Abstract
Background
For patients with metastatic non-small cell lung cancer, timely molecular testing is essential to determine the appropriate course of therapy. Initial treatment with platinum chemotherapy and/or an immune checkpoint inhibitor (ICI) is the standard of care for patients without actionable genomic alterations.
Objective
We aimed to assess treatment patterns and clinical outcomes among patients with metastatic non-small cell lung cancer, no actionable genomic alterations, and with prior ICI and platinum-based chemotherapy in a community oncology setting.
Methods
This retrospective observational study examined electronic health records from adult patients with an initial metastatic non-small cell lung cancer diagnosis without actionable genomic alterations from 2017 to 2019. Patients had received a subsequent line of therapy (LOT) [index] after discontinuing platinum-based chemotherapy plus an ICI in the previous one or two LOTs. Patient demographics and clinical characteristics were analyzed descriptively. Clinical outcomes were evaluated using Kaplan–Meier analyses.
Results
Among the study population (n = 961), the most common index LOT regimens were non-platinum-based chemotherapies (57.3%), platinum-based chemotherapies (12.9%), ICI-based chemotherapies (12.7%), platinum + ICI-based chemotherapies (9.4%), and other (7.7%). The most common post-index LOT regimens were non-platinum based (61.2%), ICI based (15.3%), platinum based (10.7%), platinum + ICI based (3.2%), and other (2.5%). Median time to treatment discontinuation, time to next treatment, and overall survival were numerically longest with index LOT ICI-based regimens (6.5, 9.9, and 18.9 months, respectively) and shortest with platinum-based regimens (2.8, 5.3, and 8.0 months, respectively) and non-platinum-based regimens (2.6, 5.0, and 7.8 months, respectively).
Conclusions
Among patients with metastatic non-small cell lung cancer without actionable genomic alterations previously treated with platinum + ICIs, non-platinum chemotherapy agents were most commonly prescribed in the index LOT. Clinical outcomes including time to treatment discontinuation, time to next treatment, and overall survival were short, highlighting the unmet need for more effective later-line treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In patients with metastatic non-small cell lung cancer without actionable genomic alterations, current treatment guidelines recommend platinum + immune checkpoint inhibitors (ICI). |
Few effective options are available after progression on platinum + ICI treatment, and retreatment with platinum ± ICI was observed. |
There is an unmet need for effective treatment for patients with metastatic non-small cell lung cancer without actionable genomic alterations previously treated with platinum + ICI. |
1 Introduction
Lung cancer is the leading cause of cancer-related death in the USA [1]. Non-small cell lung cancer (NSCLC) is the most common type of lung cancer, accounting for approximately 85–90% of lung cancer cases [2]. Non-small cell lung cancer is divided into two groups by histology: non-squamous cell carcinoma (including predominantly adenocarcinoma [54% of cases], and large cell) and squamous cell carcinoma (29% of cases) [2, 3]. The majority of patients with NSCLC present with metastatic disease (mNSCLC) at diagnosis [3].
Treatment guidelines recommend biomarker testing for patients with metastatic adenocarcinoma, large cell carcinoma, and NSCLC not otherwise specified, and consideration of testing for those with metastatic squamous cell histology, prior to the start of first-line (1L) systemic treatment for advanced or metastatic NSCLC [4]. The goal of genomic testing is to identify patients with actionable genomic alterations (AGA) for which there are existing targeted therapies. However, many patients with adenocarcinoma (56–67%) and nearly all patients with squamous cell carcinoma do not have AGAs. For these patients, targeted therapies are not recommended options [5,6,7].
For patients with mNSCLC and no AGAs, programmed death ligand-1 (PD-L1) expression level is a primary consideration when selecting 1L treatment. Guidelines recommend an immune checkpoint inhibitor (ICI) ± platinum-based chemotherapy for patients with PD-L1 ≥ 50%, and ICI + platinum-based chemotherapy for those with PD-L1 ≥ 1–49% [4]. In KEYNOTE-024, among patients with PD-L1 ≥ 50%, 1L treatment with pembrolizumab monotherapy was associated with significantly longer progression-free survival (PFS) and overall survival (OS) versus a platinum-based chemotherapy regimen (median PFS: 10.3 vs 6.0 months, hazard ratio [HR]: 0.50 [95% confidence interval (CI) 0.36, 0.68] [8]; median OS: 26.3 vs 13.4 months, HR: 0.62 [95% CI 0.48, 0.81] [9]). KEYNOTE-189 showed improved outcomes in patients with PD-L1 ≥ 1–49% who received 1L pembrolizumab + pemetrexed + platinum versus a pemetrexed-platinum combination alone (median PFS: 9.0 vs 4.9 months, HR: 0.48; 95% CI 0.40, 0.58; median OS: 22.0 months vs 10.7 months; HR, 0.56; 95% CI 0.45, 0.70) [10]. For patients with PD-L1 expression < 1% and advanced or metastatic disease, guidelines recommend 1L systemic therapy options that include either an ICI + platinum-based chemotherapy regimen or ipilimumab + nivolumab [4].
However, for patients without an AGA who have disease progression after previous treatment with ICI + platinum-based chemotherapy, few options are available. Later lines of treatment (LOT) generally employ non-platinum-based chemotherapies (e.g., gemcitabine, docetaxel monotherapy, docetaxel with ramucirumab, or albumin-bound paclitaxel). These regimens offer limited clinical benefit. In TAX 320, a cohort receiving docetaxel was compared with a cohort receiving either vinorelbine or ifosfamide (combined groups). Docetaxel 75 mg/m2 was associated with a median OS of 5.7 months, a median time to progression of 2.0 months, and a 12-month survival of 32%, and the vinorelbine or ifosfamide cohort had a median time to progression of 1.8 months and a 12-month survival of 19% [12]. In the REVEL trial, second-line docetaxel + ramucirumab was compared to docetaxel alone. Although the combination was associated with improved outcomes versus the single agent, PFS and OS in both trial arms were short (median PFS: 4.5 vs 3.0 months, HR: 0.76; 95% CI 0.68, 0.86; median OS: 10.5 vs 9.1 months, HR: 0.86; 95% CI 0.75, 0.98, respectively) [11]. Further, non-platinum-based drugs, including pemetrexed and docetaxel, have been associated with grade 3–4 hematological events [13,14,15]. In addition, some patients may repeat chemotherapy + ICI even though they had not responded to that treatment previously [16, 17]. No real-world evidence-based studies have provided evidence that rechallenging with chemotherapy + ICI has a clinical benefit [18]. Thus, there is an unmet need for patients with mNSCLC without AGAs who have not responded to previous treatment with chemotherapy + ICI.
While data from clinical trials have been used to establish treatment guidelines, the trials are often highly selective and may not be representative of patients seen in routine clinical practice. Utilization of electronic health records (EHRs) offers a method to assess a broad patient population in a real-world setting. The aim of this study was to characterize real-world treatment patterns and associated clinical outcomes among patients with mNSCLC without AGAs who had initiated a subsequent LOT after prior use of ICI and platinum-based chemotherapy.
2 Material and Methods
2.1 Data Source
This was a retrospective observational study of patients with mNSCLC. Data were obtained via programmatic database abstraction from structured data fields of The US Oncology Network iKnowMed (iKM) EHR. The US Oncology Network consists of community oncology practices representing 900 oncology providers in 500 sites of care across 40 states [19]. Vital status was supplemented with information from the Social Security Limited Access Death Master File.
2.2 Sample Selection
Patients with an initial diagnosis of mNSCLC (de-novo or recurrent) between 1 January, 2017 and 31 December, 2019 who met the eligibility criteria were included in this study (Fig. 1). Metastatic disease was defined as meeting any one of the following criteria: TNM with an M value of 1, record of metastatic location, current or prior disease status containing a reference to metastatic disease, at least one line of systemic anti-cancer treatment for metastatic disease (as opposed to adjuvant/neoadjuvant lines). Eligible patients were also ≥18 years of age, had two or more visits within The US Oncology Network after the mNSCLC diagnosis date, and were without an AGA (i.e., negative biomarker test results for EGFR, ALK, ROS1, BRAF, NTRK, MET, and RET, or no evidence of biomarker testing during the study period). Further, patients must have received a subsequent LOT (index LOT) after discontinuation of both a platinum-based chemotherapy and an ICI (concurrent or sequential) as the only one or two prior LOTs. Patients were excluded if they had been enrolled in an interventional clinical trial or had received treatment for another primary cancer at any time during the study period. All patients were followed from the index date until the earliest of 31 December, 2020, last patient record, or date of death (as recorded in the iKM EHR or Limited Access Death Master File).
2.3 LOTs and Treatment Groups
A treatment-based algorithm was applied to identify LOTs received after the mNSCLC diagnosis date using drug start and stop dates recorded in the iKM EHR. Combination regimens included any new drug that was prescribed or administered within 28 days of a previous prescription or administration. A LOT was considered to have been discontinued when all drugs in a regimen were discontinued for ≥ 120 days or when a new drug was added at > 28 days after index LOT initiation (new LOT). The LOT immediately after platinum-based chemotherapy and ICI discontinuation was defined as the index LOT.
For reporting, the index LOT was classified into five mutually exclusive, drug class-based treatment groups: (1) platinum-based chemotherapy + ICI; (2) platinum-based chemotherapy; (3) ICI monotherapy or combination therapy (other than with a platinum-based chemotherapy agent); (4) non-platinum-based chemotherapy; and (5) other (non-chemotherapy, non-ICI regimens, or targeted therapies).
2.4 Measurements and Statistical Analyses
Descriptive analyses were used to assess patient demographics at baseline (up to 12 months prior to the mNSCLC diagnosis date) and clinical characteristics. Clinical outcomes associated with the index LOT were evaluated using Kaplan–Meier analyses, including time to treatment discontinuation (TTD), time to next treatment (TTNT), and OS. For all measures, the results were reported for the full patient cohort and stratified by treatment groups. SAS software version 9.4 was used for data management and statistical analyses.
Time to treatment discontinuation was defined as the interval between the dates of index LOT initiation and discontinuation for any cause. Patients who did not discontinue the index LOT during the study observation period were censored on the study end date or the last visit date in the iKM EHR, whichever occurred first.
Time to next treatment was assessed from the date of index LOT initiation to the earlier of the next LOT initiation or death due to any cause. Patients who did not receive a subsequent LOT or were still alive by the end of the study observation period were censored on the study end date, or the last visit date in the iKM EHR, whichever occurred first. Time to next treatment has been used in prior retrospective studies as a proxy for progression-free survival (PFS) [20, 21].
Overall survival was defined as the interval between index LOT initiation and death from any cause as documented in the Limited Access Death Master File and the iKM EHR database. Patients who did not die within the study observation period were censored on the study end date or the last visit date available in the iKM EHR, whichever occurred first.
2.5 Ethics
All data were handled in compliance with the Health Insurance Portability and Accountability Act of 1996 and the Health Information Technology for Economic and Clinical Health Act. The study protocol was granted an exemption and a waiver of informed consent by the US Oncology Institutional Review Board in February 2021.
3 Results
3.1 Patient Characteristics
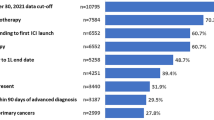
Of 13,978 patients identified with mNSCLC during the study identification period, 961 met the eligibility criteria (Fig. 2). The median (range) age was 68 (27–89) years, 46.6% (n = 448) were female, and the majority were Caucasian (74.8%, n = 719) (Table 1 and Table 1 of the Electronic Supplementary Material [ESM]). The largest proportion of patients came from the South census region of USA (38.6%, n = 371), and the lowest proportion from the Northeast census region of USA (6.2%, n = 60), consistent with the distribution of The US Oncology Network patient representation. The median (minimum, maximum) follow-up time from initiation of the index LOT to the end of the study observation period was 15.0 (0, 47.8) months.
Study attrition flowchart. ICI immune checkpoint inhibitor, LOT line of therapy, NSCLC non-small cell lung cancer. *Of 11,194 patients with no actionable genomic alterations, 6092 were identified via negative biomarker test results and 5102 were included in the study based on no evidence of biomarker testing
Nearly two-thirds (65.6%, n = 630) of patients were diagnosed with de novo metastatic disease, while 33.5% (n = 322) had recurrent disease (Table 1 and Table 2 of the ESM). Non-squamous histology was identified in 45.2% (n = 434) of patients, squamous histology in 18.9% (n = 182), and 35.9% (n = 345) did not have their histology documented in the EHR. Most patients (66.8%, n = 642) were current or former smokers. Half of patients (50.1%, n = 481) had an Eastern Cooperative Oncology Group performance status score of 0–1, although 41.2% (n = 396) of patients had no score recorded. The PD-L1 expression level was < 1%, 1–49%, and ≥ 50% for 22.1% (n = 212), 22.9% (n = 220), and 18.7% (n = 180) of patients, respectively. Approximately one third of patients (36.3%, n = 349) had a missing/undocumented PD-L1 status within the structured fields of the EHR.
3.2 Pre-index Regimens
Prior to the index LOT, 35.7% (n = 343) of patients had received one prior LOT (concurrent ICI + platinum-based chemotherapy) [Table 3 of the ESM] and 64.3% (n = 618) had received two prior LOTs with sequential use of platinum and ICI (Tables 4 and 5 of the ESM). Of those with two pre-index LOTs, 86.1% (n = 532) were treated with platinum-based chemotherapy and 13.9% (n = 86) with ICI in the first LOT.
3.3 Index LOT Regimens
Figure 3a shows the distribution of index LOT by the class-based regimen. The most commonly prescribed index treatment was a non-platinum-based regimen (57.3%, n = 551), followed by a platinum-based regimen (12.9%, n = 124), an ICI-based regimen (12.7%, n = 122), a platinum + ICI-based regimen (9.4%, n = 90), and other regimens (7.7%, n = 74). The most common regimens by agent (across all class-based regimens) were docetaxel monotherapy (23.7%, n = 228) and docetaxel + ramucirumab (14.3%, n = 137). The remaining agent-level regimens accounted for fewer than 5% of patients each (Fig. 3b).
3.4 Post-index LOT Regimens
There were 281 (29.2%) patients who had a post-index LOT regimen. Of those, most patients received a non-platinum-based regimen (61.2%, n = 172). Others included ICI-based (15.3%, n = 43), platinum-based (10.7%, n = 30), platinum + ICI-based (3.2%, n = 9), and other regimens (2.5%, n = 7) [Table 6 of the ESM].
3.5 Sequencing of Treatment Patterns
Diagrams of treatment sequencing from two LOTs prior to index through one LOT after index are shown in Fig. 4a (patients with one lot pre-index, n = 343), Fig. 4b (patients with two lots pre-index, n = 618), and Fig. 4c (post-index LOT; n = 281). Of patients with one LOT prior to the index LOT, 68.5% (n = 235/343) were treated with non-platinum regimens in the index LOT versus 51.1% (n = 316 /618) of patients with two LOTs prior to index. In contrast, only 18.4% of patients with one prior LOT were re-treated with a platinum and/or ICI regimen, whereas patients with two prior LOTs were more likely to re-utilize these agents (44.2%).
Sankey diagrams of treatment sequencing, from two lines of therapy (LOTs) prior to index through one LOT after index. a Diagram of treatment sequencing from one LOT prior to index (Index LOT−1) to index. b Diagram of treatment sequencing from two LOTs prior to index (Index LOT−2) through to index. c Diagram of treatment sequencing from index to one LOT after index (Index LOT+1). ICI immune checkpoint inhibitor
3.6 Clinical Outcomes by Class-Based Regimens
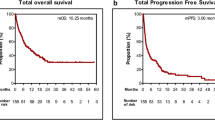
The median (95% CI) TTD for the index LOT was 3.5 months (2.8, 3.7) with 69.2% (n = 665) of patients having a treatment discontinuation event (treatment discontinuation or death) [Fig. 5a]. The non-platinum and platinum-based chemotherapy groups had the numerically shortest median (95% CI) TTD: 2.6 (2.1, 3.0) and 2.8 (2.2, 3.7) months, respectively. Patients treated with an ICI-based regimen had the numerically longest median (95% CI) TTD: 6.5 (4.3, 10.6) months.
The median (95% CI) TTNT after index LOT initiation was 5.4 (5.1, 5.8) months, with 64.8% (n = 623) of patients having a next treatment event (new LOT or death) [Fig. 5b]. The numerically shortest median (95% CI) TTNT was among patients treated with other therapies (4.6 [3.2, 6.2] months) and was slightly numerically increased for those treated with either non-platinum-based (5.0 [4.5, 5.3] months) or platinum-based (5.3 [4.5, 6.4] months) chemotherapies. Patients treated with ICI-based regimens had the numerically longest median (95% CI) TTNT (9.9 [7.4, 13.1] months).
The median (95% CI) OS from index LOT initiation was 8.8 (8.0, 9.6) months, and 50.9% (n = 489) died during the follow-up period (Fig. 5c). Similar to the measures of TTD and TTNT, median OS (95% CI) tended to be numerically lower for patients treated with chemotherapies (non-platinum 7.8 [7.0, 8.8] and platinum 8.0 [6.4, 9.2] months), and were numerically highest for those treated with ICI-based regimens (18.9 [13.0, Not Reached] months).
3.7 Clinical Outcomes by Common Agent-Based Regimens
The median (95% CI) TTD, TTNT, and OS for docetaxel monotherapy was 2.2 (2.1, 2.8), 4.5 (3.9, 5.2), and 6.9 (5.8, 8.6) months, respectively (not shown). For patients who received the combination of docetaxel + ramucirumab, the median (95% CI) TTD was 3.7 (2.3, 4.9) months, the TTNT was 5.6 (4.9, 7.2) months, and OS was 8.3 (7.2, 11.1) months.
4 Discussion
This study reports real-world treatment patterns and outcomes among 961 patients with mNSCLC, without AGA, and prior concurrent or sequential ICI and platinum-based chemotherapy as the only prior treatments. Among the study sample, the most common regimens in the index LOT were non-platinum chemotherapy, followed by platinum chemotherapy, ICI, platinum + ICI, and other regimens. For patients who did not respond to a 1L platinum ± ICI-based regimen, current treatment guidelines recommend ICI for patients who had not received ICI in a previous LOT, and non-platinum-based chemotherapy, such as docetaxel, pemetrexed, gemcitabine, docetaxel + ramucirumab, or albumin-bound paclitaxel, regardless of ICI in the previous LOT [4]. Despite poor clinical outcomes and safety profile, docetaxel monotherapy was the most common treatment followed by docetaxel + ramucirumab. While non-platinum therapies were most often used in the index LOT after platinum and ICI-based regimens, we did observe a substantial amount of retreatment with platinum and ICI despite prior discontinuation of those agents. Treatment options in post-index lines continued to be limited; retreatment with non-platinum, platinum, and ICI remained the prevalent treatment approach.
Overall survival in the real-world setting was relatively short, suggesting a need for more effective treatments in the second-line setting and beyond. For more than half of the patients who received a non-platinum chemotherapy regimen, median OS was numerically lower versus patients who received docetaxel ± ramucirumab in the REVEL trial (7.8 vs 10.0 months) [11]. Specifically, OS was 6.9 and 8.3 months in our study compared with 9.1 and 10.5 months in the REVEL trial for docetaxel monotherapy and docetaxel + ramucirumab, respectively. The discrepancies in these outcomes may be related to differences in patient populations. In our study, all patients were required to have received prior ICI and platinum chemotherapy, whereas the REVEL trial only required prior platinum use. As a result, the type of prior treatment and the number of prior LOTs differed between these populations [11]. In our study, the 12-month overall survival for patients treated with a non-platinum-based regimen was poor (33.5%) although similar to the 12-month survival as reported in TAX 320 for patients treated with docetaxel (32%) [12].
Of all class-based regimens observed, the median TTD, TTNT, and OS were longest for patients who either continued or were retreated with ICI-based regimens in the index LOT. Longer OS among patients who continued ICI after progression was also observed in a retrospective analysis of patients from the OAK study. The median post-progression OS was 12.7 months among patients who continued atezolizumab versus 8.8 months among those switching to non-protocol therapy (mostly chemotherapy or targeted therapies) [22]. However, the small number of patients who continued or were retreated with ICI in our study hinders the interpretation of survival benefit, and comparison with OAK is limited because very few patients in our study received atezolizumab. More research is needed to understand the continuation or reutilization of ICIs beyond progression.
The most frequently prescribed regimens in the index LOT were docetaxel and docetaxel + ramucirumab. However, docetaxel is known to be associated with tolerability issues [15] that negatively impact patients’ health-related quality of life [14]. In the REVEL trial, docetaxel + ramucirumab was associated with a higher risk of grade ≥ 3 hematological adverse events compared with docetaxel + placebo (neutropenia 49 vs 39%, leukopenia 14 vs 12%, febrile neutropenia 16 vs 10%). At the same time, such events were also seen in a considerable proportion of the control arm. In addition, patients in both arms had adverse events that resulted in dose adjustments, including bleeding and hypertension [11]. Such adverse events point to the need for new therapies with improved safety profiles to support the treatment of patients in later lines of therapy.
Real-world data on the subsequent LOT after ICI and platinum-based therapies for patients with advanced/metastatic NSCLC are sparse. Bains et al. performed a retrospective EHR database study that investigated treatment patterns and OS in the LOT following first-line chemotherapy and second-line programmed cell death protein 1/PD-L1. Although Bains et al. allowed the use of chemotherapy in the first LOT to be either a single agent or a combination regimen (vs requiring a platinum agent), treatment patterns in the third-line were similar to our study with single-agent non-platinum chemotherapy being the most frequently prescribed (45.5%). Other treatment groups are difficult to compare as the class-level categorization of regimens differed between studies. However, continuation of programmed cell death protein 1/PD-L1-based therapies (13.1%) and reutilization of platinum combination therapies (16.0%) were evident [23].
The continued use of non-platinum chemotherapy in the index LOT, despite unfavorable outcomes, and reutilization of platinum + ICI suggest an unmet need for more effective later-line treatments. Newer treatment options for later lines of treatment, including antibody-drug conjugates, are emerging for patients with mNSCLC without AGAs who progress after ICIs and platinum chemotherapy. Pending results of clinical trials in progress, antibody-drug conjugates may offer potential treatment options for this patient population.
The use of an EHR data source for this study yielded a relatively large population size and real-world assessment of treatment patterns after at least one or two treatments for mNSCLC in a community oncology setting. In particular, a LOT assignment based on structured EHR data can have high accuracy [24]. The iKM is utilized in community-based oncology practices throughout the country and not academic medical centers, and thus may be more representative of typical community clinical practice [25].
While the EHR data have many strengths, they also have certain limitations. Select variables, such as the PD-L1 result, safety events, and histology at baseline, may be entered into unstructured fields (e.g., progress notes) as opposed to structured fields. Specific to this study, biomarker testing and results may not have been entered into the structured fields of the EHR. Thus, some patients assumed to be without AGAs in this study may have been misclassified, potentially explaining the observed use of a limited number of targeted agents in the “other” index LOT. In general, for studies based on EHR, data are collected for clinical practice use, not research, and thus may be lacking in standardization of the collection methods, instruments, and thoroughness of reporting. Services and procedures performed outside of the associated clinics, including hospitalizations, are not included in the structured dataset. The iKM EHR used by The US Oncology Network clinics integrates evidence-based treatment guidelines, which may not represent the care that occurs across all community clinics nationally. Furthermore, medication use was reported based on injectable therapies actually administered and prescriptions written for oral therapies regardless of whether the prescriptions were fulfilled. While conclusions from EHR-based data have limitations, they are generally accepted as more representative of “typical” care than clinical trials [26].
Of note, patients eligible for this study were required to have survived between the metastatic diagnosis date and initiation of the index LOT. Because death could not occur during this time period, these patients may have had a survival advantage compared with those who were not study eligible. Future retrospective studies might consider incorporating landmark survival analyses or time-dependent Cox regression analyses to minimize the risk of immortal time bias.
Last, the follow-up period for this study ended in 2020. Since that time, two additional immunotherapies (cemiplimab and tremelimumab) have been approved by the US Food and Drug Administration for the treatment of patients with metastatic NSCLC. Further research is needed to determine the degree to which these new agents might influence provider prescribing in the LOT after Immuno-Oncology therapy and a platinum-based chemotherapy among patients with no AGAs.
5 Conclusions
In our real-world study of patients with mNSCLC without AGAs who were previously treated with platinum and ICIs, the majority of the patients were treated with a non-platinum-based chemotherapy regimen in the index LOT. Clinical outcomes such as TTD, TTNT, and OS with these treatments were poor, demonstrating a high unmet need in this patient population. Reutilization of ICIs and platinum-based chemotherapy was also common suggesting a lack of effective treatment options and highlighting the need for newer and more efficacious treatments in later lines of therapy.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33.
SEER*Explorer: an interactive website for SEER cancer statistics. Surveillance Research Program, National Cancer Institute. Data source(s): SEER incidence data, November 2022 submission (1975–2020), SEER 22 registries. April 19, 2023. Available from: https://seer.cancer.gov/statistics-network/explorer/. Accessed 26 Apr 2023.
Ganti AK, Klein AB, Cotarla I, Seal B, Chou E. Update of incidence, prevalence, survival, and initial treatment in patients with non-small cell lung cancer in the US. JAMA Oncol. 2021;7(12):1824–32.
NCCN clinical practice guidelines in oncology (NCCN Guidelines®). Non-small cell lung cancer version 3.2023. April 13, 2023. Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed 26 Apr 2023.
Tsao AS, Scagliotti GV, Bunn PA Jr, et al. Scientific advances in lung cancer 2015. J Thorac Oncol. 2016;11(5):613–38.
Bubendorf L, Lantuejoul S, de Langen AJ, Thunnissen E. Nonsmall cell lung carcinoma: diagnostic difficulties in small biopsies and cytological specimens: number 2 in the series “Pathology for the clinician” edited by Peter Dorfmuller and Alberto Cavazza. Eur Respir Rev. 2017;26(144):17007.
Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl. 4):iv192–237.
Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–33.
Reck M, Rodriguez-Abreu D, Robinson AG, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥50. J Clin Oncol. 2021;39(21):2339–49.
Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2020;38(14):1505–17.
Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384(9944):665–73.
Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens: the TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18(12):2354–62.
Bonnet M, Jouinot A, Boudou-Rouquette P, et al. Predictive factors associated with pemetrexed acute toxicity. Eur J Clin Pharmacol. 2023;79(5):635–41.
Al-Batran SE, Hozaeel W, Tauchert FK, et al. The impact of docetaxel-related toxicities on health-related quality of life in patients with metastatic cancer (QoliTax). Ann Oncol. 2015;26(6):1244–8.
Yang W, Xuan B, Chen M, et al. Comparison of efficacy and safety between immunotherapy and docetaxel monotherapy in NSCLC patients. Front Oncol. 2022;12: 883514.
Cai Z, Zhan P, Song Y, Liu H, Lv T. Safety and efficacy of retreatment with immune checkpoint inhibitors in non-small cell lung cancer: a systematic review and meta-analysis. Transl Lung Cancer Res. 2022;11(8):1555–66.
Akamatsu H, Teraoka S, Takamori S, et al. Nivolumab retreatment in non-small cell lung cancer patients who responded to prior immune checkpoint inhibitors and had ICI-free intervals (WJOG9616L). Clin Cancer Res. 2022;28(15):O1-7.
Xu Z, Hao X, Yang K, et al. Immune checkpoint inhibitor rechallenge in advanced or metastatic non-small cell lung cancer: a retrospective cohort study. J Cancer Res Clin Oncol. 2022;148(11):3081–9.
The US Oncology Network. Available from: https://www.usoncology.com/our-company. Accessed 14 June 2024.
Walker B, Boyd M, Aguilar K, et al. Comparisons of real-world time-to-event end points in oncology research. JCO Clin Cancer Inform. 2021;5:45–6.
Shah NJ, Sura SD, Shinde R, et al. Real-world treatment patterns and clinical outcomes for metastatic renal cell carcinoma in the current treatment Era. Eur Urol Open Sci. 2023;49:110–8.
Gandara DR, von Pawel J, Mazieres J, et al. Atezolizumab treatment beyond progression in advanced NSCLC: results from the randomized, phase III OAK study. J Thorac Oncol. 2018;13(12):1906–18.
Bains S, Kalsekar A, Amiri KI, Weiss J. Real-world treatment patterns and outcomes among patients with metastatic NSCLC previously treated with programmed cell death protein-1/programmed death-ligand 1 inhibitors. JTO Clin Res Rep. 2022;3(2): 100275.
Choi YC, Zhang D, Tyczynski JE. Comparison between health insurance claims and electronic health records (EHRs) for metastatic non-small-cell lung cancer (NSCLC) patient characteristics and treatment patterns: a retrospective cohort study. Drugs Real World Outcomes. 2021;8(4):577–87.
Melas M, Subbiah S, Saadat S, Rajurkar S, McDonnell KJ. The Community Oncology and Academic Medical Center Alliance in the age of precision medicine: cancer genetics and genomics considerations. J Clin Med. 2020;9(7):2125.
Nazha B, Yang JC, Owonikoko TK. Benefits and limitations of real-world evidence: lessons from EGFR mutation-positive non-small-cell lung cancer. Future Oncol. 2021;17(8):965–77.
Acknowledgements
Medical writing assistance (writing and revising of the manuscript) and statistical assistance (checking code and editing figures) were provided by Sonya Dave, PhD, and Lisa Kaspin-Powell, PhD, as employees of Ontada.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was funded by Daiichi-Sankyo, Inc.
Conflict of interest
Jerome H. Goldschmidt is an employee of The US Oncology Network, which received funding from Daiichi-Sankyo, Inc. for this study. Anupama Vasudevan and Michelle Silver were employees of Ontada at the time the study was performed. Wan-Yu Tseng, Yunfei Wang, and Janet Espirito are employees of Ontada. Jackie Kwong, Ruchit Shah, and Elizabeth Marrett are employees of Daiichi-Sankyo, Inc.
Ethics approval
Institutional review board and compliance/privacy approval was gained prior to initiation of the retrospective research. As this project involved the analysis of existing data and records, study information was analyzed in such a manner that research participants could not be directly identified. Patient informed consent was not required because of the nature of the study design. Thus, an exemption status and a waiver of informed consent were approved by The US Oncology, Inc. Institutional Review Board. Data were handled in compliance with the Health Insurance Portability and Accountability Act of 1996 and the Health Information Technology for Economic and Clinical Health Act.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The raw data used for this analysis are not publicly available because of privacy or ethical restrictions.
Code availability
Not applicable.
Author contributions
JG made substantial contributions to the conception or design of the work, approved the final version to be published, and agreed to be accountable for all aspects of the work. W-YT contributed to the interpretation of data for the work, drafted or revised the work critically for important intellectual content, approved the final version to be published, and agreed to be accountable for all aspects of the work. YW performed the acquisition, analysis, or interpretation of data for the work and approved the final version to be published. JE contributed to the conception or design of the work and/or the acquisition, analysis, or interpretation of data for the work, drafted or revised the work critically for important intellectual content, approved the final version to be published, and agreed to be accountable for all aspects of the work. AV contributed to the conception or design of the work and/or the acquisition, analysis, or interpretation of the data for the work, drafted or revised the work critically for important intellectual content, approved the final version to be published, and agreed to be accountable for all aspects of the work. MS contributed to the conception or design of the work and/or the acquisition, analysis, or interpretation of data for the work, drafted or revised the work critically for important intellectual content, approved the final version to be published, and agreed to be accountable for all aspects of the work. JK had the following contributions: conception or design of the work, interpretation of data for the work, drafted or revised the work critically for important intellectual content, approved the final version to be published, and agreed to be accountable for all aspects of the work. RS had the following contributions: interpretation of data for the work, drafted or revised the work critically for important intellectual content, approved the final version to be published, and agreed to be accountable for all aspects of the work. EM had the following contributions: conception or design of the work, interpretation of data for the work, drafted or revised the work critically for important intellectual content, approved the final version to be published, and agreed to be accountable for all aspects of the work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Goldschmidt, J.H., Tseng, WY., Wang, Y. et al. Treatment Patterns and Clinical Outcomes Among Patients with Metastatic Non-small Cell Lung Cancer Without Actionable Genomic Alterations Previously Treated with Platinum-Based Chemotherapy and Immunotherapy. Drugs - Real World Outcomes (2024). https://doi.org/10.1007/s40801-024-00440-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s40801-024-00440-3