61.905
Yokoyama K, Nagaoka A, Sakai J (2012) Effects of the hydrogen absorption conditions on the hydrogen embrittlement behavior of Ni–Ti superelastic alloy. ISIJ Int 52:255–262. https://doi.org/10.2355/isijinternational.52.255
Kuběnová M, Zálešák J, Čermák J, Dlouhý A (2013) Impact of hydrogen-assisted heat treatments on microstructure and transformation path in a Ni-rich NiTi shape memory alloy. J Alloys Compd 577:S287–S290. https://doi.org/10.1016/j.jallcom.2012.07.096
Gamaoun F, Hassine T (2014) Ageing effect and rate dependency of a NiTi shape memory alloy after hydrogen charging. J Alloys Compd 615:S680–S683. https://doi.org/10.1016/j.jallcom.2013.10.214
Snir Y, Carl M, Ley NA, Young ML (2017) Effects of hydrogen charging on the phase transformation of martensitic NiTi shape memory alloy wires. Shape Mem and Superelasticity 3:443–456. https://doi.org/10.1007/s40830-017-0127-y
Yokoyama K, Hirata Y, Sakai J (2017) After-effects induced by interactions between hydrogen and the martensite transformation in Ni–Ti superelastic alloy. Philos Mag Lett 97:350–358. https://doi.org/10.1080/09500839.2017.1363423
Baturin A, Lotkov A, Grishkov V, Rodionov I, Kudiiarov V (2018) Effect of hydrogen redistribution during aging on the structure and phase state of nanocrystalline and coarse-grained TiNi alloys. J Alloys Compd 751:359–363. https://doi.org/10.1016/j.jallcom.2018.04.045
Weiser A, Buršíková V, Jarý M, Dymáček P, Dugáček J, Frenzel J, Čermák J, Dlouhý A (2019) Strength of hydrogen-free and hydrogen-doped Ni50Ti50 shape memory platelets. Scr Mater 162:151–155. https://doi.org/10.1016/j.scriptamat.2018.10.044
Ng CW, Mahmud AS, Razali MF, Ahmad MN (2020) Effect of hydrogen embrittlement towards thermal and mechanical behavior of NiTi shape memory alloy. IOP Conf Ser: Mater Sci Eng 920:012033. https://doi.org/10.1088/1757-899X/920/1/012033
Li Z, Xiao F, Liang X, Chen H, Li Z, Jin X, Fukuda T (2019) Effect of hydrogen doping on stress-induced martensitic transformation in a Ti–Ni shape memory alloy. Metal Mater Trans A 50:3033–3037. https://doi.org/10.1007/s11661-019-05258-1
Yamaguchi N, Yokoyama K (2022) Degradation caused by self-multiplication of damage induced by an interplay between hydrogen and the martensite transformation in a Ni–Ti superelastic alloy. Philos Mag Lett 102:60–70. https://doi.org/10.1080/09500839.2021.2015080
Nam TH, Shimizu K, Saburi T, Nenno S (1989) Crystal structure of a hydride formed by electrochemical hydrogenation in a Ti–Ni–Al alloy. Mater Trans JIM 30:539–548. https://doi.org/10.2320/matertrans1989.30.539
Akamine H, Kalbekov A, Higashizono T, Matsuda M, Hata S, Nishida M (2023) Hydrogen-induced phase with martensitic-like characteristics in Ti–Ni shape memory alloys. J Alloys Compd 965:171470. https://doi.org/10.1016/j.jallcom.2023.171470
Moitra A, Solanki KN, Horstemeyer MF (2011) The location of atomic hydrogen in NiTi alloy: a first principles study. Comput Mater Sci 50:820–823. https://doi.org/10.1016/j.commatsci.2010.10.013
Karbakhsh Ravari B, Nishida M (2013) In situ SEM studies of the transformation sequence of multistage martensitic transformations in aged Ti-50.8 at.% Ni alloys. Philos Mag 93:2279–2296. https://doi.org/10.1080/14786435.2013.769070
Karbakhsh Ravari B, Kizakibaru N, Nishida M (2013) Quantitative microstructure analyses upon multistage martensitic transformation in an aged Ti–50.8at.% Ni alloy. J Alloys Compd 577:S268–S273. https://doi.org/10.1016/j.jallcom.2012.03.041
Karbakhsh Ravari B, Farjami S, Nishida M (2014) Effects of Ni concentration and aging conditions on multistage martensitic transformation in aged Ni-rich Ti–Ni alloys. Acta Mater 69:17–29. https://doi.org/10.1016/j.actamat.2014.01.028
Soejima Y, Motomura S, Mitsuhara M, Inamura T, Nishida M (2016) In situ scanning electron microscopy study of the thermoelastic martensitic transformation in Ti–Ni shape memory alloy. Acta Mater 103:352–360. https://doi.org/10.1016/j.actamat.2015.10.017
Akamine H, Soejima Y, Nakamura T, Sahar F, Fukuda T, Kakeshita T, Nishida M (2020) Isothermal martensitic transformations in an aged Ni-Rich Ti–Ni alloy containing coherent Ti3Ni4 particles. Mater Trans. https://doi.org/10.2320/matertrans.MT-MJ2019004
Soejima Y, Heima A, Akamine H, Inamura T, Nishida M (2020) Comparison of In situ SEM and TEM observations of thermoelastic martensitic transformation in Ti–Ni shape memory alloy. Mater Trans 61:2107–2114. https://doi.org/10.2320/matertrans.MT-M2020219
Nishiyama Z (1978) Conditions for martensite formation and stabilization of austenite. In: Fine ME, Meshii M, Wayman CM (eds) Martensitic transformation. Academic Press, New York, pp 276–279
Sandrock GD, Perkins AJ, Hehemann RF (1971) The premartensitic instability in near-equiatomic TiNi. Metall Trans 2:2769–2781. https://doi.org/10.1007/BF02813251
Wayman CM, Cornelis I, Shimizu K (1972) Transformation behavior and the shape memory in thermally cycled TiNi. Scr Metall 6:115–122. https://doi.org/10.1016/0036-9748(72)90261-X
Miyazaki S, Igo Y, Otsuka K (1986) Effect of thermal cycling on the transformation temperatures of Ti–Ni alloys. Acta Metall 34:2045–2051. https://doi.org/10.1016/0001-6160(86)90263-4
Tadaki T, Nakata Y, Shimizu K (1987) Thermal cycling effects in an aged Ni-rich Ti–Ni shape memory alloy. Trans JIM 28:883–890. https://doi.org/10.2320/matertrans1960.28.883
Pelton AR, Huang GH, Moine P, Sinclair R (2012) Effects of thermal cycling on microstructure and properties in Nitinol. Mater Sci Eng A 532:130–138. https://doi.org/10.1016/j.msea.2011.10.073
Zhang J, Somsen C, Simon T, Ding X, Hou S, Ren S, Ren X, Eggeler G, Otsuka K, Sun J (2012) Leaf-like dislocation substructures and the decrease of martensitic start temperatures: a new explanation for functional fatigue during thermally induced martensitic transformations in coarse-grained Ni-rich Ti–Ni shape memory alloys. Acta Mater 60:1999–2006. https://doi.org/10.1016/j.actamat.2011.12.014
Ravari BK, Mitsuhara M, Farjami S, Nishida M (2013) Effect of thermal cycling on multistage martensitic transformation in aged Ti–50.8 at.% Ni alloy. Mater Trans 54:2185–2188. https://doi.org/10.2320/matertrans.M2013309
Sibirev A, Resnina N, Belyaev S (2019) Relationship between the variation in transformation temperatures, resistivity and dislocation density during thermal cycling of Ni50Ti50 shape memory alloy. Int J Mater Res 110:387–392. https://doi.org/10.3139/146.111755
Akamine H, Heima A, Soejima Y, Mitsuhara M, Inamura T, Nishida M (2023) Where and when are dislocations induced by thermal cycling in Ti–Ni shape memory alloys? Acta Mater 244:118588. https://doi.org/10.1016/j.actamat.2022.118588
Yamashita F, Ide Y, Akamine H, Ishikawa K, Nishida M (2020) Effect of C/O ratio on phase change and stability of inclusions in Ti–Ni alloys fabricated by a commercial production process. Shape Mem Superelasticity. https://doi.org/10.1007/s40830-020-00302-1
Kudoh Y, Tokonami M, Miyazaki S, Otsuka K (1985) Crystal structure of the martensite in Ti-49.2 at.%Ni alloy analyzed by the single crystal X-ray diffraction method. Acta Metall 33:2049–2056. https://doi.org/10.1016/0001-6160(85)90128-2
Kakeshita T, Shimizu K, Nakamichi S, Tanaka R, Endo S, Ono F (1992) Effect of hydrostatic pressures on thermoelastic martensitic transformations in aged Ti–Ni and ausaged Fe–Ni–Co–Ti shape memory alloys. Mater Trans JIM 33:1–6. https://doi.org/10.2320/matertrans1989.33.1
Acknowledgements
This work was partially supported by the Japan Society for Promotion of Science (JSPS) KAKENHI [grant numbers 19H00829, 22K18907, and 23K13540] and the Japan Science and Technology Agency (JST) [grant number 20100113] under the Industry-Academia Collaborative R&D Program “Heterogeneous Structure Control: Towards Innovative Development of Metallic Structural Materials.” The sponsors had no role in the study design, in the collection, analysis, or interpretation of data, in the writing of the report, or in the decision to submit the article for publication. We thank Dr. S. Kise, Dr. F. Yamashita, and Mr. K. Ishikawa (Furukawa Techno Material) for providing the Ti-Ni alloy.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kalbekov, A., Akamine, H., Ajito, S. et al. Transformation Behaviors and Microstructure Modifications in Hydrogen-Charged Ti–Ni Shape Memory Alloy. Shap. Mem. Superelasticity (2024). https://doi.org/10.1007/s40830-024-00495-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40830-024-00495-9