Abstract
Introduction
Contemporary real-world data on advanced non-small cell lung cancer (aNSCLC) treatment patterns across programmed cell death-ligand 1 (PD-L1) expression levels and testing status are limited.
Methods
A retrospective cohort was selected of adults newly diagnosed with aNSCLC between January 1, 2018, and July 31, 2021, who initiated first-line treatments, which were described by PD-L1 status and expression levels (≥ 50%, 1–49%, < 1%). Treatment received before and after PD-L1 test results were described for patients initiating first-line treatment before PD-L1 results. For patients who initiated chemotherapy alone before PD-L1 results, the probability of receiving immune checkpoint inhibitors (ICIs) after PD-L1 results was estimated by PD-L1 level and associated factors were explored.
Results
Among 12,202 patients with aNSCLC initiating first-line treatment [54.7% male, mean (standard deviation) age 69.2 (9.4) years], the most common therapies were ICI-based regimens across PD-L1 levels, and chemotherapy alone among PD-L1-untested patients. Use of chemotherapy alone decreased between 2018 and 2019 and stabilized thereafter, accounting for 21–29% of first-line treatments across PD-L1 levels and 48% of untested patients in 2021. Of 1468 patients initiating first-line treatment before PD-L1 results, treatments remained unchanged in most patients after PD-L1 results. Among patients initiating chemotherapy alone before PD-L1 results, the probability of receiving ICIs within 45 days after test results was 40.5% [95% confidence interval (CI) 31.6–48.3%], 28.6% (95% CI 20.3–36.0%), and 22.9% (95% CI 16.9–28.4%) at PD-L1 ≥ 50%, 1–49%, and < 1%, respectively.
Conclusion
While ICI-based regimens accounted for most first-line treatments across PD-L1 levels, chemotherapy alone was initiated in > 20% of patients tested for PD-L1 and 48% of untested patients in 2021. Patients who initiated chemotherapy alone had a low probability of receiving ICIs after PD-L1 test results. These results highlight the need for understanding the role and timing of PD-L1 test results for informing treatment decisions for patients with aNSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
To better understand the role of programmed cell death-ligand 1 (PD-L1) testing in the treatment of advanced non-small cell lung cancer and how PD-L1 testing results may inform subsequent treatment. |
Since contemporary data on treatment patterns across different levels of PD-L1 expression are limited, this study used a large electronic health record–derived database to characterize the treatment landscape between 2018 and 2021 stratified by PD-L1 expression levels and testing status. |
What was learned from the study? |
Patterns of first-line treatment of advanced non-small cell lung cancer changed between 2018 and 2019 but subsequently stabilized; observed treatments were generally consistent with guidelines for use of immune checkpoint inhibitors, although in 2021, chemotherapy alone continued to be used in 21–29% of patients across PD-L1 expression levels and in 48% of untested patients. |
Most patients who initiated chemotherapy alone before receiving PD-L1 test results did not change treatment after receiving PD-L1 testing results regardless of PD-L1 expression level. |
Introduction
Non-small cell lung cancer (NSCLC) is the most common form of lung cancer, and most patients have advanced disease (aNSCLC) at initial diagnosis [1]. Platinum-based doublet chemotherapy was considered the standard of care in patients with aNSCLC who are negative for genomic aberrations including epidermal growth factor receptor (EGFR) mutation, anaplastic lymphoma kinase (ALK) translocation, or C-ROS oncogene 1 (ROS1) rearrangement [2,3,4]. The approval of immunotherapies such as immune checkpoint inhibitors (ICIs) that target programmed cell death-ligand 1 (PD-L1) changed the treatment paradigm for aNSCLC [5,6,7,8].
In 2016, the US Food and Drug Administration approved the first ICI as monotherapy for first-line treatment of patients with aNSCLC with PD-L1 expression ≥ 50%. The indication was expanded to include patients with aNSCLC and PD-L1 ≥ 1% (2019) or in combination with chemotherapy for those with non-squamous (2017) and squamous (2018) histology regardless of PD-L1 expression levels [9,10,11]. Current National Comprehensive Cancer Network (NCCN) guidelines for treatment of aNSCLC reflect these changes and recommend ICI-based therapy across all PD-L1 expression levels [12].
Characterizing the treatment landscape advances our understanding of how these therapies are used in real-world clinical settings, and could potentially inform management strategies to enhance patient outcomes. While several studies have reported patterns of first-line treatment for aNSCLC subsequent to ICI approval [13,14,15,16,17], these studies did not stratify by PD-L1 expression level and covered only the initial years after the introduction of ICIs and thus may not reflect contemporary management of patients with aNSCLC. Therefore, the objective of this study was to describe the treatment landscape and trends among patients with aNSCLC initiating first-line treatment across PD-L1 expression levels and those not undergoing PD-L1 testing. An additional objective was to understand the role of PD-L1 test results on treatment patterns by characterizing treatment changes among patients initiating first-line treatment before obtaining their PD-L1 test results.
Methods
Study Design
This was a retrospective cohort study of adult patients newly diagnosed with aNSCLC between January 1, 2018, and July 31, 2021, who initiated first-line systemic therapy in the nationwide Flatiron Health de-identified electronic health record (EHR)-derived database. The Flatiron Health EHR database is a large, longitudinal database comprising de-identified patient-level structured and unstructured data, curated via technology-enabled abstraction. During the study period (2018–2021), the de-identified data originated from approximately 280 US cancer clinics (~ 800 sites of care) [18].
The data were already de-identified and subject to obligations to prevent re-identification and protect patient confidentiality. Institutional review board approval of the study protocol for creating the aNSCLC research database was obtained by Flatiron Health before the current study was conducted and included a waiver of informed consent.
Study Populations
The study included patients who had newly diagnosed aNSCLC between January 1, 2018, and July 31, 2021, within the Flatiron Health database; the diagnosis of aNSCLC was confirmed by Flatiron Health review of pathology reports. Patients were also required to be ≥ 18 years old on the diagnosis date and to have initiated first-line systemic treatment within 90 days after the diagnosis date (index date = date of first-line treatment initiation). Patients were excluded if they had a positive biomarker test result for EGFR, ALK, or ROS1, or use of corresponding targeted therapies, or had participated in a clinical trial any time before or within 30 days post-index.
The main cohort consisted of eligible patients who either (1) had a determinable PD-L1 expression level test result before or up to 28 days after first-line systemic therapy initiation or (2) did not have PD-L1 testing or any result within the same window (i.e., untested).
From the main cohort, a sub-cohort of patients was also selected who initiated treatment before receiving PD-L1 testing results to evaluate how PD-L1 results might affect subsequent treatment. These patients were required to have no PD-L1 results before or at initiation of first-line therapy, and valid PD-L1 test results within 28 days after initiation of first-line therapy.
PD-L1 Levels
Among patients with determinable PD-L1 expression levels from a valid PD-L1 test result before or within 28 days of first-line treatment initiation, patients were categorized as having high PD-L1 expression (≥ 50%), low PD-L1 expression (1–49%), or negative for PD-L1 (< 1% expression). If multiple test results were available, PD-L1 expression level was assigned based on the highest test result.
Treatment Patterns
Lines of therapy (LOTs) were identified using Flatiron Heath oncologist-defined rules [19]. Briefly, LOT identification started from the initial aNSCLC diagnosis date, and eligible systemic treatments initiated within 28 days of the first eligible drug episode were considered one LOT. A gap of > 120 days between any two sequential drug episodes caused the LOT number to advance.
Outcomes: First-Line Treatment, Treatment Changes After PD-L1 Test Results
In the main cohort, first-line treatments were classified into platinum-based chemotherapy alone, ICI monotherapy, ICI + platinum-based chemotherapy (ICI + chemotherapy), and other therapies (i.e., any other systemic treatment that did not fall into the prior categories), regardless of whether patients also received therapy targeting vascular endothelial growth factor. In the sub-cohort, treatment regimens initiated before the test results were compared with the treatment regimen up to 45 days after receiving the PD-L1 test results.
Other Variables
Baseline demographic and clinical characteristics included age at aNSCLC diagnosis, sex (male, female), race and ethnicity (Asian, Black or African American, Hispanic or Latino, White, other race), payer type (commercial, Medicare, Medicaid, other or unknown), histology (non-squamous cell carcinoma, squamous cell carcinoma, NSCLC not otherwise specified), Eastern Cooperative Oncology Group (ECOG) performance status (0 or 1, 2–4, unknown), de novo versus recurrent aNSCLC, metastasis to bone (yes, no), metastasis to liver or bile duct (yes, no), and metastasis to brain (yes, no). ECOG performance status was assessed within 90 days before the index date, and the closest value to index date was used when multiple scores were available. Metastasis (bone, liver, brain) was defined as having any diagnosis of secondary malignancy of the corresponding site on or any time before the index date.
Statistical Analysis
Descriptive statistics for baseline characteristics included means, standard deviations (SD), medians, and 25th and 75th percentiles for continuous variables, with number and percentage presented for categorical variables. In the main cohort, demographic and clinical characteristics were described overall, by PD-L1 expression level subgroups (< 1%, 1–49%, ≥ 50%), and for those without PD-L1 testing. The sub-cohort was stratified by type of first-line treatment initiated before PD-L1 results and PD-L1 expression levels.
Descriptive analysis of the main cohort consisted of the proportion of patients with each type of first-line treatment stratified by PD-L1 expression level, with further stratification by index year to characterize changes in the treatment landscape over time. In the sub-cohort, the type of treatment received before PD-L1 results and within 45 days after PD-L1 results was evaluated and stratified by PD-L1 level (< 1%, 1–49%, ≥ 50%). Among patients who initiated chemotherapy alone before PD-L1 results, the Kaplan–Meier method was used to estimate the probability and 95% confidence interval (CI) of receiving ICI (either added to chemotherapy or as a switch to monotherapy) after receiving the PD-L1 test results, stratified by PD-L1 expression levels; log-rank tests were used to compare the groups. Cox proportional hazard models were used to derive hazard ratios (HRs) with 95% CIs to evaluate the impact of baseline demographic and clinical characteristics, including PD-L1 expression levels, on the initiation of ICIs by day 45 among patients who initiated chemotherapy alone before receiving PD-L1 test results.
Results
Patient Population
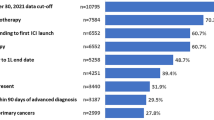
From among the 73,568 patients with NSCLC in the Flatiron Health database at the time of the study, 25,144 adults were newly diagnosed with aNSCLC during the study period, of whom 16,152 initiated first-line systemic treatment within 90 days of their aNSCLC diagnosis and 12,202 of these met all study criteria and were included in the main cohort; 1468 patients from the main cohort met the criteria for sub-cohort analysis (Fig. 1). Patients in the main cohort were primarily male (54.7%), White (66.5%), and had a mean (SD) age of 69.2 (9.4) years (Table 1). The South had the greatest representation (46.0%), and 50.6% of the patients were commercially insured. Diagnosis of aNSCLC was de novo in 75.9% of patients, with most patients having non-squamous cell carcinoma histology (64.6%) and ECOG performance status 0 or 1 (59.1%) (Table 1).
The distribution of patients across the three PD-L1 levels was comparable (23.8–26.3%), and an additional 25.1% of the main cohort consisted of untested patients (Table 1). When stratified by PD-L1 expression levels and testing status, most demographic and clinical characteristics were generally similar to the overall population (Table 1), except for a higher proportion of patients with squamous cell carcinoma among those who had no PD-L1 test relative to those across the PD-L1 levels (39.5% vs. 24.9–30.6%, respectively).
Baseline characteristics of the sub-cohort were similar to those of the main cohort overall and across PD-L1 levels; 31.9%, 27.6%, and 40.5% of patients in the sub-cohort had PD-L1 expression ≥ 50%, 1–49%, and < 1%, respectively (Table S1).
First-Line Treatment Landscape
As shown in Fig. 2, there were changes in the first-line treatment landscape from 2018 to 2019 followed by relative stability in type of first-line treatment initiated in each of the PD-L1 strata among patients with PD-L1 test results. ICIs were the predominant treatment (> 60% of patients from 2019 onward) as either monotherapy or ICI + chemotherapy. In the PD-L1 < 1% group, there was a notable reduction in chemotherapy alone between 2018 (49.0%) and 2019 (30.1%) and a corresponding increase in ICI-based treatment from 46.6% to 65.7% (Fig. 2a). In the PD-L1 1–49% group (Fig. 2b), there was a smaller but noticeable shift from chemotherapy alone (37.0%–24.3%) to ICI-based treatment regimens (59.0%–73.3%) from 2018 to 2019. Between 2019 and 2021, chemotherapy use appeared to stabilize across PD-L1 expression levels and accounted for 20.8–29.1% of first-line treatment in 2021 (Fig. 2a–c). Use of ICI + chemotherapy was most common among patients with PD-L1 < 1% and PD-L1 1–49% (Fig. 2a and b), whereas ICI monotherapy was most common (43.1–54.7%) among patients with PD-L1 ≥ 50% (Fig. 2c).
Among patients not tested for PD-L1, there were few changes in treatment patterns. Chemotherapy alone remained the most common first-line treatment between 2018 and 2021 (47.6–55.0%), followed by ICI + chemotherapy (26.1–36.9%) and ICI monotherapy (6.2–14.0%) (Fig. 2d).
Treatment Patterns Among Patients Initiating First-Line Treatment Before PD-L1 Test Results
In the sub-cohort, ICI + chemotherapy was the most common treatment before obtaining the PD-L1 test results and was received by 42.6% of patients (Table 2). ICI monotherapy was the least common treatment, received by 5.6% of patients, with chemotherapy alone and other therapies received by 35.6% and 15.7% of patients, respectively. Baseline characteristics were generally similar between patients initiating chemotherapy alone and ICI + chemotherapy, except for a lower percentage of patients with squamous cell carcinoma histology in those who received ICI + chemotherapy (20.9%) relative to chemotherapy alone (33.7%). Relative to the other treatment categories, patients who received ICI monotherapy were slightly older (mean age 73.0 years vs. 68.2–69.0 years) and more likely to have recurrent disease (40.2% vs. 9.4–14.2%) (Table 2).
At 45 days after receiving PD-L1 test results, most patients at each PD-L1 level remained on the same treatment as before the PD-L1 test results regardless of treatment type, except for those who had received other therapies (Fig. 3); 50.1–62.0% of patients who received other therapies before the test results received ICIs, as either monotherapy or ICI + chemotherapy, after receiving test results. Of the patients who received chemotherapy alone before test results, 21.1–35.3% received ICIs as either monotherapy or ICI + chemotherapy after the test results, with the highest proportion of any ICI use among those with PD-L1 ≥ 50%. Across PD-L1 levels, 28.0–30.0% of patients who received ICI monotherapy before the PD-L1 test results received other therapies or no therapy after receiving the test results.
Probability of Receiving ICIs After PD-L1 Test Results Among First-Line Chemotherapy-Treated Patients
Among the sub-cohort of patients who were receiving chemotherapy alone (n = 523) before PD-L1 test results, the probability of receiving ICIs over the 45-day period after PD-L1 test results was highest among patients with PD-L1 ≥ 50% (log-rank p = 0.0026) (Fig. 4). The probability of switching to or adding an ICI was 40.5% (95% CI 31.6–48.3%), 28.6% (95% CI 20.3–36.0%), and 22.9% (95% CI 16.9–28.4%) for patients with PD-L1 ≥ 50%, 1–49%, and < 1%, respectively.
Probability of receiving ICI therapy within 45 days after receiving PD-L1 test results among patients with newly diagnosed advanced non-small cell lung cancer who initiated chemotherapy as first-line treatment before PD-L1 test results. ICI immune checkpoint inhibitor, PD-L1 programmed cell death-ligand 1
The association of sociodemographic and clinical characteristics with receipt of ICIs after receipt of PD-L1 test results among the patients who were previously receiving chemotherapy alone are reported in Table 3. During the 45 days after receiving PD-L1 results, patients with PD-L1 ≥ 50%, bone or bone marrow metastasis, higher ECOG performance status, or receiving treatment in later years were more likely to receive ICIs. PD-L1 ≥ 50% increased the likelihood of receiving ICIs by 93% relative to PD-L1 < 1% (HR 1.93; 95% CI 1.27–2.93). The strongest association was for bone or bone marrow metastasis, with a 2.5-fold higher likelihood of receiving ICIs (HR 2.59; 95% CI 1.63–3.84) relative to no metastasis at this site, followed by receiving treatment in the years 2020 and 2021, both with a slightly more than twofold likelihood compared with 2018 [HRs of 2.32 (95% CI 1.45–3.71) and 2.02 (95% CI 1.23–3.31), respectively]. Patients with an ECOG performance status of 2–4 were 56% more likely to receive ICIs than those with ECOG performance status 0 or 1 (HR 1.56; 95% CI 0.96–2.53). Patients with squamous cell carcinoma histology were 53% less likely to receive ICIs (HR 0.47; 95% CI 0.30–0.72).
Discussion
In this large real-world cohort of patients with aNSCLC who had no known ALK, EGFR, or ROS1 aberrations and who initiated first-line treatment, we observed increased uptake of ICIs between 2018 and 2019 across PD-L1 levels. By 2021, approximately 20–30% of PD-L1-tested patients received chemotherapy alone as first-line treatment. In approximately 25% of patients, first-line treatment was initiated before PD-L1 testing, of whom nearly half received chemotherapy alone as first-line treatment during each study year. Among patients who initiated chemotherapy alone before receiving their PD-L1 test result, more than half remained on chemotherapy after receiving PD-L1 results, and only 21.1–35.3% subsequently received ICIs even in high PD-L1 expressers.
Published studies evaluating treatment patterns for aNSCLC describe a shift in treatment modalities that supports rapid acceptance and clinical uptake of ICIs immediately following their approval and incorporation into guidelines [13,14,15,16,17]. The current study showed that these shifts to ICIs have been maintained. The small to moderate increases in ICI use between 2018 and 2019 for patients with low and negative PD-L1, in contrast to the consistently higher use of ICIs among patients with PD-L1 ≥ 50% during these 2 years, likely reflects recognition of the expanded indication for these drugs and release of data on ICI combination therapy [20, 21]. Patterns of first-line treatment for patients with aNSCLC in real-world clinical settings subsequently remained stable across all PD-L1 levels through the end of the study period (October 31, 2021). Regardless of PD-L1 expression, ICIs as monotherapy or in combination with chemotherapy were the predominant first-line treatment and were initiated in 59.0–80.0% of patients. The most frequent use of ICIs was among patients with high levels of PD-L1 expression, who were also the most likely to receive ICIs as monotherapy.
These results are consistent with and expand on a previous study of treatment patterns through 2018 also using the Flatiron Health aNSCLC database [13]. Also consistent with that study, our results suggest that even if first-line treatment utilization in most patients was concordant with current recommendations, a substantial proportion of patients continued to initiate either chemotherapy alone or other non-ICI treatments as first-line therapy. While the reason for these treatment decisions could not be determined in our study, clinicians and patients may consider specific factors other than treatment guidelines when deciding not to use ICIs. In particular, 24.1% of patients with aNSCLC had recurrent disease rather than a de novo diagnosis, and it is possible that patients with recurrent disease who progressed to advanced-stage disease were less likely to use ICI as first-line therapy if they had previously received these drugs. In our study, patients with recurrent (vs. de novo) aNSCLC who received chemotherapy alone before PD-L1 test results had a lower, albeit nonsignificant, likelihood of receiving ICI after receiving PD-L1 test results. Another potential reason for not using ICIs may be contraindications such as the presence of genomic aberrations; however, this study excluded patients with ALK, EGFR, and ROS1 aberrations or who received targeted treatments for such aberrations. While autoimmune disease or immunosuppressive treatments may also impact treatment choices, other real-world studies have shown that ICI use is not uncommon in these patients [22,23,24]. Finally, socioeconomic barriers may also impact choice of therapy, and additional studies are needed to further explore the factors influencing treatment decisions.
In contrast to patients with known PD-L1 levels, chemotherapy alone was the most frequent first-line therapy (47.6–55.0%) among patients not tested for PD-L1 expression across all years. While there appeared to be a small shift to greater use of ICI + chemotherapy between 2018 and 2019, with a concomitant decline in chemotherapy alone, the treatment paradigm appeared to remain stable in subsequent years. Approximately 20% of patients did not undergo testing for PD-L1, which was slightly less than the 29.6% previously reported for an earlier time period [14]. Reasons for not testing have not been well studied and may include frailty, comorbidities, and overall benefit–risk assessment. Adhering to recommendations for PD-L1 testing, when appropriate, with timely testing and accurate reporting could help inform and optimize first-line treatment choices for aNSCLC.
The reasons for not initiating ICIs after receiving positive PD-L1 results were unknown, but may be due to a lack of awareness by providers, difficulty accessing appropriate testing or medication, or financial factors. Among patients who initiated chemotherapy alone before PD-L1 results, approximately half remained on chemotherapy regardless of PD-L1 testing results. Chemotherapy is associated with well-recognized adverse events that are reported by patients to negatively impact function and quality of life in a manner that may offset potential treatment benefits [25,26,27]. Additionally, clinical studies have demonstrated that ICIs convey clinically meaningful overall survival benefits with lower rates of specific toxicities, and improve patient-reported outcomes versus chemotherapy [5,6,7,8].
Patients with high PD-L1 expression levels, which accounted for approximately one third of all patients tested for PD-L1, were more likely to receive ICIs after receiving test results. This observation was not surprising, given that the initially approved indication for first-line use was for patients expressing high levels of PD-L1. However, even among patients who subsequently received a PD-L1 ≥ 50% test result after initiating chemotherapy, only 40% received ICI within the subsequent 45 days. The strongest association appeared to be for the presence of bone or bone marrow metastasis, with an almost 2.5-fold higher likelihood of receiving ICI therapy at 45 days. Despite poorer prognosis reported among patients with aNSCLC and bone metastasis, partial or complete response to ICIs has been reported [28,29,30]. Having squamous cell carcinoma histology was associated with a significantly lower likelihood of ICI, which could be partially due to the later approval of ICI + chemotherapy in this group relative to those with non-squamous cell carcinoma.
Limitations
Several study limitations should be considered, including that EHRs do not capture the full range of clinical variables (e.g., physician or patient preference), and data on some variables that may contribute to treatment decisions were incomplete or unavailable (e.g., ECOG performance status). Additional research is warranted to understand the determinants of treatment choices, especially the use of chemotherapy alone in the first line among patients with aNSCLC without ALK, EGFR, or ROS1 genomic aberrations. Studies are also needed to evaluate the impact on treatment outcomes of enhanced treatment decision-making based on timely biomarker testing. As information on LOTs are not routinely recorded in the EHR, LOT characterization is driven by Flatiron Health’s proprietary oncologist-defined algorithms and may not accurately reflect the actual LOTs. Another limitation is that the sample size for the sub-cohort was relatively small. Finally, the Flatiron Health database predominantly reflects community oncology centers, with only a small proportion of patients attending academic medical centers, limiting the generalizability of the results to the broader population of patients with aNSCLC.
Conclusions
This real-world study showed that ICI use accounted for most first-line aNSCLC treatment, concordant with guideline recommendations; however, a substantial proportion of patients across PD-L1 expression levels were initiated on non-ICI therapies, especially chemotherapy alone. Up to 2021, one out of five patients did not get tested for PD-L1, of whom almost half initiated chemotherapy alone as first-line treatment. Furthermore, a substantial portion of patients who initiated therapy before PD-L1 test results were found to receive chemotherapy alone and most of these remained on this regimen despite subsequently receiving positive PD-L1 results. Overall, these results highlight the fact that despite meaningful uptake in PD-L1 testing and use of ICIs as first-line therapy for aNSCLC, many patients do not receive guideline-directed biomarker testing or guideline-directed therapy based on their biomarker status; adherence to guidelines for biomarker testing may help optimize personalized treatment for aNSCLC.
References
Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Updated version published 15 September 2020 by the ESMO Guidelines Committee 2018 [cited 2022 March 12]. Available from: https://www.esmo.org/content/download/347819/6934778/1/ESMO-CPG-mNSCLC-15SEPT2020.pdf.
Besse B, Adjei A, Baas P, et al. 2nd ESMO Consensus Conference on Lung Cancer: non-small-cell lung cancer first-line/second and further lines of treatment in advanced disease. Ann Oncol. 2014;25(8):1475–84.
Reck M, Rabe KF. Precision diagnosis and treatment for advanced non-small-cell lung cancer. N Engl J Med. 2017;377(9):849–61.
Ettinger DS, Wood DE, Aggarwal C, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 1.2020. J Natl Compr Canc Netw. 2019;17(12):1464–72.
Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–33.
Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–30.
Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383(14):1328–39.
Sezer A, Kilickap S, Gumus M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet. 2021;397(10274):592–604.
U.S. Food and Drug Administration. Pembrolizumab (KEYTRUDA) Checkpoint Inhibitor 2016 [cited 2022 March 12]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/pembrolizumab-keytruda-checkpoint-inhibitor.
U.S. Food and Drug Administration. FDA approves pembrolizumab in combination with chemotherapy for first-line treatment of metastatic squamous NSCLC 2018 [cited 2022 March 12]. Available from: https://www.fda.gov/drugs/fda-approves-pembrolizumab-combination-chemotherapy-first-line-treatment-metastatic-squamous-nsclc.
U.S. Food and Drug Administration. FDA approves atezolizumab with nab-paclitaxel and carboplatin for metastatic NSCLC without EGFR/ALK aberrations 2019 [cited 2022 March 12]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-atezolizumab-nab-paclitaxel-and-carboplatin-metastatic-nsclc-without-egfralk.
National Comprehensive Cancer Network (NCCN). Non-Small Cell Lung Cancer. Version 3.2020 2020 [cited 2022 March 12]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
Leapman MS, Presley CJ, Zhu W, et al. Association of programmed cell death ligand 1 expression status with receipt of immune checkpoint inhibitors in patients with advanced non-small cell lung cancer. JAMA Netw Open. 2020;3(6): e207205.
Nadler E, Arondekar B, Aguilar KM, et al. Treatment patterns and clinical outcomes in patients with advanced non-small cell lung cancer initiating first-line treatment in the US community oncology setting: a real-world retrospective observational study. J Cancer Res Clin Oncol. 2021;147(3):671–90.
Lester J, Escriu C, Khan S, et al. Retrospective analysis of real-world treatment patterns and clinical outcomes in patients with advanced non-small cell lung cancer starting first-line systemic therapy in the United Kingdom. BMC Cancer. 2021;21(1):515.
O’Connor JM, Fessele KL, Steiner J, et al. Speed of adoption of immune checkpoint inhibitors of programmed cell death 1 protein and comparison of patient ages in clinical practice vs pivotal clinical trials. JAMA Oncol. 2018;4(8): e180798.
Carroll R, Bortolini M, Calleja A, et al. Trends in treatment patterns and survival outcomes in advanced non-small cell lung cancer: a Canadian population-based real-world analysis. BMC Cancer. 2022;22(1):255.
Ma X, Long L, Moon S, Adamson BJS, Baxi SS. Comparison of Population Characteristics in Real-World Clinical Oncology Databases in the US: Flatiron Health, SEER, and NPCR. medRxiv. 2020:2020.03.16.20037143.
Khozin S, Carson KR, Zhi J, et al. Real-World Outcomes of Patients with Metastatic Non-Small Cell Lung Cancer Treated with Programmed Cell Death Protein 1 Inhibitors in the Year Following U.S. Regulatory Approval. Oncologist. 2019;24(5):648–56.
Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–92.
Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med. 2018;379(21):2040–51.
Kehl KL, Yang S, Awad MM, et al. Pre-existing autoimmune disease and the risk of immune-related adverse events among patients receiving checkpoint inhibitors for cancer. Cancer Immunol Immunother. 2019;68(6):917–26.
Chen L, Walker MS, Zhi J, et al. Real-world prevalence of autoimmune disease (AD) among patients (pts) receiving immune checkpoint inhibitors (ICI) in ASCO’s CancerLinQ database. Journal of Clinical Oncology. 2019;37(15_suppl):6583.
Leonardi GC, Gainor JF, Altan M, et al. Safety of programmed death-1 pathway inhibitors among patients with non-small-cell lung cancer and preexisting autoimmune disorders. J Clin Oncol. 2018;36(19):1905–12.
Blinman P, Alam M, Duric V, McLachlan SA, Stockler MR. Patients’ preferences for chemotherapy in non-small-cell lung cancer: a systematic review. Lung Cancer. 2010;69(2):141–7.
Whisenant MS, Williams LA, Garcia Gonzalez A, et al. What Do patients with non-small-cell lung cancer experience? Content domain for the md anderson symptom inventory for lung cancer. JCO Oncol Pract. 2020;16(10):e1151–60.
Islam KM, Anggondowati T, Deviany PE, et al. Patient preferences of chemotherapy treatment options and tolerance of chemotherapy side effects in advanced stage lung cancer. BMC Cancer. 2019;19(1):835.
Landi L, D’Inca F, Gelibter A, et al. Bone metastases and immunotherapy in patients with advanced non-small-cell lung cancer. J Immunother Cancer. 2019;7(1):316.
Cortellini A, Tiseo M, Banna GL, et al. Clinicopathologic correlates of first-line pembrolizumab effectiveness in patients with advanced NSCLC and a PD-L1 expression of ≥ 50. Cancer Immunol Immunother. 2020;69(11):2209–21.
Asano Y, Yamamoto N, Hayashi K, et al. Complete response of bone metastasis in non-small cell lung cancer with pembrolizumab: two case reports. Anticancer Res. 2021;41(3):1693–9.
Acknowledgements
Funding
This study was funded by Regeneron Pharmaceuticals, Inc. Regeneron also funded the journal’s Rapid Service and Open Access Fees.
Medical Writing, Editorial, and Other Assistance
Medical writing support in the preparation of this publication was provided by E. Jay Bienen, PhD, an independent medical writer, and funded by Regeneron Pharmaceuticals, Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Concept and design: Wenzhen Ge, Ning Wu, Ruben G.W. Quek, Jean-Francois Pouliot, Hilary Dietz, Jessica J. Jalbert, James Harnett, Scott J. Antonia. Data analysis: Ning Wu, Jinjie Liu. Drafting of the article: Wenzhen Ge, Ning Wu, Ruben G.W. Quek, Jean-Francois Pouliot, Jinjie Liu, Hilary Dietz, JessicaJ. Jalbert, James Harnett, Scott J. Antonia. Review and final approval of the article: Wenzhen Ge, Ning Wu, Ruben G.W. Quek, Jean-Francois Pouliot. Jinjie Liu, Hilary Dietz, Jessica J. Jalbert, James Harnett, Scott J. Antonia.
Disclosures
Wenzhen Ge, Ning Wu, Ruben G. W. Quek, Jean-Francois Pouliot, Jessica J. Jalbert, and James Harnett are employees and shareholders of Regeneron Pharmaceuticals, Inc. Jinjie Liu is an employee of Genesis Research, which provides consulting services to Regeneron Pharmaceuticals, Inc. Hilary Dietz receives research support from Regeneron Pharmaceuticals, Inc. Scott J. Antonia has provided consulting and advisory support to AstraZeneca, Bristol-Myers Squibb, Merck, CBMG, Memgen, RAPT, Venn, Achilles Therapeutics, Celsius, Samyang Biopharma, GlaxoSmithKline, and Amgen; receives funding support as travel and accommodation expenses from Bristol-Myers Squibb, Merck, Rapt, Achilles Therapeutics, Celsius, GlaxoSmithKline, and Amgen; and receives institutional research funding from Novartis, outside the submitted work.
Compliance with Ethics Guidelines
Institutional Review Board (WCG IRB, Puyallup, WA) approval of the study protocol was obtained before study conduct and included a waiver of informed consent.
Data Availability
The data that support the findings of this study have been originated by Flatiron Health, Inc. and are not publicly available, in order to safeguard the terms that ensure that the data remain deidentified. These de-identified data may be made available upon request and are subject to a license agreement with Flatiron Health; interested researchers should contact DataAccess@flatiron.com to determine licensing terms.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ge, W., Wu, N., Quek, R.G.W. et al. Characterizing the Shifting Real-World Treatment Landscape by PD-L1 Testing Status and Expression Level in Advanced Non-Small Cell Lung Cancer. Adv Ther 39, 4645–4662 (2022). https://doi.org/10.1007/s12325-022-02260-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02260-9