Abstract
Bismuth containing nanomaterials recently received increasing attention with respect to environmental applications because of their low cost, high stability and nontoxicity. In this work, Bi–Bi2O2CO3 heterojunctions were fabricated by in-situ decoration of Bi nanoparticles on Bi2O2CO3 nanosheets via a simple hydrothermal synthesis approach. X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) were used to confirm the morphology of the nanosheet-like heterostructure of the Bi–Bi2O2CO3 composite. Detailed ultrafast electronic spectroscopy reveals that the in-situ decoration of Bi nanoparticles on Bi2O2CO3 nanosheets exhibit a dramatically enhanced electron-hole pair separation rate, which results in an extraordinarily high photocatalytic activity for the degradation of a model organic dye, methylene blue (MB) under visible light illumination. Cycling experiments revealed a good photochemical stability of the Bi–Bi2O2CO3 heterojunction under repeated irradiation. Photocurrent measurements further indicated that the heterojunction incredibly enhanced the charge generation and suppressed the charge recombination of photogenerated electron-hole pairs.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Highlights
-
A facile low cost hydrothermal technique was employed to synthesize of Bi-Bi2O2CO3, and Bi nanoparticles was decorated in-situ on Bi2O2CO3.
-
The heterostructure exhibits enhanced electron-hole separation and improves visible-light photocatalytic activity effectively.
2 Introduction
Photocatalysis technology has attracted enormous interest because of its potential to soften and release the global energy crisis and environmental pollution [1–6]. Although various types of semiconductor photocatalyst have been developed, their applications are impeded by a high recombination rate of electron–hole pairs and low efficiency of solar light absorption in the photocatalysis [7, 8]. A tremendous effort has been made to optimize the electronic band structure allowing an efficient electron–hole separation, which has been acknowledged to be a key factor in enhancing solar energy conversion [9–14]. The development of heterojunction systems has also been understood since it is beneficial for electron transfer to improve electron–hole pair separation, and therefore resulting in an excellent photocatalytic activity under solar light illumination [15, 16].
Recently, economic and abundant bismuth-containing semiconductors have been attracted large attention for diverse applications, especially in the area of energy conversion and environmental treatment [17, 18, 19, 20, 21]. The bismuth subcarbonate (Bi2O2CO3) is one of the most interesting semiconductor with a large bang gap of 3.3 eV. It belongs to the layered Aurivillius-related oxide family, consisting of Bi2O2 2+ layers sandwiched between two slabs of CO3 2− layers [22]. However, the use of Bi2O2CO3 in light harvesting applications is very limited because it can absorb only UV light. To overcome this drawback, several 3D hierarchical Bi2O2CO3 architectures composed of nanosheets, nanoplates and microspheres have been developed [23, 24]. The coupling of Bi2O2CO3 with other materials to construct heterojunctions has also been shown as an advantages approach to improve the visible light responsive activity and to facilitate the separation of photogenerated electron–hole pairs. Different low band gap semiconductors and polymers have been used to improve the photocatalytic activity of Bi2O2CO3. Liu et al. constructed hierarchical graphene–Bi2O2CO3 composites which exhibit a significantly enhanced visible light photocatalytic performance [25]. Good visible light photocatalytic activity toward the degradation of Rhodamine B was reported by Zhang et al. for n–n heterostructured Bi2O2CO3/Bi2WO6 [26]. Zhou’s group reported that PANI decorated Bi2O2CO3 nanosheets exhibited a four to half times better photocatalytic activity for degradation of Rhodamine B in comparison to Bi2O2CO3 nanosheets under visible light illumination [27]. Recently, p–n heterojunction Ag2O/Bi2O2CO3 photocatalysts was shown to manifest an excellent visible light activity for degradation of MB and methyl orange [28].
Recently surface plasmon resonance (SPR) of noble metal nanoparticles (Ag or Au) was reported for improving the activity of semiconductor photocatalysts efficiently [29, 30]. In comparison with the high cost of noble-metals, Bi nanoparticles are inexpensive and show comparable SPR [31]. Recently, two reports on Bi nanoparticles demonstrate that they are useful for catalysis and sensing applications [32, 33]. Dong et al. showed that plasmonic Bi nanoparticles can be used for NO removal [34]. Several Bi nanoparticles based nanocomposites like Bi/BiOCl, Bi/Bi2O3, and Bi/BiOI exhibit enhanced photocatalytic activity comparing to their counterpart [35–37]. Recently, Bi nanoparticles based heterojunctions with semiconductor have been an intense research area due to their enhanced charge separation and improved photocatalytic efficacies [38–40]. However, Bi nanoparticles decorated Bi2O2CO3 nanosheets have not been considered up to date.
In the present study, we developed an in situ decoration of Bi nanoparticles on Bi2O2CO3 nanosheets via a one-pot hydrothermal method. From time-resolved fluorescence spectroscopy, we observed that an ultrafast electron transfer process in the Bi–Bi2O2CO3 heterojunction reveals an excited state electron transfer from Bi2O2CO3 to Bi. The novel Bi-decorated Bi2O2CO3 nanosheets exhibited a dramatically enhanced photocatalytic activity towards MB degradation comparing to pure Bi2O2CO3 nanosheets because of the SPR effect of Bi nanoparticles and an efficient separation of electron–hole pairs in the Bi–Bi2O2CO3 heterojunction. We also observed that Bi–Bi2O2CO3 exhibited a good recyclability with respect to degradation of MB, which is significant for real world applications.
3 Experimental Section
3.1 Reagents
Bismuth nitrate pentahydrate [Bi(NO3)3·5H2O], cityl trimethyl ammonium bromide (CTAB), sodium carbonate (Na2CO3), and methylene blue (MB) were purchased from Sigma Aldrich. All other chemicals employed were of analytical grade and used without further purification.
3.2 Synthesis of Bi–Bi2O2CO3 Heterojunction
In a typical synthesis of Bi–Bi2O2CO3, 0.2 millimol Bi(NO3)3·5H2O was first dissolved in 20 mL 1 M HNO3 (denoted as solution A). Meanwhile, 1.6 millimol Na2CO3 and 50 mg CTAB were dissolved in 20 mL ethanol–water mixture (denoted as solution B). Then, solution B was added into solution A under stirring for 30 min at 30 °C. The resulting mixture was transferred into a 20 mL Teflon-lined stainless-steel autoclave and was placed into an oven to react at 180 °C for 6 h. The system was then cooled to ambient temperature naturally. The final product was collected and washed with distilled water and absolute alcohol at least five times. As-prepared samples were dried at 60 °C for 6 h. The reductive nature of EtOH and CTAB allowed an in situ formation of Bi nanoparticles on the Bi2O2CO3 nanosheet. As a result, a heterojunction structure consisting of Bi2O2CO3 sheets and metallic Bi nanoparticles has been produced. For the synthesis of Bi2O2CO3 nanosheets we follow a preparation as reported by Zhou et al. [27].
3.3 Characterization Methods
Field emission scanning electron microscopy (FESEM, QUANTA FEG 250) was used to investigate the surface morphology of the samples and samples were performed by applying a diluted drop of samples on a silicon wafer. Transmission electron microscopy (TEM) grids were prepared by applying a diluted drop of the samples to carbon-coated copper grids. The particle sizes were determined from micrographs recorded at a magnification of 100,000X using an FEI (Technai S-Twin, operating at 200 kV) instrument. X-ray diffraction (XRD) patterns of the samples were recorded by employing a scanning rate of 0.02° S−1 in the 2θ range from 20° to 80° using a PANalytical XPERTPRO diffractometer equipped with Cu K α radiation (at 40 mA and 40 kV). For optical experiments, the steady-state absorption and emission were carried out with a Shimadzu UV-2600 spectrophotometer and a Jobin–Yvon Fluoromax-3 fluorimeter, respectively. Picosecond-resolved spectroscopic studies were carried out using a commercial time correlated single photon counting (TCSPC) setup from Edinburgh Instruments (instrument response function, IRF = 80 ps, excitation at 375 nm). The details of experimental setting up and methodology were described in our earlier reports [41, 42].
3.4 Photocatalytic Performance Measurements
The photocatalysis activity of the samples were evaluated in terms of photodegradation of MB which was taken as a model pollutant in water. The photodegradation reaction of MB (initial concentration C 0 = 0.5 × 10−5 M) was carried out in a 10 mm optical path quartz cell reactor containing 2 mL of a model MB solution with a concentration of 0.5 g L−1 of the photocatalyst in deionized water (DI). The suspension was irradiated with a mercury lamp, λ ≥ 400 nm (under visible light) and absorbance data were collected continuously by using a reported setting [4]. The percentage degradation (%DE) of MB was determined by Eq. 1:
where I 0 is the initial absorption intensity of MB at λ max = 660 nm and I is the absorption intensity after irradiation.
3.5 Photocurrent Measurements
Photocurrent measurements were done in a dye-sensitized solar cell (DSSC) setup [43]. To prepare the working and counter electrodes for the photocurrent responses, FTO glasses were ultrasonically cleaned in soap-suds, deionized water, and acetone, respectively. For preparation of the counter electrode, platinum (Pt) was deposited on the FTO substrates by thermal decomposition of 10 mM platinum chloride (in isopropanol) at 385 °C for 30 min. Bi2O2CO3 and Bi–Bi2O2CO3 were used as the photoelectrode. The two electrodes were placed on top of each other with a single layer of 60 μm thick Surlyn (Solaronix) as a spacer between the two electrodes. A liquid electrolyte composed of 0.5 M lithium iodide (LiI), 0.05 M iodine (I2) and 0.5 M 4-tert-butylpyridine (TBP) in acetonitrile was used as the hole conductor and filled in the inter electrode space using capillary force through two small holes (diameter = 1 mm) predrilled on the counter electrode. Finally, the two holes were sealed by using another piece of Surlyn to prevent a leakage of the electrolyte from the cell. In all our experiments, the active area of the DSSCs was fixed to 1 cm2.
4 Results and Discussion
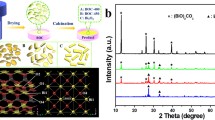
Figure 1a shows XRD patterns of the as-synthesized Bi2O2CO3 and Bi–Bi2O2CO3. The diffraction pattern of Bi2O2CO3 is perfectly indicated as a tetragonal Bi2O2CO3 phase. After the addition of ethanol, the XRD pattern of the Bi–Bi2O2CO3 sample is also indexed to the Bi2O2CO3 phase (JCPDS card no. 41-1488) [44]. No characteristic peak for Bi nanoparticles in Bi–Bi2O2CO3 was observed, probably due to low content of Bi. Similar results were reported in previously literatures based on metal/semiconductor photocatalyst [35, 45, 46]. As shown in Fig. 1b, c, the SEM images of Bi2O2CO3 and Bi-Bi2O2CO3 reveal a large sheet-like morphology with a width from 50 to 600 nm. After decoration of Bi on the Bi2O2CO3 nanosheets, no significant structural and morphological change was observed. The smooth sheet-like morphology of Bi–Bi2O2CO3 indicates a uniform distribution of Bi nanoparticles on the surface of Bi2O2CO3. Morphology and crystallinity of Bi2O2CO3 and Bi–Bi2O2CO3 were further examined via TEM and HRTEM as shown in Fig. 2a–d. The TEM image of Bi–Bi2O2CO3 shows a uniform distribution of Bi nanoparticles on the surface of the Bi2O2CO3 nanosheets. The HRTEM images of Bi2O2CO3 and Bi–Bi2O2CO3 exhibit a high crystallinity of Bi2O2CO3 nanosheet and Bi nanoparticles as given in Fig. 2c, d. The inter-planar distance between the fringes are about 0.276 and 0.32 nm, which correspond to the (110) crystal plane of Bi2O2CO3 and (012) crystal plane of Bi nanoparticles, respectively [38, 47]. The selected area electron diffraction (SAED) pattern obtained from the HRTEM images (Fig. 2e, f) demonstrates further the well-crystallinity. From the EDAX measurement shown in Fig. 2g, the at.% ratio of Bi and O is 1:5 for Bi2O2CO3 whereas 2:3 for Bi–Bi2O2CO3. The XPS studies of Bi2O2CO3 and Bi–Bi2O2CO3 were well documented in earlier studies [18, 27, 31, 48]. In those studies, they concluded that the O 1s peak centered at 530.5 eV ascribed to Bi–O bonds in Bi2O2CO3, while peaks at 284.8 and 288.7 eV were the characteristic peaks of adventitious carbon species and CO3 2− in Bi2O2CO3. Peaks around 157.0 and 162.3 eV were assigned to the formation of Bi metal present in the heterostructure. The other characterizations on Bi2O2CO3 and Bi–Bi2O2CO3 related materials [18, 27, 31, 48] including HRTEM and EDAX are consistent with our experimental observations.
Figure 3a shows UV–Vis absorption spectra of Bi2O2CO3 and Bi–Bi2O2CO3. The Bi2O2CO3 shows absorption peak at 360 nm and long tail over 800 nm due to scattering of the nanoparticles presented in the solution, which consists with earlier reports [49, 50]. After formation of heterojunction Bi–Bi2O2CO3, an enhancement of absorption in the visible region was observed due to the presence of Bi nanoparticles, and surface plasmon absorption around 500 nm was found as shown in Fig. 3b. The SPR of non-noble metal Bi in the near ultraviolet and visible region were reported by different groups [51, 52]. Notably, such absorption enhancement in the visible region is also according to the color change of the samples as shown in the inset of Fig. 3a, b. Thus, formation of Bi nanoparticles on the surface of Bi2O2CO3 nanosheets results in an enhancement of absorption over the entire UV–Vis region. The photoluminescences of Bi2O2CO3 and Bi–Bi2O2CO3 exhibit emission around 400–550 nm upon excitation at 365 nm as shown in Fig. 3c, e. Picosecond-resolved fluorescence was studied to investigate the detailed photophysical properties of the heterostructure after decoration of Bi nanoparticles on the Bi2O2CO3 nanosheets. The fluorescence decay of Bi2O2CO3 and Bi–Bi2O2CO3 was determined at 460 nm upon excitation by 375 nm laser source (Fig. 4) and tabulated in Table 1.
The fluorescence decay of Bi2O2CO3 shows two components of 343 ps and 3.5 ns along with an average lifetime of 1.25 ns. After decoration of Bi nanoparticles, the average time of Bi–Bi2O2CO3 decreases to 0.70 ns. Thus, the faster component of 50 ps is attributed to the excited state electron transfer from Bi2O2CO3 to Bi. The obvious decrease in fluorescence lifetime of the heterostructures suggests that the decoration of Bi nanoparticles on the Bi2O2CO3 nanosheets can act as electron sink and therefore contribute to electron–hole separation. Such kind of metal-semiconductor heterojunctions facilitates in a remarkable way of the decline in the recombination of electron–hole pairs and is useful to enhance solar energy utilization [53–55].
The photocatalytic activities of Bi2O2CO3 and Bi–Bi2O2CO3 were evaluated by photodegradation of the model organic contaminant MB under visible light illumination. However, in our case the as-prepared Bi2O2CO3 and Bi–Bi2O2CO3 have insignificant photocatalytic activity in dark (Fig. 5a). During the photocatalytic reaction, MB forms a well-known colorless product leucomethylene blue (LMB) [56, 57] as expressed in Eq. 2.
a Photocatalytic degradation of MB under visible light illumination. b Photocatalytic degradation of MB by Bi2O2CO3 at different wavelength. c Ct/C0 versus time with various concentrations of methylene blue by Bi2O2CO3. d Langmuir–Hinshelwood plot (L–H) for photocatalytic degradation of methylene blue using Bi2O2CO3 (solid line is the model fitting and solid circles are experimental data). e Photodegradation of MB over Bi2O2CO3 and Bi–Bi2O2CO3 under conventional condition, presence of H2O2 and N2 into the solution. f A recyclability study of Bi–Bi2O2CO3 under visible light illumination
Figure 5a shows changes in MB concentration as a function of time in presence and absence of photocatalysts. With our experimental time window, MB has less than 10% degradation under light illumination in the absence of photocatalysts. In contrast, Bi2O2CO3 nanosheets show an enhanced photocatalytic activity and 60% of MB was degraded after 60 min illumination. One can see that presence of Bi nanoparticles on the Bi2O2CO3 nanosheets further enhance photocatalytic activity (100%) compared to Bi2O2CO3 nanosheets (60%). Figure 5b shows photocatalysis of methylene blue (MB) at different wavelength by Bi2O2CO3. Insignificant photocatalysis at 650 nm (MB absorbance maxima 660 nm) indicates that MB is unable to photosensitize Bi2O2CO3. Thus photocatalysis predominately takes place via sensitization of Bi2O2CO3. In order to find out the effect of the surface on photocatalysis, the Langmuir–Hinshelwood (L–H) kinetics was studied using different concentrations of MB (Fig. 5c). As shown in Fig. 5d, a significant deviation of the model (solid line) from experimental data is evident. The observation indicates that surface adsorption of the model pollutant plays insignificant role in the photodegradation. In order to investigate the catalytic pathway, we further studied the photocatalytic activity of Bi2O2CO3 in the presence of a radical initiator (H2O2) and radical quencher (N2 bubbling) separately (Fig. 5e). In fact, in the presence of H2O2 under solar light illumination increases generation of OH· which eventually increases the photocatalytic activity of Bi2O2CO3 for degradation of MB. This demonstrates the role of reactive oxygen species (ROS) in the degradation of MB [58]. The photodegradation efficiency of Bi2O2CO3 decreases with N2 bubbling in the solution, so O2 primarily acts as an efficient electron trap, leading to the generation of O −2 radicals during photocatalytic reaction [59]. From the application point of view, photochemical stability and durability of photocatalysts are significant during photocatalytic reaction [60]. To further test photocatalytic performance of the as-prepared heterojunction Bi–Bi2O2CO3 photocatalyst, recycling experiment was carried out under repeated irradiation. Figure 5f shows the repeated photocatalytic activity of Bi–Bi2O2CO3. The results indicate that the rate remains similar after four consecutive cycles, implying that the obtained Bi–Bi2O2CO3 heterojunction photocatalyst has high stability and no photocorrosion occurs during the photodegradation of MB. Photocurrent measurement was carried out under solar light illumination to investigate the efficient electron–hole separation. The photocurrent of Bi–Bi2O2CO3 heterostructures is much higher than that of Bi2O2CO3 (see Fig. 6). This implies that the heterojunction shows an improved separation of photogenerated electron–hole pairs and can greatly facilitate its photocatalytic activity.
There are several reports which indicate that the enhancement in photocatalytic performance can be ascribed to the synergetic effects of many factors, such as hierarchical structure, surface area, interfacial charge transfer, and efficient separation of photoinduced electrons and holes [61–65]. In the present study, the enhanced photocatalytic performance for the Bi–Bi2O2CO3 photocatalyst can be ascribed to the formation of heterojunction between Bi nanoparticles and the surface of Bi2O2CO3 nanosheets. Furthermore, the Fermi level of Bi nanoparticles which acts as electron acceptors can be estimated to be about −0.17 eV as calculated by the work function of metallic bismuth of 4.22 eV [66–68]. Since the Fermi level of metallic Bi (−0.17 eV) is lower than the conduction band of Bi2O2CO3 (−1.40 eV) [27], the photogenerated electrons would probably transfer from Bi2O2CO3 to the deposited Bi nanoparticles and therefore promote the separation of photo-generated electrons and holes, effectively. After the separation of electrons and holes, these two kinds of photogenerated charge carriers would be transformed into reactive species that are responsible for promoting photocatalytic activity. Based on the above investigations, a schematic illustration is proposed as shown in Scheme 1.
5 Conclusion
We successfully synthesized Bi–Bi2O2CO3 heterojunction by a one-step hydrothermal method. Detailed spectroscopic investigations reveal that ultrafast photo-induced charge separation in the Bi–Bi2O2CO3 heterojunction is conducive for enhanced solar energy conversion. As a potential prototype application, we found enhanced photocatalytic activity of the heterostructure using MB as a model organic contaminant under visible light illumination. The efficient separation of photoinduced electron–hole pairs in the heterojunction was further proved by photocurrent measurement. Moreover, this work not only provides cost effective procedure to prepare efficient photocatalyst with high stability but also opens up a new field for bismuth containing heterostructures with several future applications.
References
M.N. Chong, B. Jin, C.W.K. Chow, C. Saint, Recent developments in photocatalytic water treatment technology: a review. Water Res. 44(10), 2997–3027 (2010). doi:10.1016/j.watres.2010.02.039
M.R. Hoffmann, S.T. Martin, W. Choi, D.W. Bahnemann, Environmental applications of semiconductor photocatalysis. Chem. Rev. 95(1), 69–96 (1995). doi:10.1021/cr00033a004
S. Dong, J. Feng, M. Fan, Y. Pi, L. Hu, X. Han, M. Liu, J. Sun, Recent developments in heterogeneous photocatalytic water treatment using visible light-responsive photocatalysts: a review. RSC Adv. 5(19), 14610–14630 (2015). doi:10.1039/C4RA13734E
Sunil P. Lonkar, Vishnu V. Pillai, Samuel Stephen, Ahmed Abdala, Vikas Mittal, Facile in situ fabrication of nanostructured graphene-CuO hybrid with hydrogen sulfide removal capacity. Nano-Micro Lett. 8(4), 312–319 (2016). doi:10.1007/s40820-016-0090-8
P. Kar, S. Sardar, S. Ghosh, M.R. Parida, B. Liu, O.F. Mohammed, P. Lemmens, S.K. Pal, Nano surface engineering of Mn2O3 for potential light-harvesting application. J. Mater. Chem. C 3(31), 8200–8211 (2015). doi:10.1039/C5TC01475A
T.K. Maji, D. Bagchi, P. Kar, D. Karmakar, S.K. Pal, Enhanced charge separation through modulation of defect-state in wide band-gap semiconductor for potential photocatalysis application: Ultrafast spectroscopy and computational studies. J. Photochem. Photobiol. A 332, 391–398 (2017). doi:10.1016/j.jphotochem.2016.09.017
Y. Wang, Q. Wang, X. Zhan, F. Wang, M. Safdar, J. He, Visible light driven type II heterostructures and their enhanced photocatalysis properties: a review. Nanoscale 5(18), 8326–8339 (2013). doi:10.1039/c3nr01577g
J. Liqiang, S. Xiaojun, S. Jing, C. Weimin, X. Zili, D. Yaoguo, F. Honggang, Review of surface photovoltage spectra of nano-sized semiconductor and its applications in heterogeneous photocatalysis. Sol. Energy Mater. Sol. Cells 79(2), 133–151 (2003). doi:10.1016/S0927-0248(02)00393-8
H. Huang, X. Li, J. Wang, F. Dong, P.K. Chu, T. Zhang, Y. Zhang, Anionic group self-doping as a promising strategy: band-gap engineering and multi-functional applications of high-performance CO32-doped Bi2O2CO3. ACS Catal. 5(7), 4094–4103 (2015). doi:10.1021/acscatal.5b00444
H. Huang, X. Han, X. Li, S. Wang, P.K. Chu, Y. Zhang, Fabrication of multiple heterojunctions with tunable visible-light-active photocatalytic reactivity in BiOBr–BiOI full-range composites based on microstructure modulation and band structures. ACS Appl. Mater. Interfaces 7(1), 482–492 (2015). doi:10.1021/am5065409
H. Huang, K. Xiao, Y. He, T. Zhang, F. Dong, X. Du, Y. Zhang, In situ assembly of BiOI@Bi12O17Cl2 p-n junction: charge induced unique front-lateral surfaces coupling heterostructure with high exposure of BiOI {001} active facets for robust and nonselective photocatalysis. Appl. Catal. B 199, 75–86 (2016). doi:10.1016/j.apcatb.2016.06.020
H. Huang, K. Liu, K. Chen, Y. Zhang, Y. Zhang, S. Wang, Ce and F comodification on the crystal structure and enhanced photocatalytic activity of Bi2WO6 photocatalyst under visible light irradiation. J. Phys. Chem. C 118(26), 14379–14387 (2014). doi:10.1021/jp503025b
Q. He, S. Huang, C. Wang, Q. Qiao, N. Liang, M. Xu, W. Chen, J. Zai, X. Qian, The role of Mott-Schottky heterojunctions in Ag–Ag8SnS6 as counter electrodes in dye-sensitized solar cells. Chem. Sus. Chem. 8(5), 817–820 (2015). doi:10.1002/cssc.201403343
S. Huang, Q. He, J. Zai, M. Wang, X. Li, B. Li, X. Qian, The role of Mott-Schottky heterojunctions in PtCo-Cu2ZnGeS4 as counter electrodes in dye-sensitized solar cells. Chem. Commun. 51(43), 8950–8953 (2015). doi:10.1039/C5CC02584B
L. Jin, G. Zhu, M. Hojamberdiev, X. Luo, C. Tan, J. Peng, X. Wei, J. Li, P. Liu, A plasmonic Ag–AgBr/Bi2O2CO3 composite photocatalyst with enhanced visible-light photocatalytic activity. Ind. Eng. Chem. Res. 53(35), 13718–13727 (2014). doi:10.1021/ie502133x
G. Zhu, M. Hojamberdiev, K.-I. Katsumata, X. Cai, N. Matsushita, K. Okada, P. Liu, J. Zhou, Heterostructured Fe3O4/Bi2O2CO3 photocatalyst: synthesis, characterization and application in recyclable photodegradation of organic dyes under visible light irradiation. Mater. Chem. Phys. 142(1), 95–105 (2013). doi:10.1016/j.matchemphys.2013.06.046
A. Hameed, T. Montini, V. Gombac, P. Fornasiero, Surface phases and photocatalytic activity correlation of Bi2O3/Bi2O4-x nanocomposite. J. Am. Chem. Soc. 130(30), 9658–9659 (2008). doi:10.1021/ja803603y
S. Xiao, Y. Li, J. Hu, H. Li, X. Zhang, L. Liu, J. Lian, One-step synthesis of nanostructured Bi-Bi2O2CO3-ZnO composites with enhanced photocatalytic performance. Cryst. Eng. Comm. 17(20), 3809–3819 (2015). doi:10.1039/C5CE00338E
P. Cai, S. Zhou, D. Ma, S. Liu, W. Chen, S. Huang, Fe2O3-modified porous BiVO4 nanoplates with enhanced photocatalytic activity. Nano-Micro Lett. 7(2), 183–193 (2015). doi:10.1007/s40820-015-0033-9
W. Wang, J. Wang, Z. Wang, X. Wei, L. Liu, Q. Ren, W. Gao, Y. Liang, H. Shi, p-n junction CuO/BiVO4 heterogeneous nanostructures: synthesis and highly efficient visible-light photocatalytic performance. Dalton Trans. 43(18), 6735–6743 (2014). doi:10.1039/c3dt53613k
Y. Zhang, G. Zhu, M. Hojamberdiev, J. Gao, J. Hao, J. Zhou, P. Liu, Synergistic effect of oxygen vacancy and nitrogen doping on enhancing the photocatalytic activity of Bi2O2CO3 nanosheets with exposed {001} facets for the degradation of organic pollutants. Appl. Surf. Sci. 371, 231–241 (2016). doi:10.1016/j.apsusc.2016.02.210
C. Greaves, S.K. Blower, Structural relationships between Bi2O2CO3 and β-Bi2O3. Mater. Res. Bull. 23(7), 1001–1008 (1988). doi:10.1016/0025-5408(88)90055-4
P. Madhusudan, J. Zhang, B. Cheng, G. Liu, Photocatalytic degradation of organic dyes with hierarchical Bi2O2CO3 microstructures under visible-light. CrystEngComm 15(2), 231–240 (2013). doi:10.1039/C2CE26639C
T. Zhao, J. Zai, M. Xu, Q. Zou, Y. Su, K. Wang, X. Qian, Hierarchical Bi2O2CO3 microspheres with improved visible-light-driven photocatalytic activity. CrystEngComm 13(12), 4010–4017 (2011). doi:10.1039/c1ce05113j
P. Madhusudan, J. Yu, W. Wang, B. Cheng, G. Liu, Facile synthesis of novel hierarchical graphene-Bi2O2CO3 composites with enhanced photocatalytic performance under visible light. Dalton Trans. 41(47), 14345–14353 (2012). doi:10.1039/c2dt31528a
Y.-S. Xu, W.-D. Zhang, Anion exchange strategy for construction of sesame-biscuit-like Bi2O2CO3/Bi2MoO6 nanocomposites with enhanced photocatalytic activity. Appl. Catal. B 140–141, 306–316 (2013). doi:10.1016/j.apcatb.2013.04.019
Z. Zhao, Y. Zhou, F. Wang, K. Zhang, S. Yu, K. Cao, Polyaniline-decorated {001} facets of Bi2O2CO3 nanosheets: in situ oxygen vacancy formation and enhanced visible light photocatalytic activity. ACS Appl. Mater. Interfaces 7(1), 730–737 (2015). doi:10.1021/am507089x
N. Liang, M. Wang, L. Jin, S. Huang, W. Chen et al., Highly efficient Ag2O/Bi2O2CO3 p-n heterojunction photocatalysts with improved visible-light responsive activity. ACS Appl. Mater. Interfaces 6(14), 11698–11705 (2014). doi:10.1021/am502481z
S. Peng, L. Li, H. Tan, Y. Wu, R. Cai et al., Monodispersed Ag nanoparticles loaded on the PVP-assisted synthetic Bi2O2CO3 microspheres with enhanced photocatalytic and supercapacitive performances. J. Mater. Chem. A 1(26), 7630–7638 (2013). doi:10.1039/c3ta10951h
R. Wang, X. Li, W. Cui, Y. Zhang, F. Dong, In situ growth of Au nanoparticles on 3D Bi2O2CO3 for surface plasmon enhanced visible light photocatalysis. New J. Chem. 39(11), 8446–8453 (2015). doi:10.1039/C5NJ01882J
F. Dong, Q. Li, Y. Sun, W.-K. Ho, Noble metal-like behavior of plasmonic Bi particles as a cocatalyst deposited on (BiO)2CO3 microspheres for efficient visible light photocatalysis. ACS Catal. 4(12), 4341–4350 (2014). doi:10.1021/cs501038q
H. Safardoust-Hojaghan, M. Salavati-Niasari, M.H. Motaghedifard, S.M. Hosseinpour-Mashkani, Synthesis of micro sphere-like bismuth nanoparticles by microwave assisted polyol method; designing a novel electrochemical nanosensor for ultra-trace measurement of Pb2+ ions. New J. Chem. 39(6), 4676–4684 (2015). doi:10.1039/C5NJ00532A
Z. Wang, C. Jiang, R. Huang, H. Peng, X. Tang, Investigation of optical and photocatalytic properties of bismuth nanospheres prepared by a facile thermolysis method. J. Phys. Chem. C 118(2), 1155–1160 (2014). doi:10.1021/jp4065505
F. Dong, T. Xiong, Y. Sun, Z. Zhao, Y. Zhou, X. Feng, Z. Wu, A semimetal bismuth element as a direct plasmonic photocatalyst. Chem. Commun. 50(72), 10386–10389 (2014). doi:10.1039/C4CC02724H
D. Chen, M. Zhang, Q. Lu, J. Chen, B. Liu, Z. Wang, Facile synthesis of Bi/BiOCl composite with selective photocatalytic properties. J. Alloys Compd. 646, 647–654 (2015). doi:10.1016/j.jallcom.2015.06.113
Y. Huang, W. Wang, Q. Zhang, J.-J. Cao, R.-J. Huang, W. Ho, S.C. Lee, In situ fabrication of α-Bi2O3/(BiO)2CO3 nanoplate heterojunctions with tunable optical property and photocatalytic activity. Sci. Rep. 6, 23435 (2016). doi:10.1038/srep23435
C. Chang, L. Zhu, Y. Fu, X. Chu, Highly active Bi/BiOI composite synthesized by one-step reaction and its capacity to degrade bisphenol A under simulated solar light irradiation. Chem. Eng. J. 233, 305–314 (2013). doi:10.1016/j.cej.2013.08.048
M. Gao, D. Zhang, X. Pu, H. Li, D. Lv, B. Zhang, X. Shao, Facile hydrothermal synthesis of Bi/BiOBr composites with enhanced visible-light photocatalytic activities for the degradation of rhodamine B. Sep. Purif. Technol. 154, 211–216 (2015). doi:10.1016/j.seppur.2015.09.063
Y. Chen, D. Chen, J. Chen, Q. Lu, M. Zhang, B. Liu, Q. Wang, Z. Wang, Facile synthesis of Bi nanoparticle modified TiO2 with enhanced visible light photocatalytic activity. J. Alloys Compd. 651, 114–120 (2015). doi:10.1016/j.jallcom.2015.08.119
X. Liu, H. Cao, J. Yin, Generation and photocatalytic activities of Bi@Bi2O3 microspheres. Nano Res. 4(5), 470–482 (2011). doi:10.1007/s12274-011-0103-3
S. Sardar, P. Kar, S. Sarkar, P. Lemmens, S.K. Pal, Interfacial carrier dynamics in PbS-ZnO light harvesting assemblies and their potential implication in photovoltaic/photocatalysis application. Sol. Energ. Mat. Sol. Cells 134, 400–406 (2015). doi:10.1016/j.solmat.2014.12.032
P.K. Sarkar, N. Polley, S. Chakrabarti, P. Lemmens, S.K. Pal, Nanosurface energy transfer based highly selective and ultrasensitive “Turn on” fluorescence mercury sensor. ACS Sens. 1(6), 789–797 (2016). doi:10.1021/acssensors.6b00153
S. Sardar, P. Kar, H. Remita, B. Liu, P. Lemmens, S. Kumar Pal, S. Ghosh, Enhanced charge separation and FRET at heterojunctions between semiconductor nanoparticles and conducting polymer nanofibers for efficient solar light harvesting. Sci. Rep. 5, 17313 (2015). doi:10.1038/srep17313
Y. Sun, Z. Zhao, F. Dong, W. Zhang, Mechanism of visible light photocatalytic NOx oxidation with plasmonic Bi cocatalyst-enhanced (BiO)2CO3 hierarchical microspheres. Phys. Chem. Chem. Phys. 17(16), 10383–10390 (2015). doi:10.1039/C4CP06045H
J.L.T. Chen, V. Nalla, G. Kannaiyan, V. Mamidala, W. Ji, J.J. Vittal, Synthesis and nonlinear optical switching of Bi2S3 nanorods and enhancement in the NLO response of Bi2S3@Au nanorod-composites. New J. Chem. 38(3), 985–992 (2014). doi:10.1039/c3nj01380d
S.P. Lim, A. Pandikumar, N.M. Huang, H.N. Lim, Facile synthesis of Au@TiO2 nanocomposite and its application as a photoanode in dye-sensitized solar cells. RSC Adv. 5(55), 44398–44407 (2015). doi:10.1039/C5RA06220A
G. Cheng, H. Yang, K. Rong, Z. Lu, X. Yu, R. Chen, Shape-controlled solvothermal synthesis of bismuth subcarbonate nanomaterials. J. Solid State Chem. 183(8), 1878–1883 (2010). doi:10.1016/j.jssc.2010.06.004
G. Zhu, Y. Liu, M. Hojamberdiev, J. Han, J. Rodriguez, S.A. Bilmes, P. Liu, Thermodecomposition synthesis of porous β-Bi2O3/Bi2O2CO3 heterostructured photocatalysts with improved visible light photocatalytic activity. New J. Chem. 39(12), 9557–9568 (2015). doi:10.1039/C5NJ01462J
S. Lin, L. Liu, Y. Liang, W. Cui, Z. Zhang, Oil-in-water self-assembled synthesis of Ag@AgCl nano-particles on flower-like Bi2O2CO3 with enhanced visible-light-driven photocatalytic activity. Materials 9(6), 486 (2016). doi:10.3390/ma9060486
J. Siegel, O. Kvítek, P. Ulbrich, Z. Kolská, P. Slepička, V. Švorčík, Progressive approach for metal nanoparticle synthesis. Mater. Lett. 89, 47–50 (2012). doi:10.1016/j.matlet.2012.08.048
S. Weng, B. Chen, L. Xie, Z. Zheng, P. Liu, Facile in situ synthesis of a Bi/BiOCl nanocomposite with high photocatalytic activity. J. Mater. Chem. A 1(9), 3068–3075 (2013). doi:10.1039/c2ta01004f
J. Toudert, R. Serna, M. Jiménez de Castro, Exploring the optical potential of nano-bismuth: tunable surface plasmon resonances in the near ultraviolet-to-near infrared range. J. Phys. Chem. C 116(38), 20530–20539 (2012). doi:10.1021/jp3065882
S.K. Cushing, N. Wu, Progress and perspectives of plasmon-enhanced solar energy conversion. J. Phys. Chem. Lett. 7(4), 666–675 (2016). doi:10.1021/acs.jpclett.5b02393
J. Li, S.K. Cushing, F. Meng, T.R. Senty, A.D. Bristow, N. Wu, Plasmon-induced resonance energy transfer for solar energy conversion. Nat. Photon. 9(9), 601–607 (2015). doi:10.1038/nphoton.2015.142
R. Costi, A.E. Saunders, E. Elmalem, A. Salant, U. Banin, Visible light-induced charge retention and photocatalysis with hybrid CdSe–Au nanodumbbells. Nano Lett. 8(2), 637–641 (2008). doi:10.1021/nl0730514
C. Yogi, K. Kojima, N. Wada, H. Tokumoto, T. Takai, T. Mizoguchi, H. Tamiaki, Photocatalytic degradation of methylene blue by TiO2 film and Au particles-TiO2 composite film. Thin Solid Films 516(17), 5881–5884 (2008). doi:10.1016/j.tsf.2007.10.050
A. Mills, J. Wang, Photobleaching of methylene blue sensitised by TiO2: an ambiguous system? J. Photochem. Photobiol. A 127(1–3), 123–134 (1999). doi:10.1016/S1010-6030(99)00143-4
A. Giri, N. Goswami, M. Pal, M.T. Zar Myint, S. Al-Harthi, A. Singha, B. Ghosh, J. Dutta, S.K. Pal, Rational surface modification of Mn3O4 nanoparticles to induce multiple photoluminescence and room temperature ferromagnetism. J. Mater. Chem. C 1(9), 1885–1895 (2013). doi:10.1039/c3tc00709j
Y. Park, S.-H. Lee, S.O. Kang, W. Choi, Organic dye-sensitized TiO2 for the redox conversion of water pollutants under visible light. Chem. Commun. 46(14), 2477–2479 (2010). doi:10.1039/b924829c
Z. Ai, W. Ho, S. Lee, L. Zhang, Efficient photocatalytic removal of NO in indoor air with hierarchical bismuth oxybromide nanoplate microspheres under visible light. Environ. Sci. Technol. 43(11), 4143–4150 (2009). doi:10.1021/es9004366
F. Dong, Y. Sun, M. Fu, Z. Wu, S.C. Lee, Room temperature synthesis and highly enhanced visible light photocatalytic activity of porous BiOI/BiOCl composites nanoplates microflowers. J. Hazard. Mater. 219–220, 26–34 (2012). doi:10.1016/j.jhazmat.2012.03.015
Q. Xiang, J. Yu, M. Jaroniec, Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 41(2), 782–796 (2012). doi:10.1039/C1CS15172J
Q. Li, H. Liu, F. Dong, M. Fu, Hydrothermal formation of N-doped (BiO)2CO3 honeycomb-like microspheres photocatalysts with bismuth citrate and dicyandiamide as precursors. J. Colloid Interface Sci. 408, 33–42 (2013). doi:10.1016/j.jcis.2013.07.040
S. Yu, H. Huang, F. Dong, M. Li, N. Tian, T. Zhang, Y. Zhang, Synchronously achieving plasmonic Bi metal deposition and I– doping by utilizing BiOIO3 as the self-sacrificing template for high-performance multifunctional applications. ACS Appl. Mater. Interfaces 7(50), 27925–27933 (2015). doi:10.1021/acsami.5b09994
Y. Guo, Y. Zhang, N. Tian, H. Huang, Homogeneous {001}-BiOBr/Bi heterojunctions: facile controllable synthesis and morphology-dependent photocatalytic activity. ACS Sustain. Chem. Eng. 4(7), 4003–4012 (2016). doi:10.1021/acssuschemeng.6b00884
V. Subramanian, E.E. Wolf, P.V. Kamat, Catalysis with TiO2/gold nanocomposites. Effect of metal particle size on the fermi level equilibration. J. Am. Chem. Soc. 126(15), 4943–4950 (2004). doi:10.1021/ja0315199
S. Trasatti, Electronegativity, work function, and heat of adsorption of hydrogen on metals. J. Chem. Soc. Faraday Trans. 1 68, 229–236 (1972). doi:10.1039/f19726800229
Y. Yu, C. Cao, H. Liu, P. Li, F. Wei, Y. Jiang, W. Song, A Bi/BiOCl heterojunction photocatalyst with enhanced electron-hole separation and excellent visible light photodegrading activity. J. Mater. Chem. A 2(6), 1677–1681 (2014). doi:10.1039/C3TA14494A
Acknowledgements
P. Kar thanks Council of Scientific and Industrial Research (CSIR, India) for fellowship. T.K. Maji thanks of DST, INSPIRE for fellowship. We thank DST, India for financial grant (SB/S1/PC-011/2013), DAE (India) for financial grant 2013/37P/73/BRNS, NTH-School “Contacts in Nanosystems: Interactions, Control and Quantum Dynamics”, the Braunschweig International Graduate School of Metrology (IGSM), and DFG-RTG 1952/1, Metrology for Complex Nanosystems.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kar, P., Maji, T.K., Nandi, R. et al. In-Situ Hydrothermal Synthesis of Bi–Bi2O2CO3 Heterojunction Photocatalyst with Enhanced Visible Light Photocatalytic Activity. Nano-Micro Lett. 9, 18 (2017). https://doi.org/10.1007/s40820-016-0118-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40820-016-0118-0