Abstract
Introduction
In children with epilepsy, comorbidities are frequent. In dyslexia, comorbidities are increasingly acknowledged. Little is known about temporal aspects (dyslexia antedating or postdating epilepsy onset, time interval), epilepsy types, and dyslexia phenotypes.
Method
From over 1000 files of children with epilepsy, 51 cases were retrospectively identified with a formal diagnosis of dyslexia. Ages at diagnoses of dyslexia and epilepsy, epilepsy variables, and dyslexia-related neuro-cognition (phoneme deletion and rapid letter naming) were recorded.
Analyses
Temporal variables, epilepsy variables, and neuro-cognition were analyzed with chi-squared, t tests, ancova, and generalized linear models.
Results
Duration of epilepsy to diagnosis of dyslexia ranged from − 5.5 years (dyslexia antedating epilepsy) to 10.1 years. In 35% of the children, diagnosis of dyslexia antedated the emergence of epilepsy. Dyslexia was seen across seizure types, with some preference for temporal lobe and rolandic epilepsy; rates for antedating and postdating dyslexia were similar. Notably, encephalopathic development was also seen. No specific dyslexia phenotype was seen. Children with dyslexia diagnoses after or in close temporal relationship to diagnoses of epilepsy (shortly before or after) scored lower on phonology and naming.
Conclusion
Antedating and postdating dyslexia can be seen in all epilepsy types. In a natural setting, dyslexia may antedate or postdate the emergence of epilepsy by several years. Around the time the epilepsy is about to surface, scores on dyslexia-related neuro-cognitive tasks are lowest, suggesting a bidirectional effect of the seizure condition on cognition. Encephalopathic development may be mimicking dyslexia criteria in some cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In children with epilepsy, reading problems are a common comorbidity. In children with severe reading disorders (dyslexia), the presence of comorbidities, like disorders in language, mathematics, attention or emotional development, is progressively being acknowledged (Daucourt et al., 2020; Snowling & Melby-Lervåg, 2016; Tijms et al., 2021). Associated with the recognition of comorbidities in dyslexia, questions emerge on the relationships between the conditions: as to whether the different conditions develop in parallel, if one condition is primary or whether one develops as a secondary consequence of the other, how they influence each other, and what comorbidity should be treated first (Moll et al., 2020). Studies on comorbidities in epilepsy similarly focus on the possibility that conditions may be co-occurring by chance; one may be causing the other (directly or indirectly), or one condition may be having the other as a result; the conditions may share (genetic) risk factors; or they may have reciprocal, bidirectional relationships (Keezer et al., 2016). These questions encompass causal and temporal relationships on comorbidities and may also be considered relevant in the context of epilepsy and dyslexia. Research on comorbidities, however, seldom concerns dyslexia and epilepsy. The present paper will focus on dyslexia and epilepsy co-occurring in children.
Reading, Reading Disorders, and Dyslexia
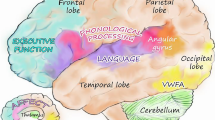
Reading words involves transforming the written letters (graphemes) or small clusters of letters into their verbal form (phonemes), blending them into a word and conveying meaning to the word. The reading process is an interplay of visual and, mainly, phonological and language abilities; eventually, reading processes become speeded, automatic, and effortless, and expert readers recognize and comprehend written words fluently (Castles et al., 2018; Landerl et al., 2022). Three main brain areas in the left hemisphere have been identified to play a role in the reading process, and to show alterations in connectivity patterns in dyslexia: the dorsal temporo-parietal region, the inferior frontal region, and, particularly, the ventral occipito-temporal region (Pugh et al., 2000; Richlan, 2020; Richlan et al., 2013; Simos et al., 2000; Vanderauwera et al., 2018; Vellutino et al., 2004). The occipito-temporal and the inferior frontal region show strong interconnectivity and are activated in word and nonword reading and early stages of reading development (Pugh et al., 2000; Vanderauwera et al., 2018).
Dyslexia
Children vary in their ability to learn to read; children having severe reading disorders or dyslexia remain persistently at the lower end in terms of reading accuracy and speed relative to their age and grade (Hulme & Snowling, 2016; Moll et al., 2020; Tijms et al., 2021). The categorical diagnosis of dyslexia involves both inclusionary and exclusionary criteria. Inclusionary criteria may be necessary or explanatory. Necessary criteria confirm the presence of a severe reading disorder. Explanatory criteria verify underlying weaknesses pointing toward a dyslexia phenotype. They comprise (1) necessary criterion reading disorder: performance in two domains (word reading, nonword reading, or spelling) at the lowest 6.7% (5.7 to 10%); (2) explanatory criterion on neuro-cognitive abilities: problems in phoneme processing, specifically phoneme awareness, like phoneme deletion, suggesting a phonological weakness phenotype; or naming problems, like rapid letter naming, suggesting a rapid naming weakness phenotype, or both (de Groot et al., 2015; Hulme & Snowling, 2016; Landerl et al., 2022; Vanasse et al., 2005); and (3) familial: if present, a familial risk factor (de Jong et al., 2016; Ferrer et al., 2022; Hulme & Snowling, 2016; Snowling & Melby-Lervåg, 2016). Exclusionary criteria indicate that the problems in reading should not be secondary to, or primarily attributable to, improper schooling, visual impairment, very low IQ, or a neurological disorder (Diagnostic and Statistical Manual of Mental Disorders, DSM-5; APA, 2013).
Reading Disorders in Children with Epilepsy
In children with epilepsy, reading problems are often seen. Studies including various types of epilepsy (Germanò et al., 2005; Vanasse et al., 2005) have found that children with epilepsy lagged two years behind in reading compared to norms, regardless of the type of epilepsy. Using the criterion which is also applied for dyslexia—reading scores at or below − 1.5 SD of the expected norm (Hulme & Snowling, 2016)—elevated risk of reading disorders (6.5 to 38.3%) in children with epilepsy was described (Cheng et al., 2022; Fastenau et al., 2008). Language and reading problems can be seen across generalized and focal seizure types (Jackson et al., 2019). Risk factors and underlying neuro-cognitive abilities, like rapid naming and phonological abilities, have also been found compromised across epilepsy types, with no clear dyslexia phenotype (Chaix et al., 2006; Selassie et al., 2008; Smith et al., 2015; Vega et al., 2015).
While dyslexia and compromised dyslexia-related neuro-cognitive abilities have been described in various epilepsy types, the two epilepsy syndromes most frequently associated with reading problems have been temporal lobe epilepsy (TLE) and benign (self-limited) epilepsy with centro-temporal spikes (BECTS, SeLECTS or rolandic epilepsy). Evidence for reading problems in TLE was inconsistent for both word reading and underlying phonological subskills (Lah et al., 2017; Vanasse et al., 2005). With few exceptions (Jackson et al., 2019), reading problems as well as rapid naming and phonological problems in children with BECTS have been described consistently (Germanò et al., 2020; Northcott et al., 2007; Vega et al., 2015). In a meta-analysis on children with BECTS (Smith et al., 2015), the authors confirmed that children with BECTS have significant problems with single-word reading as well as phonological processing. Children with BECTS scored 0.7 SD lower in reading and 0.5 SD lower on phonological tasks than comparison groups.
Less attention has been given to the possible encephalopathic effect that seizure conditions may have on reading and underlying phonological abilities. Seizure conditions are known to have potential encephalopathic effects, that is, epileptic activity may be associated to specific or generalized cognitive disorders or even cognitive loss, and therefore also to learning disorders (Berg et al., 2010; Covanis, 2012; Spencer et al., 2022; van Iterson et al., 2014). Reliable cognitive loss of FS-IQ at retesting was documented in 16.4% of a heterogeneous sample of children with epilepsy (van Iterson et al., 2013). In addition, syndromes associated with reading disorders, like BECTS or Panayiotopoulos, may be considered to be at the milder end of a spectrum of disorders, and may evolve into more severe, encephalopathic, forms, like the epileptic encephalopathy ESES (Fejerman et al., 2000; Roulet-Perez & Mayor, 2018). ESES, or electrical status epilepticus in sleep (Berg et al., 2010; Covanis, 2012), is thought to occur in less than 2% of children with epilepsy (Aaberg et al., 2016). A characteristic feature of ESES is decline of cognitive function, including developmental arrest and loss of already acquired abilities, which may occur before clinical seizures emerge, may start around the time of reading acquisition, and may be global or specific (Fejerman et al., 2000; van den Munckhof et al., 2015; van Iterson et al., 2021). As such, ESES could be also thought to affect reading acquisition or lead to acquired, rather than developmental, reading disorders (Roulet-Perez & Mayor, 2018).
Time-Related Aspects in Epilepsy and Dyslexia
Language and learning disorders may be seen in children before or around the diagnosis of epilepsy (Jackson et al., 2019; Overvliet et al., 2011; Schouten et al., 2001), and may continue after the epilepsy has resolved (Germanò et al., 2020), suggesting that the seizures may not be the only factor affecting reading disorders.
Given that epilepsy often surfaces in preschool years (Germanò et al., 2005; Northcott et al., 2007; van Iterson et al., 2014; van Iterson et al., 2015; Vanasse et al., 2005), a diagnosis of epilepsy is likely to antedate the diagnosis of dyslexia. Little is known, however, on dyslexia antedating the emergence of seizures.
Purpose
The purpose of the present paper was to study the co-occurrence of dyslexia in a sample of children with epilepsy, focusing on the temporal (i.e., time related) relationship between the diagnosis of epilepsy and the diagnosis of dyslexia. Within this temporal context, the paper aimed at addressing both types of epilepsy and dyslexia phenotypes.
Following the natural course of a sample of children with epilepsy receiving educational support, the research questions of the present study were (1) in what time sequence does a diagnosis of dyslexia present in children with epilepsy: do diagnoses of dyslexia antedate and postdate diagnoses of epilepsy? (2) Is dyslexia more prevalent in children with TLE and in BECTS than in other epilepsy types, as suggested by the literature? Is dyslexia diagnosed in epileptic encephalopathies? How do epilepsy types relate to temporal aspects? (3) Are dyslexia-related neuro-cognitive abilities (phoneme deletion; rapid letter naming) associated with temporal aspects, suggestive of a specific phenotype?
Methods
The medical, educational, and neuropsychological files of > 1500 children with epilepsy who had received specialized educational support for school-aged children and students with epilepsy (LWOE De Waterlelie) between 2018 and 2022 were retrospectively reviewed by the first author for their reference to dyslexia. The support provided by the LWOE has a low threshold, and is therefore accessible for children with epilepsy in the educational setting. The LWOE De Waterlelie is associated with the Netherlands’ tertiary epilepsy center Stichting Epilepsie Instellingen Nederland (SEIN). The children receiving special provisions were not restricted to those of SEIN, and could also be under the care of specialists from elsewhere. Children were in regular or in special education, and at the time of the study ranged from 3.5 to > 20 years of age. In order to focus on school-aged children, and to establish rates of occurrence of dyslexia, only children between 7 and 20 years of age were considered. Also, those in schools for children with moderate and severe intellectual disabilities were excluded. Thus, the sample from which the children with dyslexia were identified comprised 1182 children (see flow diagram, Fig. 1). Median chronological age = 11; interquartile range 9 to 14 years.
The children were selected for their reference to a diagnosis of dyslexia. This information was retrieved from formal dyslexia assessments, or, alternatively, from categorical statements in medical, neuropsychological, or school reports, or parent interviews. For a diagnosis of dyslexia, a stepwise concerted procedure between the school and the psychologist specialized in dyslexia assessment was required. In the first stage, the children had to show persistently low scores (− 1.28 SD or lowest 10%) on reading documented with consecutive school evaluations before being granted access to further formal dyslexia assessment. In the next stage, they had to be formally diagnosed with standardized tests following the criteria of the Netherlands (de Jong et al., 2016). This diagnosis of dyslexia includes necessary inclusionary (word reading, nonword reading, or spelling below − 1.28 or − 1.5 SD, two out of three), explanatory (phonological or rapid naming difficulties or both), and exclusionary criteria (dyslexia as a primary, rather than secondary disorder). Age at diagnosis of dyslexia was taken from dyslexia reports or parental information. The presence of familial risk for dyslexia was recorded as well. Beyond the categorical diagnosis, in addition, quantitative information of the diagnostic dyslexia evaluation was collected wherever available. This information related to number of months of formal reading instruction (10 months per school year) reading level achieved (also in months) and test scores (converted to mean = 100, SD = 15) on word reading (Brus & Voeten, 1979), nonword reading (van den Bos et al., 1999), phoneme deletion and rapid letter naming (Blomert & Vaessen, 2009; Kort et al., 2005).
Information on epilepsy was collected from medical or neuropsychological reports and related to age at onset of epilepsy, seizure type (focal or generalized), topographic region (frontal, temporal, occipital), type of epilepsy (e.g., BECTS, Panayiotopoulos), side of seizure onset (left or right hemisphere), presence of MRI abnormalities after neuroimaging (MRI+ if abnormalities were reported, MRI− if no abnormalities were reported or no MRI was done), genetic findings based on referral for epilepsy genetics (genetic+ if positive findings were reported after genetic testing, genetic– if no genetic findings were reported or no genetic testing had been done), status epilepticus (SE) reported in the history, ESES reported in the history (resolved or still active), number of antiseizure medications (ASM) taken, and ASM at dyslexia assessment. “Encephalopathic development” was defined as either (a history of) ESES or a decline of FS-IQ at retesting—reliable cognitive loss of at least 14 points on FS-IQ—or both. Information on cognitive functioning (psychological testing most closely to diagnosis of dyslexia) was collected from psychological reports. They related to age at testing and results on verbal abilities, non-verbal abilities and processing speed, the three main scales/ index scores of the Wechsler Scales, as well as Full-Scale IQ (FS-IQ). The children had been tested with the Wechsler Intelligence Scale for Children, third edition WISC-IIINL and fifth edition WISC-VNL (Wechsler, 2005, 2018). The missing IQ data for one case with antedating dyslexia was replaced by the group mean. For most children, only one IQ measurement was available; wherever more than one test had been taken, the test closest to the diagnosis of dyslexia was taken for the main analyses; data on retesting were used to determine cognitive loss.
Ethics
Written informed consent was obtained from the parents for all children in care of LWOE De Waterlelie. The study complies with the Helsinki Declaration. The present study is observational retrospective file research, not subject to additional ethical approval of the CCMO (Central Committee on Research on Human Subjects) in the Netherlands.
Analyses
In total, reference to dyslexia was found in n = 68 children. Of these, for n = 51, further dyslexia assessment had been done; they constituted the sample with dyslexia in the present study. For a subset of n = 18, quantitative diagnostic data were available.
The large sample of n = 1182 eligible cases was used to establish rates of occurrence, rate of children with dyslexia, and rate of children with dyslexia in ESES. The values were contrasted to the reference values from the literature. To establish whether rates of dyslexia were elevated in BECTS and TLE, they were contrasted to dyslexia in “other epilepsies” from the eligible sample.
To identify children with dyslexia predating epilepsy, the difference between age at diagnosis of dyslexia minus age at onset of epilepsy was calculated as “duration of dyslexia to epilepsy”; negative values denoted that the diagnosis of dyslexia antedated the diagnosis of epilepsy; conversely, positive values indicated that the diagnosis of epilepsy was first. The sample with dyslexia was accordingly split into two groups: “antedating dyslexia” (diagnosis of dyslexia antedating the diagnosis of epilepsy) and “postdating dyslexia” (diagnosis of dyslexia postdating the diagnosis of epilepsy). The two groups were contrasted in terms of epilepsy and neuropsychological variables using chi-squared (or Fisher exact tests) and t tests (Tables 1 and 2).
To study whether duration of epilepsy to diagnosis of dyslexia was associated with sex, FS-IQ, or familial dyslexia, or whether duration of epilepsy was associated with epilepsy variables, ancova was done. Duration of epilepsy to dyslexia was entered as the dependent variable; together with sex, FS-IQ, and familial dyslexia, epilepsy variables (seizure onset side or site, epilepsy types) were entered as independent variables. Epilepsy variables (scored as 0 = absent or 1 = present) were included if they were present at least in 20% of the sample (n > 8 children, see Tables 1 and 2). An exception was made for encephalopathic development, which was represented by eight children only, and was included as well. The analysis was redone for postdating dyslexia only, adding number of ASM (drug load) at time of dyslexia assessment as a predictor.
Neuro-Cognitive Patterns
To study whether a pattern of relative weakness suggestive of a particular dyslexia phenotype was seen, the scores on word versus nonword reading and phoneme deletion versus letter naming were analyzed. At group level, with paired-sample t tests, means of word versus nonword reading, as well as means of phoneme deletion versus letter naming were compared. At an individual level, a cutoff was set at a 23-point difference (1.5 SDs) for a “significant” intraindividual difference between word and nonword reading (and, separately, between phoneme deletion and rapid letter naming). Rates of children showing no significant difference or a relative weakness in one or the other task were contrasted with chi-squared.
Then, focusing on the contribution of time-related variables (group: antedating or postdating dyslexia; duration of epilepsy to diagnosis of dyslexia) to the neuro-cognitive variables, data were analyzed with generalized linear models with normal identity function. This was done separately for word reading, nonword reading, phoneme deletion, and rapid letter naming. In model 0, only sex was entered as a predictor. In model 1, FS-IQ was added. Thereafter, group (antedating or postdating dyslexia) and duration of epilepsy to diagnosis of dyslexia were entered as predictors (model 2a); alternatively, an interaction term of duration of epilepsy and group (model 2b); and squared duration of epilepsy to dyslexia (model 2c). Finally (model 3), for the word and nonword reading only, phoneme deletion and letter naming were entered as predictors. Given the small sample sizes for the epilepsy variables in this subset of data, epilepsy types were not entered. With Akaike’s information criterion (AIC), the consecutive models were checked for improvement of the model. The most parsimonious model was pursued, and non-significant variables were excluded. To make a preliminary estimate as to whether drug load would have an effect on neuro-cognitive scores, and whether this would be dependent of sex, for postdating dyslexia only, the analyses were redone, first adding number of ASMs at dyslexia assessment and then replacing sex and ASM by the ASM by sex interaction term.
Results
The data on antedating and postdating dyslexia for the sample of 51 children with dyslexia are shown in Tables 1 and 2 and Fig. 2. The data and analyses for the subset of 18 children with data on dyslexia assessment are shown in Tables 3 and 4 and Fig. 3a, b.
The 51 children represented 4.3% from the sample of 1182 children, significantly lower (Χ21181,1 = 10.8, p = .001) than the 6.7% of cases with dyslexia expected based on psychometric criteria (Tijms et al., 2021). From the sample, in n = 18 (35.3%), the diagnosis of dyslexia antedated the diagnosis of epilepsy; in n = 33 (64.7%), dyslexia postdated the diagnosis of epilepsy (Table 1). Figure 2 displays the duration of epilepsy up to the diagnosis of dyslexia; point 0 on the x axis denotes the diagnosis of epilepsy. The duration ranged from − 5.5 years (negative values denoting antedating dyslexia) to + 10.1 years. In antedating dyslexia, dyslexia had been diagnosed a mean of − 2.0 years (SD = 1.4) before epilepsy onset, with a range of − 5.5 years to − 1 month. In postdating dyslexia, dyslexia had been diagnosed a mean of 4.7 (SD = 2.8) years after the onset of epilepsy, with a range of 0.8 to 10.1 years. Figure 2 also shows that in seven children, the diagnoses of dyslexia and epilepsy were given within 1 year of each other: in four children, dyslexia antedated; in three, the diagnostic process was ongoing when the epilepsy started—in these children, epileptogenic process and the reading disorder could be considered largely overlapping.
Epilepsy Variables, TLE, and BECTS
Taken together, children with BECTS (n = 14) or TLE (n = 11) constituted 49.0% of the sample with dyslexia, with left hemisphere onset in eight, right hemisphere onset in three children, and bilateral or not specified in 14. Contrasted to the eligible sample of 1182 children, elevated rates of dyslexia were reported in BECTS (11.7% versus 3.5% in “other epilepsy types”; Χ21181,1 = 17.2, p < .001), but not in TLE (6.7% versus 3.9%; Χ21181,1 = 2.57, p = .109). Epilepsy with temporo-occipital seizure onset was seen in two children (one left hemisphere, one not specified), both with antedating dyslexia with seizures beginning two or more years after diagnosis of dyslexia, while left hemisphere BECTS and occipital involvement was reported in one child with postdating dyslexia.
Comparison of the sample with antedating and postdating dyslexia (Tables 1 and 2) revealed that, as expected, mean age at onset of epilepsy (AOE antedating dyslexia = 10.9 years; SD = 2.3; postdating dyslexia = 5.7; SD = 3.4) was higher in the children with antedating dyslexia (F50,1 = 33.2, p < .001, η2 = 0.41). Mean age at diagnosis of dyslexia (antedating dyslexia = 8.9 years; SD = 1.3; postdating dyslexia = 10.4; SD = 2.6) was higher in postdating dyslexia (F50,1 = 5.1, p = .028, η2 = 0.09). No differences were seen between antedating dyslexia and postdating dyslexia on rates of any of the epilepsy variables (seizure type, seizure onset side or site). That is to say, that children with TLE, BECTS, or any other epilepsy were as likely to be diagnosed with dyslexia before or after the diagnosis of epilepsy. Familial risk for dyslexia was reported in 29.4% of the children; no differences were seen between the two groups. No differences were seen in rates according to sex, placement in special education, ASM tried, or IQ scores between the two groups.
ancova revealed an association between duration of epilepsy to a diagnosis of dyslexia and FS-IQ (β = − 0.111, SE = 0.05, p = .038, ηp2 = .10, meaning higher FS-IQ in earlier diagnoses) as well as with TLE (β = − 4.467, SE = 1.65, p = .010, ηp2 = .16), but not for BECTS (β = − 1.490, SE = 1.59, p = .354), or any other epilepsy variable (left hemisphere, overall partial seizures; absences, overall generalized seizures; MRI+, encephalopathic development, sex, or familial risk for dyslexia). In TLE, duration ranged from − 5.5 years to +6.0 years (mean = − 0.09, SD = 4.0 years); in BECTS, duration ranged from − 2.6 years to +6.9 years (mean = 2.49, SD = 3.1 years). Post hoc analyses, for antedating and postdating dyslexia separately, showed that TLE was associated with the largest intervals between antedating dyslexia and emergence of TLE seizures (p = .009); other variables showed no meaningful associations. Based on postdating dyslexia only (n = 33), in a separate ancova, and adding drug load as a variable, no significant associations were found.
Redoing the aforementioned analyses with the larger sample of 68 children (that is, including children meeting the criterion of persistent low scores on reading and a reference of suspected for dyslexia, but no formal assessment) showed overall similar results, as well as two conspicuous differences. The 68 children represented 5.7% of the sample of 1182 children; an underrepresentation of dyslexia in epilepsy was no longer seen (p = .193). Also, the difference in age at diagnosis of dyslexia was no longer seen (p = .146), possibly suggesting that the higher age in postdating dyslexia was associated with reluctance to diagnose children with epilepsy as having comorbid dyslexia.
To downsize the differences in age at onset between the groups, the aforementioned analyses were also redone setting age at onset of epilepsy at 7 years or older for all children. For this largely reduced sample, the overall conclusions did not change. The remaining n = 27 children with dyslexia (17 antedating, 10 postdating) represented 4.2% of the reduced comparison sample of 647 children, significantly less than expected (p = .010). Age at epilepsy onset was similar (p = .226) for antedating and postdating dyslexia. Relative to antedating dyslexia, mean duration of epilepsy to dyslexia was still significantly longer (p < .001), and mean age at testing for dyslexia was still significantly higher (p = .016) in postdating dyslexia. Scores on IQ scales did not differ, and rates of epilepsy types also were overall similar. Thus, the reduced sample again showed that given similar age at onset of epilepsy, epilepsy type, and IQ, diagnosis of dyslexia tended to be given later in children with epilepsy.
Encephalopathic Development
An epileptic encephalopathy was seen in eight children (15.7%). Five children (9.8%) presented with ESES (Table 2), significantly more than the expected 2% for a sample with epilepsy (Χ250,1 = 15.8, p < .001). Of these, two children received the diagnosis of dyslexia while ESES was still active; one of these showed (ongoing) reliable cognitive loss. Three additional children showed reliable loss at retesting.
Dyslexia-Related Neuro-Cognitive Abilities
Preliminary analysis revealed no differences between the subset of children with available dyslexia assessment (n = 18) and the remaining children (n = 33). No differences were seen in terms of mean age at onset of epilepsy, age at dyslexia testing, duration of epilepsy to dyslexia, and FS-IQ. Except for genetic+ (p = .005, dyslexia assessment was present for all with reported genetic+ findings), no differences were seen in rates of children according to sex, epilepsy side, site or type, findings on neuroimaging, ESES, or SE, or ASM.
Word and Nonword Reading
Paired-sample t test revealed no group-mean differences between levels of word and nonword reading (p = .672). Intraindividual differences between word and nonword reading ranged from − 20 to + 25 points. With a cutoff at 23 points, 16 children showed no specific pattern; two children with postdating dyslexia showed a weakness in nonword reading. Rates were overall similar for antedating and postdating dyslexia (Χ218,2 =1.43, p = .231). Generalized linear models showed that, for word and nonword reading, no model improved the (non-significant) model 0, indicating that none of the variables (sex, group: antedating versus postdating dyslexia, duration of epilepsy to the diagnosis of dyslexia, squared duration, interaction term duration by group, phoneme deletion or rapid letter naming) was contributing to word or nonword reading scores.
Phoneme Deletion and Rapid Letter Naming
Paired-sample t test between levels of phoneme deletion and rapid letter naming also showed no differences (p = .318), indicating that at group level, both dyslexia-related neuro-cognitive tasks were equally affected. Intraindvidual differences between phoneme deletion and letter naming ranged from − 35 to +35 points. Twelve children showed no specific phenotype; in antedating dyslexia, two children showed a relative weakness in phoneme deletion and two in rapid letter naming; in postdating dyslexia, one child showed a weakness in phoneme deletion and one in letter naming. No differences in rates of phenotypes were seen between antedating and postdating dyslexia (Χ218,2 = 2.92, p = .232).
The results of generalized linear models are shown in Table 4. For phoneme deletion, model 2b (sex, interaction group × duration) improved earlier models. In antedating dyslexia, longer duration between the diagnoses of dyslexia and epilepsy (note that duration has negative values in antedating dyslexia) was associated with higher (i.e., better) phoneme deletion scores (p = .018); while in postdating dyslexia, longer duration of epilepsy was (also) associated with higher phoneme deletion scores (p = .012). While not significantly improving earlier models, model 2c showed a significant term for squared duration of epilepsy (p = .011). Overall, the results suggested lowest scores on phoneme deletion around the time of emergence of the seizures. Figure 3a provides an illustration of an unadjusted squared function of duration of epilepsy to diagnosis of dyslexia, explaining 29.5% of the variance of phoneme deletion.
For rapid letter naming, model 2a (duration of epilepsy to diagnosis and group) showed the best fit, with higher scores for antedating dyslexia (p = .004) and longer duration of epilepsy to diagnosis of dyslexia (p = .002). The results suggest that after the emergence of epilepsy, scores on letter naming were lowest, while they increased when the time elapsed between epilepsy onset and diagnosis of dyslexia became longer. Later models did not provide a better fit, but model 2c again showed a significant term for squared duration of epilepsy (p = .021). Figure 3b shows an unadjusted squared function of duration to epilepsy to diagnosis of dyslexia, explaining 15.5% of the variance.
The additional analyses for postdating dyslexia only, albeit based on a reduced sample of n = 11 children, showed overall similar results: for word and nonword reading, consecutive generalized linear models did not show improved fit, but a significant term for FS-IQ (higher IQ associated with better word reading) and sex (girls outperformed boys in nonword reading) emerged. No significant effects of drug load or ASM × sex were seen. For phoneme deletion and rapid naming, as earlier, duration and squared duration to epilepsy showed significantly improved models (ps < .008) relative to the base models. ASM or ASM × sex was not significant. For rapid letter naming, the best fitting model included higher IQ, longer duration of epilepsy to diagnosis of dyslexia, and male sex; all were significantly (ps < .001) associated with better naming scores.
Discussion
The present paper addressed comorbidities in epilepsy, focusing on dyslexia and the temporal relationship of the two diagnoses, in an observational study. Based on a retrospective file review of more than a thousand cases with epilepsy receiving specialized school services for children with epilepsy, 51 children were identified with dyslexia. Notably, 35.3% of the sample had already been diagnosed with dyslexia before the epilepsy emerged. As expected, children with antedating dyslexia had an older age of onset of epilepsy. Otherwise, no differences between the children with dyslexia before or after the onset of epilepsy in terms of type, side, site of epilepsy, or cognitive level were found, suggesting that dyslexia may be seen in any type of epilepsy, before or after the epilepsy emerges. Sex, familial risk factors, or antiseizure medication did not play a significant role in the temporal relationships between dyslexia and epilepsy. Several children were identified with epilepsy with encephalopathic effects, like cognitive loss or a past history of ESES, and some were diagnosed with dyslexia while ESES was still active. No evidence was seen for a specific dyslexia phenotype associated with antedating or postdating dyslexia. Noticeably, underlying dyslexia-related neuro-cognitive abilities, like phoneme deletion and rapid letter naming abilities, were lower in children in closer proximity to epilepsy onset, suggesting that the epilepsy was exerting a negative influence. The detrimental effect of the seizures was seen on phoneme deletion before and after epilepsy onset.
Epilepsy and Antedating Versus Postdating Dyslexia
Rates of antedating or postdating dyslexia were similar across epilepsy types. Also, duration of epilepsy to diagnosis of dyslexia was similar across epilepsy variables. Only antedating dyslexia followed by TLE was associated with a longer interval between dyslexia and epilepsy. Together, children with TLE or BECTS comprised half of the cases with dyslexia. Contrasted to the original sample of which the children with dyslexia were drawn, elevated rates of children with dyslexia were seen in BECTS, confirming earlier studies (Smith et al., 2015). Elevated rates were not seen for TLE, and temporo-occipital involvement was not often encountered in the present sample, only partly in line with earlier studies (Lah et al., 2017; Richlan et al., 2013; Vanasse et al., 2005). These findings could be understood based on earlier results indicating that more brain areas are involved in early than in proficient reading (Vanderauwera et al., 2018), and therefore, possibly “any” type of seizure activity or epileptogenic process may involucrate part of the reading network. Several children were identified with ESES. ESES may be seen as having a spectrum of severity, with global or specific cognitive impact; like BECTS, ESES has been found to involve disturbed thalamocortical networks, which may be associated with dyslexia-related neurocognitive disorders, like phonological problems (Spencer et al., 2022; van den Munckhof et al., 2020).
Both naming and phonological difficulties have been reported earlier, and they are often lowered together, particularly in more severe reading disorders (de Groot et al., 2015; Selassie et al., 2008; Smith et al., 2015). In the present study, by definition, word and nonword reading were low, and while some variability between scores on phoneme deletion and rapid letter naming was seen within individuals, phenotypes were not often seen.
Temporal Aspects, Causality, and Bidirectionality
The present paper showed that, in a natural setting, the diagnosis of dyslexia may either antedate or postdate the diagnosis of epilepsy, also by several years, independent of seizure type. Seizure onset zone may differ from the areas known to be implicated in reading, as exemplified by the wide range of seizure types as well as the low number of children with temporo-occipital epilepsy present in the sample, suggesting a disturbance of the larger reading network. These findings could also support the notion that epilepsy and dyslexia may run in parallel independent of each other at least in some cases (Keezer et al., 2016; Moll et al., 2020). In other cases, genetic aspects may play a role. For both dyslexia (Mascheretti et al., 2017) as well as epilepsies commonly associated with dyslexia, in particular BECTS (Pal et al., 2010; Strug et al., 2012), predisposing genetic factors have been found. Noteworthy, the genetic loci identified for dyslexia without epilepsy and dyslexia in BECTS, however, have been found to differ (Strug et al., 2012). Regardless of whether shared risk factors exist between dyslexia and epilepsy, however, in BECTS as well as in other epilepsy types, the present study suggests that the epilepsy which a child is predisposed to have may be seen to emerge after or before the reading disorder, with wide time intervals between the diagnosis of dyslexia and the diagnosis of epilepsy.
In discussing causal and temporal relationships between epilepsy and comorbidities (Keezer et al., 2016), the authors indicated that one condition could be directly or indirectly influencing the other, or showing a bidirectional effect. The present study supports the notion that, when dyslexia and epilepsy exist together in a child, the epilepsy has an added impact on underlying neuro-cognitive abilities, like phoneme deletion and, especially, rapid letter naming abilities. The study provides evidence for a bidirectional effect of the epileptogenic process (Pitkänen et al., 2015). When diagnostic testing for dyslexia occurs in close proximity to the emergence of epilepsy, neuro-cognitive abilities associated with dyslexia, particularly phoneme deletion, appear to be more severely compromised. That is, scores on neuro-cognitive tasks are lowered not only (shortly) after the onset of seizures, but the impact of the seizure condition which is about to emerge can already be seen in lowered scores on neuro-cognitive tasks shortly before the emergence of seizures.
Diagnostic Bias in Dyslexia in Epilepsy
The rate of children diagnosed with dyslexia was lower than the expected rate in the general population (Tijms et al., 2021), and clearly lower than the rates reported earlier (Fastenau et al., 2008). Andell (2021) has warned for the risk of one condition gaining precedence over the other, leading to underdiagnosis and lack of treatment of comorbidities in epilepsy. Earlier, the DSM-5 (APA, 2013) had articulated an exclusionary criterion for a “neurological disorder” in dyslexia (p 67). Though the thoroughly formulated criterion (de Jong et al., 2016) does not exclude dyslexia in epilepsy, it may have contributed to a hesitation to diagnose dyslexia in epilepsy, leading to underdiagnosis. Reanalysis after inclusion of children with highly suspected (but not further diagnosed) dyslexia no longer showed underrepresentation of dyslexia in epilepsy, while reanalysis of a subset of children with age at onset of epilepsy above 7 years confirmed that given similar age at onset of epilepsy, age at diagnosis of dyslexia was higher in postdating dyslexia.
In contrast, in the present sample, an elevated rate of children with epileptic encephalopathies, particularly ESES, was conspicuous. It underlines that in some children with epilepsy, the cognitive arrest characteristic in ESES (van den Munckhof et al., 2015) may be mimicking features that are at the core of the diagnosis of dyslexia: lack of progress in reading, persistence over time, unresponsiveness to instruction, and possibly slowness in naming tasks. The identification of various children with resolved ESES on the EEG and stabilized FS-IQ across assessments, who qualified for the diagnosis of dyslexia, may further support the notion that resolution of ESES need not imply recovery of specific functions that were affected (van Iterson et al., 2021). It remains open up to what extend the children would profit from targeted dyslexia treatment.
Treatment
Treatment of epilepsy should be targeted by experts in the field of epilepsy and treatment of dyslexia by experts in the field of dyslexia. Earlier studies on neurodevelopmental comorbidities (Denton et al., 2020; Tuchman et al., 2010) have underlined that each disorder should be treated in its own right, also in cases where the two conditions are associated with each other. Even in the light of a comorbidity, intensive reading intervention remains the best means to improve reading (Denton et al., 2020). While cognitive profiles have been found to differ in the presence of reading disorders, epilepsy, or both (van Iterson et al., 2015), academic profiles have described as being similar (Currie et al., 2018; Germanò et al., 2020), suggesting that treatment of dyslexia could follow the same steps also in children epilepsy.
Assets and Weaknesses of the Study
Compromised neuropsychological function have already been described in the early stages of epilepsy (Jackson et al., 2019), and after longer duration of epilepsy (Hermann et al., 2008; van Iterson et al., 2014). An asset of the present study is that it takes this information a step further. The study does so, reporting on children, formally diagnosed before the onset of epilepsy, based on academic and neuropsychological measures. As such, apart from adding to the present body of knowledge on cognitive comorbidities after epilepsy onset, the study also provides new information on comorbidities antedating epilepsy, exemplified on an important academic topic: severe reading disorders. In addition, the paper provides evidence for a bidirectional relationship between the seizure condition and compromised dyslexia-related neuro-cognition.
An asset of the present study, adding to its ecological validity, is that the children with dyslexia were drawn from a large data set of children with epilepsy. The LWOE De Waterlelie is one of the two services providing educational support for children with epilepsy nationwide, and therefore represents half the country. The services are easily accessible and can be requested by schools, parents, and physicians; they are inclusionary in terms of familial conditions, types of developmental and scholastic problems, as epilepsy types. Access to medical care, educational services, tailored instruction as well as dyslexia assessment could be considered largely similar for all children in the study.
A study which is retrospective by nature has associated weaknesses. The present study addressed dyslexia as a comorbidity in epilepsy, reviewing retrospectively files of a large heterogenous sample of school children. While efforts were made to collect data comprehensively, not all files included the full assessment on dyslexia, leading to small sample sizes in part of the analyses. No differences were seen between the children with or without full assessment, suggesting that the subset could be considered fairly representative, but future studies should include larger samples. Future studies could also focus on larger and more homogeneous samples, in terms of diagnosis of dyslexia and in terms of age at epilepsy onset, to further elucidate the relationship between the emergence of seizures and the effect on neuro-cognition. Also, the inclusion of a more comprehensive battery of neurocognitive tasks would aid in further studying neurocognition around the time of epilepsy onset.
The present study concentrated on time-related variables, while the impact of other variables would also merit further research. For example, parental education or socioeconomic status (SES) was not taken into account. SES is known to affect reading as well as abilities underlying learning to read (Waters et al., 2021), like language or working memory, which can also be compromised in children with epilepsy, differentially across age groups (Caplan et al., 2009; van Iterson & de Jong, 2018). It is unknown, however, whether variables like SES may also have a differential impact on antedating versus postdating dyslexia. Given the LWOE monitoring of all children with epilepsy in the sample, it seems unlikely that SES would have significantly affected the timing of postdating dyslexia assessment, but it remains unknown how these variables could have impacted diagnoses in antedating dyslexia.
Drug load is known to affect neurocognition (Helmstaedter et al., 2010). In the present study, no significant contribution of ASM was found, but, particularly when analyzing neurocognitive variables, sample sizes were small. Moreover, while no impact was seen of ASM in association with sex, this topic would warrant further research, also in the light of differences in treatment regimen between boys and girls. Increased knowledge of teratogenic effects of some types of ASM used by women during pregnancy on the later neurocognitive development of the child (Huber-Mollema et al., 2020), has led to adjustments in treatment choices according to sex, already in school-aged girls. In the present study, while not associated with drug load, for girls, higher scores were seen on nonword reading, and for boys on rapid letter naming.
Conclusions
In children with epilepsy, severe dyslexia as formally defined (APA, 2013; Tijms et al., 2021) can also be seen. In a substantial proportion of the children with dyslexia in epilepsy, the diagnosis of dyslexia was found to antedate the diagnosis of epilepsy, with up to 5 years. Thus, a spectrum emerges where dyslexia can be seen to antedate or postdate epilepsy, independent of epilepsy type. In severe epilepsies and encephalopathies, developmental arrest may mimic dyslexia. When the diagnosis of dyslexia and epilepsy are in close proximity, that is, during the epileptogenic process, lower scores on phonological and rapid naming tasks suggest a bidirectional effect of the seizures on dyslexia-related cognition.
Summary and Clinical Implications
Children with epilepsy often have reading difficulties; in some, these are so severe as to qualify for dyslexia: they are persistent over time, also with additional reading instruction, and associated with underlying neuro-cognitive weaknesses, like phoneme deletion or rapid letter naming. While some types of epilepsies, like BECTS, are more likely to be associated with dyslexia, it is important to note that dyslexia can be seen and diagnosed in any type of epilepsy. Type of epilepsy appeared to be unrelated to whether the dyslexia was diagnosed before or after the emergence of seizures. For example, while BECTS is preferentially associated with dyslexia, dyslexia may already have been diagnosed before the epilepsy, suggesting ingrained underlying neurodevelopmental difficulties of which the dyslexia may be seen first. Some delay was seen in providing children with epilepsy with a diagnosis of dyslexia. This delay appears at least partly justifiable, given that the most vulnerable period in the child’s dyslexia-related neuro-cognitive functioning is shortly after the emergence of seizures. At this stage, the attention is turned to achieving seizure amelioration. Passed this stage, however, given tailored medication and compliance, a child with epilepsy who qualifies for dyslexia according to the criteria could obtain a diagnosis. A word of caution should be given in the cases with encephalopathic conditions, overall cognitive arrest, or cognitive loss. In these children, the main focus is on treating and closely monitoring the encephalopathic condition and overall cognitive development. In some rare cases, the effects may be circumscribed and the child may still qualify for a specific reading disorder. When already acquired reading abilities are lost, it would be more appropriate to speak of acquired, rather than developmental, dyslexia.
Clinically, assessment of dyslexia will likely be feasible in most children with epilepsy, provided that that the children fulfill the psychometric criteria for dyslexia, that there is adherence to tailored epilepsy treatment, and that ongoing encephalopathic effects of epilepsy have been ruled out. Assessment of dyslexia in children with epilepsy should always include comprehensive neuropsychological assessment, particularly assessment of intelligence. Present neuro-cognitive findings should be contrasted to earlier evaluations in order to establish cognitive change. In addition, the clinician should be aware that arrest in reading development could be associated with (worsening of) the epileptic condition.
Regardless of the feasibility of a formal diagnosis of dyslexia, it is of utmost importance to provide specialized services to children with epilepsy and reading—or other cognitive or learning—disorders. Reading and specialized reading instruction is the most important vehicle for improving reading in all children with reading problems.
Specialized educators should stay vigilant for changes in responsiveness to reading instruction that may be pointing to changes in the epileptic condition.
References
Aaberg, K. M., Bakken, I. J., Lossius, M. I., Søraas, C. L., Håberg, S. E., Stoltenberg, C., . . . Chin, R. (2016). Comorbidity and childhood epilepsy: a nationwide registry study. Pediatrics, 138(3), e20160921.
Åndell Jason, E. (2021). Neurodevelopmental and psychiatric comorbidities negatively affect outcome in children with unprovoked seizures—A non-systematic review. Acta Paediatrica, 110(11), 2944–2950.
American Psychiatric Association, D. S. M. T. F., American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders: DSM-5 (Vol. 5, No. 5). Washington, DC: American Psychiatric Association.
Berg, A. T., Berkovic, S. F., Brodie, M. J., Buchhalter, J., Cross, J. H., van Emde Boas, W., . . . Scheffer, I. E. (2010). Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia, 51(4), 676-685.
Blomert, L., & Vaessen, A. (2009). 3DM Differential Diagnostics for Dyslexia. Cognitive analysis of reading and spelling. Boom Test Publishers.
Brus, B. T., & Voeten, M. J. M. (1979). Een minuut test (One Minute Reading Test). Swets & Zeitlinger.
Caplan, R., Siddarth, P., Vona, P., Stahl, L., Bailey, C., Gurbani, S., . . . Donald Shields, W. (2009). Language in pediatric epilepsy. Epilepsia, 50(11), 2397-2407.
Castles, A., Rastle, K., & Nation, K. (2018). Ending the reading wars: reading acquisition from novice to expert. Psychological Science in the Public Interest, 19(1), 5–51.
Chaix, Y., Laguitton, V., Lauwers-Cances, V., Daquin, G., Cances, C., Démonet, J.-F., & Villeneuve, N. (2006). Reading abilities and cognitive functions of children with epilepsy: influence of epileptic syndrome. Brain and Development, 28(2), 122–130.
Cheng, D., Miao, X., Wu, H., Chen, C., Chen, Q., & Zhou, X. (2022). Dyscalculia and dyslexia in Chinese children with idiopathic epilepsy: different patterns of prevalence, comorbidity, and gender differences. Epilepsia Open, 7(1), 160–169.
Covanis, A. (2012). Epileptic encephalopathies (including severe epilepsy syndromes). Epilepsia, 53((suppl.4)), 114–126.
Currie, N. K., Lew, A. R., Palmer, T. M., Basu, H., De Goede, C., Iyer, A., & Cain, K. (2018). Reading comprehension difficulties in children with rolandic epilepsy. Developmental Medicine & Child Neurology, 60(3), 275–282.
Daucourt, M. C., Erbeli, F., Little, C. W., Haughbrook, R., & Hart, S. A. (2020). A meta-analytical review of the genetic and environmental correlations between reading and attention-deficit/hyperactivity disorder symptoms and reading and math. Scientific Studies of Reading, 24(1), 23–56.
de Groot, B. J., van den Bos, K. P., van der Meulen, B. F., & Minnaert, A. E. (2015). Rapid naming and phonemic awareness in children with reading disabilities and/or specific language impairment: differentiating processes? Journal of Speech, Language, and Hearing Research, 58(5), 1538–1548.
de Jong, P. F., De Bree, E. H., Henneman, K., Kleijnen, R., Loykens, E., Rolak, M., . . . Wijnen, F. N. K. (2016). Dyslexie. Diagnostiek en behandeling (Dyslexia. Diagnosis and Treatment). The Netherlands: Stichting Dyslexie Nederland, SDN.
Denton, C. A., Tamm, L., Schatschneider, C., & Epstein, J. N. (2020). The effects of ADHD treatment and reading intervention on the fluency and comprehension of children with ADHD and word reading difficulties: a randomized clinical trial. Scientific Studies of Reading, 24(1), 72–89.
Fastenau, P. S., Shen, J., Dunn, D. W., & Austin, J. K. (2008). Academic underachievement among children with epilepsy: proportion exceeding psychometric criteria for learning disability and associated risk factors. Journal of Learning Disabilities, 41, 195–207.
Fejerman, N., Caraballo, R., & Tenembaum, S. N. (2000). Atypical evolutions of benign localization-related epilepsies in children: are they predictable? Epilepsia, 41(4), 380–390.
Ferrer, E., Shaywitz, B. A., Holahan, J. M., & Shaywitz, S. E. (2022). Family history is not useful in screening children for dyslexia. Journal of Pediatric Neuropsychology, 8, 15–21.
Germanò, E., Gagliano, A., Arena, C., Cedro, C., Vetri, L., Operto, F. F., . . . Roccella, M. (2020). Reading–writing disorder in children with idiopathic epilepsy. Epilepsy & Behavior, 111, 107118.
Germanò, E., Gagliano, A., Magazù, A., Sferro, C., Calarese, T., Mannarino, E., & Calamoneri, F. (2005). Benign childhood epilepsy with occipital paroxysms: neuropsychological findings. Epilepsy Research, 64(3), 137–150.
Helmstaedter, C., Schoof, K., Rossmann, T., Reuner, G., Karlmeier, A., & Kurlemann, G. (2010). Introduction and first validation of EpiTrack Junior, a screening tool for the assessment of cognitive side effects of antiepileptic medication on attention and executive functions in children and adolescents with epilepsy. Epilepsy Behav, 19(1), 55–64.
Hermann, B., Jones, J., Seth, R., Koehn, M., Becker, T., Fine, J., . . . Seidenberg, M. (2008). Growing up with epilepsy: a two-year investigation of cognitive development in children with new onset epilepsy. Epilepsia, 49(11), 1847-1858.
Huber-Mollema, Y., van Iterson, L., Oort, F. J., Lindhout, D., & Rodenburg, R. (2020). Neurocognition after prenatal levetiracetam, lamotrigine, carbamazepine or valproate exposure. Journal of Neurology, 267, 1724–1736.
Hulme, C., & Snowling, M. J. (2016). Reading disorders and dyslexia. Current opinion in pediatrics, 28(6), 731.
Jackson, D., Jones, J., Hsu, D., Stafstrom, C., Lin, J., Almane, D., . . . Hermann, B. (2019). Language function in childhood idiopathic epilepsy syndromes. Brain and Language, 193, 4-9.
Keezer, M. R., Sisodiya, S. M., & Sander, J. W. (2016). Comorbidities of epilepsy: current concepts and future perspectives. The Lancet Neurology, 15(1), 106–115.
Kort, W., Schittekatte, M., Van den Bos, K., Vermeir, G., Lutje Spelberg, H., Verhaeghe, P., & van der Wild, S. (2005). Dyslexie Screening Test (DST-NL). Manual. Adapted from: AJ Fawcett & RI Nicolson.
Lah, S., Castles, A., & Smith, M. L. (2017). Reading in children with temporal lobe epilepsy: a systematic review. Epilepsy & Behavior, 68, 84–94.
Landerl, K., Castles, A., & Parrila, R. (2022). Cognitive precursors of reading: A cross-linguistic perspective. Scientific Studies of Reading, 26(2), 111–124.
Mascheretti, S., De Luca, A., Trezzi, V., Peruzzo, D., Nordio, A., Marino, C., & Arrigoni, F. (2017). Neurogenetics of developmental dyslexia: from genes to behavior through brain neuroimaging and cognitive and sensorial mechanisms. Translational Psychiatry, 7(1), e987–e987.
Moll, K., Snowling, M. J., & Hulme, C. (2020). Introduction to the special issue “comorbidities between reading disorders and other developmental disorders”. Scientific Studies of Reading, 24(1), 1–6.
Northcott, E., Connolly, A. M., Berroya, A., McIntyre, J., Christie, J., Taylor, A., . . . Bye, A. M. (2007). Memory and phonological awareness in children with benign rolandic epilepsy compared to a matched control group. Epilepsy Research, 75(1), 57-62.
Overvliet, G., Aldenkamp, A., Klinkenberg, S., Vles, J., & Hendriksen, J. (2011). Impaired language performance as a precursor or consequence of Rolandic epilepsy? Journal of the Neurological Sciences, 304(1-2), 71–74.
Pal, D., Li, W., Clarke, T., Lieberman, P., & Strug, L. (2010). Pleiotropic effects of the 11p13 locus on developmental verbal dyspraxia and EEG centrotemporal sharp waves. Genes, Brain and Behavior, 9(8), 1004–1012.
Pitkänen, A., Lukasiuk, K., Dudek, F. E., & Staley, K. J. (2015). Epileptogenesis. Cold Spring Harbor Perspectives in Medicine, 5(10), a022822.
Pugh, K. R., Mencl, W. E., Jenner, A. R., Katz, L., Frost, S. J., Lee, J. R., . . . Shaywitz, B. A. (2000). Functional neuroimaging studies of reading and reading disability (developmental dyslexia). Mental Retardation and Developmental Disabilities Research Revieuws, 6(3), 207-213.
Richlan, F. (2020). The functional neuroanatomy of developmental dyslexia across languages and writing systems. Frontiers in Psychology, 155, 1–8.
Richlan, F., Kronbichler, M., & Wimmer, H. (2013). Structural abnormalities in the dyslexic brain: a meta-analysis of voxel-based morphometry studies. Human Brain Mapping, 34(11), 3055–3065.
Roulet-Perez, E., & Mayor, C. (2018). Childhood epilepsy with centro-temporal spikes (rolandic epilepsy) and written language. Developmental Medicine & Child Neurology, 60(3), 219–219.
Schouten, A., Oostrom, K., Jennekens-Schinkel, A., & Peters, A. C. (2001). School career of children is at risk before diagnosis of epilepsy only. Developmental Medicine & Child Neurology, 43(8), 575–576.
Selassie, G. R. H., Viggedal, G., Olsson, I., & Jennische, M. (2008). Speech, language, and cognition in preschool children with epilepsy. Developmental Medicine & Child Neurology, 50(6), 432–438.
Simos, P. G., Breier, J. I., Fletcher, J. M., Foorman, B. R., Bergman, E., Fishbeck, K., & Papanicolaou, A. C. (2000). Brain activation profiles in dyslexic children during non-word reading: a magnetic source imaging study. Neuroscience Letters, 290(1), 61–65.
Smith, A. B., Bajomo, O., & Pal, D. K. (2015). A meta-analysis of literacy and language in children with rolandic epilepsy. Developmental Medicine & Child Neurology, 57(11), 1019–1026.
Snowling, M. J., & Melby-Lervåg, M. (2016). Oral language deficits in familial dyslexia: a meta-analysis and review. Psychological Bulletin, 142(5), 498.
Spencer, E. R., Chinappen, D., Emerton, B. C., Morgan, A. K., Hämäläinen, M. S., Manoach, D. S., . . . Chu, C. J. (2022). Source EEG reveals that Rolandic epilepsy is a regional epileptic encephalopathy. Neuroimage: Clinical, 33, 102956.
Strug, L. J., Addis, L., Chiang, T., Baskurt, Z., Li, W., Clarke, T., . . . Novotny, E. J. (2012). The genetics of reading disability in an often excluded sample: novel loci suggested for reading disability in rolandic epilepsy. PloS one, 7(7), e40696.
Tijms, J., de Bree, E. H., de Jong, P. F., Loykens, E., & Reij, R. (2021). Protocol Dyslexie Diagnostiek en Behandeling - versie 3.0. (Protocol Dyslexia; Diagonstics and Treatment, 3.0). The Netherlands: Nederlands Kwaliteitsinstituut Dyslexia, NKD.
Tuchman, R., Alessandri, M., & Cuccaro, M. (2010). Autism spectrum disorders and epilepsy: moving towards a comprehensive approach to treatment. Brain and Development, 32, 719–730.
van den Bos, K., Lutje Spelberg, H., Scheepstra, A., & De Vries, J. (1999). De Klepel (test of nonword reading). Pearson.
van den Munckhof, B., van Dee, V., Sagi, L., Caraballo, R. H., Veggiotti, P., Liukkonen, E., . . . Bulteau, C. (2015). Treatment of electrical status epilepticus in sleep: a pooled analysis of 575 cases. Epilepsia, 56(11), 1738-1746.
van den Munckhof, B., Zwart, A. F., Weeke, L. C., Claessens, N. H., Plate, J. D., Leemans, A., . . . Benders, M. J. (2020). Perinatal thalamic injury: MRI predictors of electrical status epilepticus in sleep and long-term neurodevelopment. Neuroimage: Clinical, 26, 102227.
van Iterson, L., Augustijn, P. B., de Jong, P. F., & van der Leij, A. (2013). Establishing reliable cognitive change in children with epilepsy: the procedures and results for a sample with epilepsy. Journal of Psychoeducational Assessment, 31(5), 448–458.
van Iterson, L., & de Jong, P. F. (2018). Development of verbal short-term memory and working memory in children with epilepsy: developmental delay and impact of time-related variables. A cross-sectional study. Epilepsy & Behavior, 78, 166–174.
van Iterson, L., De Jong, P. F., & Zijlstra, B. J. (2015). Pediatric epilepsy and comorbid reading disorders, math disorders, or autism spectrum disorders: impact of epilepsy on cognitive patterns. Epilepsy and Behavior, 44, 159–168.
van Iterson, L., Vrij, S., Sie, L. T. L., Augustijn, P. B., Rooze, A. C. S., & Jansen, F. E. (2021). Acquired visual agnosia as an uncommon presentation of epileptic encephalopathy in a 6-year-old boy with CSWS. Epilepsy & Behavior Reports, 16, 1–7.
van Iterson, L., Zijlstra, B. J., Augustijn, P. B., van der Leij, A., & de Jong, P. F. (2014). Duration of epilepsy and cognitive development in children: a longitudinal study. Neuropsychology, 28(2), 212–221.
Vanasse, C., Béland, R., Carmant, L., & Lassonde, M. (2005). Impact of childhood epilepsy on reading and phonological processing abilities. Epilepsy & Behavior, 7(2), 288–296.
Vanderauwera, J., De Vos, A., Forkel, S. J., Catani, M., Wouters, J., Vandermosten, M., & Ghesquière, P. (2018). Neural organization of ventral white matter tracts parallels the initial steps of reading development: a DTI tractography study. Brain and Language, 183, 32–40.
Vega, Y. H., Smith, A., Cockerill, H., Tang, S., Agirre-Arrizubieta, Z., Goyal, S., . . . McGinnity, C. (2015). Risk factors for reading disability in families with rolandic epilepsy. Epilepsy & Behavior, 53, 174-179.
Vellutino, F. R., Fletcher, J. M., Snowling, M. J., & Scanlon, D. M. (2004). Specific reading disability (dyslexia): What have we learned in the past four decades? Journal of Child Psychology and Psychiatry, 45(1), 2–40.
Waters, N. E., Ahmed, S. F., Tang, S., Morrison, F. J., & Davis-Kean, P. E. (2021). Pathways from socioeconomic status to early academic achievement: the role of specific executive functions. Early Childhood Research Quarterly, 54, 321–331.
Wechsler, D. (2005). WISC-IIINL. Wechsler Intelligence Scale for Children. In Derde editie NL. Handleiding en Verantwoording. The Psychological Corporation / Nederlands Dienstencentrum NIP.
Wechsler, D. (2018). WISC-V-NL Wechsler Intelligence Scale for Children-V. Pearson.
Acknowledgements
The authors wish to thank Deb Pal for his thoughtful comments on the earlier drafts.
Author information
Authors and Affiliations
Contributions
Loretta van Iterson: designed the study, collected, analyzed and interpreted the data, and drafted the article. Peter F. de Jong: revised the draft critically, commented on the design, and gave final approval to the version to be submitted.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van Iterson, L., de Jong, P.F. Dyslexia Antedating and Postdating Epilepsy Onset. J Pediatr Neuropsychol 9, 141–156 (2023). https://doi.org/10.1007/s40817-023-00146-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40817-023-00146-4