Abstract
Introduction
Statins and fibrates are important means of preventing cardiovascular diseases, particularly when administered in combination as part of various therapeutic strategies. In this study, we explored the risks associated with various combinations of these drugs.
Objective
We aimed to evaluate the risk of 1-year hospitalization with acute kidney injury, liver injury, pancreatitis, or rhabdomyolysis related to the concurrent administration of statins and fibrates.
Methods
We performed a retrospective cohort study using data from the Shizuoka Kokuho Database, focusing on patients prescribed statins, fibrates, or a combination. Four drug exposure patterns were evaluated: adding statins to fibrates (exposure 1), switching from fibrates to statins (exposure 2), adding fibrates to statins (exposure 3), and switching from statins to fibrates (exposure 4). Hospitalization for the specified conditions within 1 year was the outcome. Propensity score matching was used to create balanced cohorts for comparison.
Results
We studied 269,226 statin users and 16,282 fibrate users. After propensity score matching, there were 498 participants in the group of exposure 1, matched with 2988 in the fibrate-only group; 1180 in the group of exposure 2, matched with 7080 in the fibrate-only group; 1183 in group of exposure 3, matched with 11,830 in the statin only group; and 1356 in group of exposure 4, matched with 13,560 in the statin only group. The 1-year hospitalization rate with liver injury was higher in the group of exposure 1 than in the fibrate-only group (1.2% vs 0.3%, p < 0.01), in the group of exposure 2 than in the fibrate-only group (0.9% vs 0.3%, p < 0.01), and in the group of exposure 4 than in the statin-only group (0.6% vs 0.2%, p = 0.02). There was also a higher risk of 1-year hospitalization with acute kidney injury in group of exposure 1 than in the fibrate-only group (1.3% vs 0.3%, p = 0.01) but not in evaluations of exposure 2, 3, and 4. However, there were no differences in the risks of 1-year hospitalization with pancreatitis or rhabdomyolysis among the matched groups.
Conclusions
We have demonstrated higher risks of 1-year hospitalization with liver injury or acute kidney injury associated with the use of combinations of statins and fibrates. This underscores the need for a cautious approach to the prescribing of such drug combinations and the importance of monitoring patients for potential adverse events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The concurrent use of statins and fibrates increases the risk of 1-year hospitalization with liver injury, particularly when a statin is added to a fibrate or when patients are switched from a fibrate to a statin. |
The addition of a statin to a fibrate increases the incidence of 1-year hospitalization with acute kidney injury, but switching from a fibrate to a statin does not. |
There are no differences in the risk of 1-year hospitalization with pancreatitis or rhabdomyolysis when a statin is added to a fibrate or when patients are switched from a fibrate to a statin. |
1 Introduction
Statins and fibrates are effective means of reducing circulating lipid levels, and play vital roles in the primary and secondary prevention of cardiovascular diseases [1]. Statins, which inhibit the 3-hydroxy-3-methylglutaryl-coenzyme A reductase pathway, consistently and substantially lower low-density lipoprotein-cholesterol levels, and thereby substantially reduce the incidence of atherosclerotic events [2,3,4,5]. Numerous trials have demonstrated their efficacy in reducing cardiovascular risk, and they have become established as fundamental tools in dyslipidemia management [6,7,8]. However, previous research has primarily focused on reducing high triglyceride levels, a risk factor that is gaining recognition in cardiovascular disease management [9,10,11]. Fibrates primarily act by activating peroxisome proliferator-activated receptor alpha, triggering metabolic changes that lead to a decrease in circulating triglyceride levels, which is especially beneficial for patients with hypertriglyceridemia [12,13,14].
Lipid-lowering agents are highly effective when used alone, but concerns have been raised regarding the concurrent use of statins and fibrates. The use of both statins and fibrates is associated with well-documented adverse effects, such as myopathy and rhabdomyolysis (statins [15,16,17], fibrates [18, 19]). In particular, an alarming report was published in 2001 regarding 31 patients who experienced fatal rhabdomyolysis while taking cerivastatin, 12 of whom were also taking the fibrate gemfibrozil [20]. As a result, the use of cerivastatin was discontinued in 2001 and the use of combinations of statins and fibrates was prohibited. In 2018, the package inserts for statins and fibrates were revised to state a “contraindication in principle” for their use in patients with laboratory data indicating abnormal renal function. A list of the statins and fibrates that are currently authorized for use in Japan [21], excluding cerivastatin and gemfibrozil, is provided in Table 1 of the Electronic Supplementary Material (ESM).
A multitude of studies have evaluated the safety profile of combinations of statins and fibrates. The ACCORD study [22], a randomized controlled trial of 5518 patients with type 2 diabetes mellitus who were taking simvastatin, showed that its use in combination with fenofibrate posed no additional risk of muscle or liver damage to that associated with monotherapy. Other smaller randomized controlled trials have also been performed [23], and although the authors reported no differences in the incidences of adverse events, the small sample sizes of tens to hundreds of patients might have been insufficient to identify rare adverse events. In addition, numerous observational studies of large databases have been conducted [24, 25], in which patients undergoing combination therapy showed high incidences of rhabdomyolysis, renal impairment, and pancreatitis.
Clinical practice is associated with diverse exposure patterns to combinations of statin and fibrate therapy. The ACCORD study [22] investigated the effects of the addition of a fibrate to pre-existing statin therapy. In addition, there have been several studies of the effects of initiating statin therapy in patients who were already taking a fibrate [26]. Moreover, some patients are switched from one therapy to another, rather than initiating combination therapy, although this has been less frequently studied [27]. Therefore, it is important to thoroughly characterize the effects of a range of drug exposure patterns, in order to assess the safety of the use of combinations of statins and fibrates, a range of which exist in clinical practice.
Thus, in the present study, we evaluated the risks of 1-year hospitalization with acute kidney injury, liver injury, pancreatitis, and rhabdomyolysis in patients who had been prescribed either a statin or a fibrate, particularly when one of these drugs had been added to the existing therapy; and compared these risks with those of monotherapy.
2 Methods
2.1 Data Source
We studied data from the Shizuoka Kokuho Database (SKDB). The SKDB is an administrative claims database for the beneficiaries of the municipal government insurance program (National Health Insurance and Late-stage Medical Care System for older people) in Shizuoka Prefecture. Shizuoka Prefecture is home to approximately 3.6 million residents and is climatically and demographically typical of Japan. The SKDB contains data comprising a regional population-based longitudinal cohort of 2,571,418 individuals who reside in Shizuoka. The data pertaining to all the enrolled individuals were preprocessed, involving thorough cleaning and anonymization [28]. This dataset included basic information regarding the subscriber list (e.g., sex, age, zip code, observation period, and the reason for disenrollment, including death) and claims from public health insurance organizations (via the National Health Insurance system for individuals aged < 75 years and via the Late-stage Elderly Medical Care System for individuals aged > 75 years). The applicability of the SKDB to real-world risk factor analysis is underscored by the inclusion of precise data regarding mortality and follow-up attrition, sourced from the Basic Resident Registration System. The SKDB has been used as a data source in several previous studies. [29,30,31].
2.2 Study Design, Population, and Group Classifications
Figure 1 provides a detailed illustration of the study design. The cohort for the present study was derived from the SKDB and was studied between 1 April, 2012 and 30 September, 2020, which align with the data collection period of the SKDB. The date of enrollment was recorded as either the date of entry into the insurance system covered by the SKDB or 1 April, 2012, whichever occurred later.
Study schema. Cohort entry was defined as the date of registration with the health insurance provider or 1 April, 2012, whichever occurred later. The index data were collected on the day that the participants were first prescribed a statin or a fibrate (the added-statin-to-fibrate group, the added-fibrate-to-statin group, the switched-fibrate-to-statin group, and the switched-statin-to-fibrate group) or 1 year after the date that a statin was first prescribed, according to the database (the statin-only and fibrate-only groups). The follow-up period was defined as the interval between the index date and the first of (1) 1 year after the index date, (2) the end of the study (30 September, 2020), (3) the date of withdrawal from the health insurance system, or (4) the occurrence of an outcome. SKDB Shizuoka Kokuho Database
We studied two distinct groups, one consisting of participants who were prescribed statins and the other comprising those who had been prescribed fibrates during the baseline period. As shown in Fig. 1, the baseline period included the exclusion assessment window, outcome washout window, and covariate assessment window, which was defined as the year prior to the index date for the added-statin-to-fibrate, switched-statin-to-fibrate, added-fibrate-to-statin, and switched-fibrate-to-statin groups; and 1 year after the cohort entry date for the statin-only and fibrate-only groups. The prescriptions issued for each drug were categorized on the basis of the specific drug code (Table 1 of the ESM). Patients aged younger than 18 years at the index date and those who were hospitalized for chronic kidney disease, chronic hepatitis, cirrhosis, liver cancer, pancreatitis, and rhabdomyolysis during the baseline period were excluded from the study cohort. The participants who were prescribed fibrates were placed into one of three groups: those who added a statin to a fibrate, those who were switched from a fibrate to a statin, and those who continued their fibrate. We then compared the added-statin-to-fibrate and the switched-fibrate-to-statin groups with the fibrate-only group.
Similarly, the participants who were prescribed a statin were allocated to three groups: those who added a fibrate to a statin, those who switched from a statin to a fibrate, and those who continued their statin. The added-fibrate-to-statin and switched-statin-to-fibrate groups were compared with the statin only group. Figure 2 illustrates the exposure patterns for each drug in each cohort.
2.3 Inclusion Criteria for Drug Prescription Groups
The inclusion criteria for the added-statin-to-fibrate, switched-fibrate-to-statin, and fibrate-only groups were prescriptions of fibrates issued within the study period and administration for a minimum of 60 days during the preceding 90 days. Similarly, the criteria for the added-fibrate-to-statin, switched statin-to-fibrate, and statin-only groups were new fibrate prescriptions during the study and at least 60 days of statin administration within the preceding 90 days.
For inclusion in the added-statin-to-fibrate or added-fibrate-to-statin groups, the participants required at least 60 days of overlapping prescriptions between the index date and 90 days later. This methodology was based on that of earlier studies of the same database [32, 33] and emulated the design of a clinical trial [34].
2.4 Outcome Measures and Follow-Up Period
The primary outcomes were hospitalization within 1 year with acute kidney injury or liver injury. The secondary outcomes were pancreatitis and rhabdomyolysis. These were identified using the International Classification of Diseases, Tenth Edition or specific disease codes (Table 2 of the ESM), as described previously [25]. The follow-up period began (the index date) on the date of the initial prescription of a combination of a statin and a fibrate, the date of drug switching, or 1 year after the first prescription recorded in the database. This period ended at the earliest of the following: the occurrence of an outcome, the completion of 1 year of follow-up, censoring, death, or the conclusion of the period of coverage of the database.
2.5 Potential Confounders
The potential confounders identified in the study were age, sex, diseases classified according to the Charlson and Elixhauser scores, and the administration of non-statin and non-fibrate medication for dyslipidemia. Drugs approved for the treatment of dyslipidemia in Japan, as listed in Table 1 of the ESM, were considered. The number of days covered by prescriptions for statins or fibrates during the 90 days prior to the index date was included in the analysis as a potential confounder, to account for prevalent user bias.
2.6 Statistical Analysis
The covariate data for each cohort are summarized using means with standard deviations for continuous data and frequencies with percentages for categorical data. A multivariate logistic regression model was used to estimate the propensity scores for each individual, and this included the candidate confounders listed above. Specific covariates were omitted from the modeling process because they were not evaluated. One-to-k matching with a caliper of 0.2 was performed using the propensity scores to construct the cohorts for analysis. K was set to be an integer ≤ 10 after propensity score matching; therefore, the number of participants in each group (the added-fibrate-to-statin, switched-statin-to-fibrate, added-statin-to-fibrate, and switched-fibrate-to-statin groups) was ≥ 90% of the total number of patients before matching. Standardized mean differences of < 10% in the baseline characteristics of the participants among the groups were used to ensure a balanced distribution. Four matched cohorts were created: the added-statin-to-fibrate and fibrate only groups, the switched-fibrate-to-statin and the fibrate-only groups, the added-fibrate-to-statin group and the statin-only groups, and the switched-statin-to-fibrate and the statin-only groups.
Survival time analysis was performed using cumulative incidence curves, with the differences between the two groups being evaluated using the Gray test and death being considered a competing risk. Point estimates and 95% confidence intervals for each cumulative incidence were calculated. Because data regarding covariates were not randomly absent among the participants, simple imputation for the missing data was not performed. P < 0.05 was regarded as indicating statistical significance. Statistical analyses were conducted using EZR Version 1.61 (Saitama Medical Center, Jichi Medical University, Tochigi, Japan) [35] or SAS Version 9.4 (SAS Institute Inc., Cary, NC, USA).
2.7 Ethics
The present study used anonymized data from the SKDB [28]. The Ethical Guidelines for Medical and Biological Research Involving Human Subjects do not require researchers to obtain informed consent from individuals when they use data that have been anonymized. The Ethics Committee of the Shizuoka Graduate University of Public Health approved the study protocol (#SGUPH_2021_001_059).
3 Results
3.1 Composition of the Cohort Prescribed Fibrates and Matching
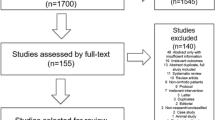
Figure 3 presents a flow diagram illustrating the study cohort. After applying the exclusion criteria, 16,282 patients who were prescribed a fibrate were identified. Of these, 548 were placed into the added-statin-to-fibrate group, 1291 into the switched-fibrate-to-statin group, and 11,212 into the fibrate-only group (Table 1). After propensity score matching, there were 498 participants in the added-statin-to-fibrate group, 2988 in the fibrate-only group, 1180 in the switched-fibrate-to-statin group, and 7080 in the fibrate-only group. Tables 3 and 4 of the ESM list the characteristics of the participants in the fibrate-prescribed groups before and after propensity score matching.
3.2 Statin-Prescribed Cohorts and Matching
Within the baseline period, 269,226 individuals were identified as having been prescribed a statin. In this cohort, the added-fibrate-to-statin group comprised 1197 participants, the switched-statin-to-fibrate group comprised 1387 participants, and the statin-only group, which served as the control group, consisted of 187,614 participants (Table 2). After propensity score matching, there were 1183 and 11,830 participants in the added-fibrate-to-statin group and the statin-only group, respectively; and 1356 and 13,560 participants in the switched-statin-to-fibrate group and the statin-only group, respectively. The characteristics of the statin-prescribed groups, both before and after matching, are listed in Tables 5 and 6 of the ESM.
3.3 Effects of Adding or Switching to a Statin in the Participants Initially Prescribed a Fibrate
Table 7 of the ESM and Table 1 show the frequency of each outcome (acute kidney injury, liver injury, pancreatitis, and rhabdomyolysis) before and after the matching process. Table 1 presents the Gray test results and shows the differences in the cumulative incidences of outcomes in pairs of matched groups. Figure 4 shows the cumulative incidence curves for each outcome for the various groups.
Cumulative incidence curves for the outcomes of the matched cohorts, used to evaluate the effects of adding a statin or switching a fibrate to a statin. To evaluate the effects of adding a statin in participants initially prescribed a fibrate, the cumulative incidence rates for hospitalization within 1 year with a acute kidney injury, b hepatic injury, c pancreatitis, and d rhabdomyolysis are shown. To evaluate the effects of a switch to a statin from a fibrate, the cumulative incidence rates for hospitalization within 1 year with e acute kidney injury, f hepatic injury, g pancreatitis, and h rhabdomyolysis are also shown
There was a difference in the incidence of 1-year hospitalization with hepatic injury between two of the groups prescribed fibrates. An analysis of the matched cohort for the evaluation of the effects of adding a statin to a fibrate revealed six events in the added-statin-to-fibrate group (n = 498) and nine in the fibrate-only group (n = 2988, Table 1), equivalent to 1-year cumulative incidences of 1.2% and 0.3%, respectively (p < 0.01, Fig. 4b). When the switched-fibrate-to-statin group was compared with the fibrate-only group, the event numbers were 10/1180 and 20/7080 (Table 1), equivalent to 1-year cumulative incidences of 0.9% and 0.3%, respectively (p < 0.01, Fig. 4f). In addition, there was a difference in the incidence of 1-year hospitalization with acute kidney injury between the added-statin-to-fibrate (n = 498) and the fibrate-only (n = 2988) groups: there were six and ten events (Table 1) and cumulative incidences of 1.3% and 0.3%, respectively (p = 0.01, Fig. 4a). However, there was no difference in the matched cohort used to evaluate the switch from a fibrate to a statin (Fig. 4e). Furthermore, there were no differences in the 1-year hospitalization of pancreatitis or rhabdomyolysis between the added-statin-to-fibrate and switched-fibrate-to-statin groups (Fig. 4c, d, g, h).
3.4 Effects of Adding or Switching to a Fibrate in the Participants Initially Prescribed a Statin
The characteristics of the participants prescribed a statin before and after matching are shown in Table 8 of the ESM and Table 2. The cumulative incidence curves for each outcome for each of the groups are shown in Fig. 5. In the matched cohort to investigate the effects of switching from a statin to a fibrate, there was a higher incidence of 1-year hospitalization with hepatic injury in the switched-statin-to-fibrate group than in the statin-only group. The groups included 8 (/1356) and 33 (/13,560) events (Table 2), with corresponding 1-year cumulative incidences of 0.6% and 0.2% (p = 0.02, Fig. 5f). However, there were no differences in the incidences of 1-year hospitalization with acute kidney injury, pancreatitis, or rhabdomyolysis between the matched groups (Fig. 5e, g, h). Furthermore, in the matched cohort used to determine the effects of adding a fibrate to a statin, there were no differences in the incidences of 1-year hospitalization with acute kidney injury, liver injury, pancreatitis, or rhabdomyolysis between the two groups (Fig. 5a–d).
Cumulative incidence curves for the outcomes of the matched cohorts, used to evaluate the effects of adding a fibrate or switching a statin to a fibrate. To evaluate the effects of adding a fibrate in participants initially prescribed a statin, the cumulative incidence rates for hospitalization within 1 year with a acute kidney injury, b hepatic injury, c pancreatitis, and d rhabdomyolysis are shown. To evaluate the effects of switching to a fibrate from a statin, the cumulative incidence rates for hospitalization within 1 year with e acute kidney injury, f hepatic injury, g pancreatitis, and h rhabdomyolysis are also shown
4 Discussion
The findings of the present study of the SKDB significantly contribute to existing knowledge regarding the implications of the administration of combinations of statins and fibrates. The higher risk of 1-year hospitalization with liver injury in participants prescribed a fibrate when a statin was added (1.2% vs 0.3%) or when they are switched from a fibrate to a statin (0.9% vs 0.3%) emphasizes the potential hepatotoxic effects of this combination. In addition, the higher risk of 1-year hospitalization with acute kidney injury associated with the addition of a statin to a fibrate (1.3% vs 0.3%) highlights the need for careful consideration before such drug combinations are prescribed. However, we did not identify differences in the incidences of 1-year hospitalization with pancreatitis or rhabdomyolysis, suggesting that the higher risks are specific to liver and kidney injuries. These findings should help guide clinicians toward more informed and safer prescribing practices.
Notably, switching from a statin to a fibrate was associated with a higher risk of 1-year hospitalization with liver injury than continuing statin use alone. Statin-associated side effects are well-documented contributors to the high incidence of treatment discontinuation [36,37,38]. Given that the present study focused on existing statin users, it is conceivable that those patients who were more predisposed toward adverse events may have already discontinued their use. However, the identification of such dropouts within a database presents a challenge, and this scenario represents a form of selection bias known as prevalent user bias [39] that affects the conclusion that switching could be associated with a risk of 1-year hospitalization with liver injury for patients prescribed a statin. Thus, it is difficult to definitively conclude that switching increases the risk of hepatic injury.
For participants prescribed a fibrate, the introduction of a statin was associated with a higher incidence of 1-year hospitalization with acute kidney injury. Fibrates are known to reversibly increase creatinine levels, but they have not previously been reported to increase the risk of renal injury [40, 41]. Furthermore, in several randomized controlled trials, statins have not been demonstrated to impair renal function [42,43,44]. It has often been reported that acute kidney injury induced by statins predominantly occurs in conjunction with rhabdomyolysis. Consequently, it is plausible that a proportion of these instances of acute kidney injury may be attributable to rhabdomyolysis.
This study differs from previous studies with respect to the specific statins and fibrates that were evaluated, as listed in Table 1 of the ESM. Notably, we excluded cerivastatin, which is known to be associated with a high incidence of rhabdomyolysis [45], and gemfibrozil, which is recognized to interact with cerivastatin [46]. As a result, the incidence of muscle-related adverse events identified in the present study, including of rhabdomyolysis, was lower than that previously documented.
The matched cohorts that were created in the present study were designed to emulate a randomized controlled trial [34]. The incidences of the outcomes were calculated using a per-protocol set that comprised participants who were prescribed one of the relevant drugs for a minimum of 60 days within the initial 90-day period. The analysis did not include instances of the discontinuation of drug administration, such as because of death or dropout, within the 90-day exposure study window, because this may have introduced bias into the estimation of the incidences of the outcomes. Furthermore, the follow-up period was limited to 1 year, the shortest duration feasible. However, we believe the associated bias is minimal, owing to our focus on the initial 90-day exposure assessment window and the subsequent application of censoring.
The present study has also shed light on the potential interactions between statins and fibrates. Several study designs [47,48,49] have been used to identify drug–drug interactions, including studies of the interactions between sulfonylureas and co-trimoxazole [47]. It was known that sulfonylureas induce hypoglycemia, unlike co-trimoxazole [47]. In patients who are prescribed sulfonylureas, a higher incidence of hypoglycemia in those who are also administering co-trimoxazole may indicate an interaction between these two drugs. When interpreting the findings of the present study through its design lens, the outcomes obtained when a fibrate is added in patients prescribed a statin might be indicative of no interaction, given that fibrate use is not generally associated with liver injury.
Nevertheless, an analogous interaction was not identified in the present study. When considering outcomes such as acute kidney injury, rhabdomyolysis, or pancreatitis, the identification of interactions is challenging because the administration of a fibrate influences the outcome of the disease. Furthermore, no interactions were identified in participants prescribed a fibrate because statins affect the outcomes [50, 51].
Instead, we discuss here the interactions between statins and fibrates in terms of pharmacokinetics and pharmacodynamics. Pharmacokinetic research has shown that gemfibrozil increases the circulating concentration of statins by inhibiting glucuronidation, which increases the incidence of adverse events [52]. In a randomized crossover study, the combination of cerivastatin and gemfibrozil was shown to increase the amount of cerivastatin absorbed into blood (area under the curve) by 5.6-fold and increase the peak plasma concentration of cerivastatin by approximately three-fold [46]. However, similar pharmacokinetic interactions have not been identified for other fibrates, such as fenofibrate [53,54,55,56], bezafibrate [57], and pemafibrate [58]. These findings are consistent with the results of the present study. Mitochondrial damage has been identified as the primary mechanism underpinning statin-induced liver injury [15], and although the exact mechanism by which fibrates induce hepatic dysfunction remains uncertain, the results of animal studies have suggested that mitochondrial damage might also play a role [59]. Considering this similarity in mechanism, the potential effects of concurrent statin and fibrate use on the incidence of liver injury cannot be disregarded.
These study results reflect current practices in Japan regarding the addition of statins and fibrates or switching between the two for the treatment of dyslipidemia. The findings reveal that a fibrate is also administered in < 1% of patients who are prescribed a statin. Moreover, the data show that only a small number of patients change from administering a statin to administering a fibrate or vice versa. This is probably attributable to the large number of prescriptions issued for ezetimibe and eicosapentaenoic acid, as indicated in Tables 4 and 6 of the ESM. The participants more frequently changed from administering a statin to one of these medications, rather than a fibrate in the present study.
The 90-day period was the same as that used in previous research [59] and is the time frame within which adverse events are typically most prevalent. Beyond 90 days, the continuity of prescriptions could not be assured, which led us to limit the follow-up period to 1 year in an effort to minimize the misclassification of the exposures. Furthermore, because the objective of the study was to assess the clinical effects of the interventions of interest, a sensitivity analysis regarding the study duration was not conducted. We decided that the chosen duration was adequate for our purposes, considering the intentional nature of the study [60].
The present study has several distinct strengths over previous studies, especially observational studies. First, we used the SKDB, a comprehensive database including data relating to a large population, to construct groups of 544–1384 patients reflecting the four exposure patterns. Second, because the SKDB is a medical claims database, it accurately reflects real-world clinical prescribing practices and can provide valuable insight into medication-switching patterns, an aspect of real-world therapy that has not previously been explored in intervention studies. Third, the database includes accurate entries, specific dates of outcomes, and monthly drug prescription records for each individual. This enabled the creation of an analysis cohort, facilitating survival time analyses that took into account both censoring and competing risks.
The present study also had some limitations. First, the definition of outcomes using International Classification of Diseases, Tenth Edition or disease codes lacks specificity. In previous studies [25], diseases were defined using laboratory values, such as a creatine kinase activity > 10 times the upper reference limit for a diagnosis of rhabdomyolysis and an alanine aminotransferase activity > 3 times the upper reference limit for a diagnosis of liver injury. However, in the database used, laboratory data were available only for some of the participants; therefore, we limited the definition to involve the necessity for hospitalization to increase the specificity of the definitions. Second, ideally, we would have employed a new-user design to facilitate more precise causal inferences to be made, but this was challenging because of the limited sample size; therefore, the analysis was conducted in a cohort comprising prevalent users. In our preliminary study, only 394 new patients in the database were found to have started prescribed statins and fibrates simultaneously, a group size that was determined to be insufficient to detect differences in the incidences of adverse events, given that these were all < 1%. Third, the large difference in the lengths of our exposure assessment period (90 days) and observation period (1 year) may limit the ability to ascertain causal relationships between therapy and late adverse events such as liver injury and acute kidney injury. In particular, it is difficult to ascertain causal effects if the therapy was ceased on day 90 and adverse events occurred much later than day 90. Future studies might benefit from treating therapy exposure as a time-dependent variable to assess its impact more accurately. Fourth, the validity of outcome measures used in this study has not been evaluated, as no studies have specifically evaluated the accuracy of the reasons or conditions for hospitalization in the Japanese claims database. Fifth, the study relied on prescription data obtained from an insurance claims database, which may not have accurately represented the use of the medication. Sixth, although the study included participants prescribed six statins and four fibrates with distinct pharmacodynamic and pharmacokinetic properties (Table 1 of the ESM), we did not analyze the individual characteristics of these drugs, but rather concentrated on the effect of the class as a whole. A decision was made not to conduct subgroup analyses because of the small sample size for such a detailed evaluation. Seventh, given the observational nature of the study, there may have been unknown covariates that were not adjusted for, leaving open the potential for confounding. Notably, residual confounding factors such as medication adherence, smoking, history of alcohol consumption for liver disease, exercise history, and trauma related to rhabdomyolysis were not available in the database; these limitations should be considered when interpreting our results. Finally, as we only studied Japanese people aged 18 years and older, our results might not be the same for people of other races and those aged younger than 18 years.
5 Conclusions
By using a comprehensive set of data from the SKDB, we have provided insight into the risk profile associated with the concomitant administration of statins and fibrates. The present findings indicate that there is higher risk of 1-year hospitalization with liver injury when statins are added to fibrate therapy or when fibrate therapy is changed to statin therapy. These imply that existing prescribing protocols should be re-evaluated. While our findings suggest a potential increase in the 1-year risk of hospitalization because of liver injury when switching from fibrate to statin therapy, it is important to note that these results may be influenced by prevalent user bias, indicating the need for further research. Moreover, the higher risk of 1-year hospitalization with acute kidney injury associated with the addition of statins to fibrate treatment regimens emphasizes the necessity for meticulous individualized treatment approaches. Although the present study did not show higher risks of 1-year hospitalization with pancreatitis or rhabdomyolysis, the additional risks associated with liver and kidney injury merit clinical attention. These outcomes emphasize the importance of rigorous monitoring alongside the use of these lipid-lowering agents in combination.
References
Dembowski E, Davidson MH. A review of lipid management in primary and secondary prevention. J Cardiopulm Rehabil Prev. 2009;29:2–12.
Blumenthal RS. Statins: effective antiatherosclerotic therapy. Am Heart J. 2000;139:577–83.
Rosenson RS, Tangney CC. Antiatherothrombotic properties of statins. JAMA. 1998;279:1643–50.
Rosenson RS. Statins in atherosclerosis: lipid-lowering agents with antioxidant capabilities. Atherosclerosis. 2004;173:1–12.
Farnier M, Davignon J. Current and future treatment of hyperlipidemia: the role of statins. Am J Cardiol. 1998;82:3J-10J.
Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78.
Cholesterol Treatment Trialists’ (CTT) Collaborators. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–90.
Armitage J, Baigent C, Barnes E, Betteridge DJ, Blackwell L, Blazing M, et al. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407–15.
Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626–35.
Schwartz GG, Abt M, Bao W, DeMicco D, Kallend D, Miller M, et al. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J Am Coll Cardiol. 2015;65:2267–75.
Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, et al. Triglycerides and the risk of coronary heart disease. Circulation. 2007;115:450–8.
Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, et al. Helsinki heart study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. N Engl J Med. 1987;317:1237–45.
Katsiki N, Nikolic D, Montalto G, Banach M, Mikhailidis DP, Rizzo M. The role of fibrate treatment in dyslipidemia: an overview. Curr Pharm Des. 2013;19:3124–31.
Ida S, Kaneko R, Murata K. Efficacy and safety of pemafibrate administration in patients with dyslipidemia: a systematic review and meta-analysis. Cardiovasc Diabetol. 2019;18:38. https://doi.org/10.1186/s12933-019-0845-x.
Averbukh LD, Turshudzhyan A, Wu DC, Wu GY. Statin-induced liver injury patterns: a clinical review. J Clin Transl Hepatol. 2022;10:543–52.
Omar MA, Wilson JP, Cox TS. Rhabdomyolysis and HMG-CoA reductase inhibitors. Ann Pharmacother. 2001;35:1096–107.
Thompson PD. Statin-associated myopathy. JAMA. 2003;289:1681.
Zhou J, Li D, Cheng Q. Fenofibrate monotherapy-induced rhabdomyolysis in a patient with post-pancreatitis diabetes mellitus. Medicine. 2020;99: e20390.
Wu J, Song Y, Li H, Chen J. Rhabdomyolysis associated with fibrate therapy: review of 76 published cases and a new case report. Eur J Clin Pharmacol. 2009;65:1169–74. https://doi.org/10.1007/s00228-009-0723-7.
Charatan F. Bayer decides to withdraw cholesterol lowering drug. BMJ. 2001;323:359.
Pharmaceuticals and Medical Devices Agency. Medical drug approval information. https://www.pmda.go.jp/review-services/drug-reviews/review-information/p-drugs/0019.html. Accessed 28 Dec 2023.
ACCORD Study Group, Ginsberg HN, Elam MB, Lovato LC, Crouse JR 3rd, Leiter LA, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–74.
Zhao S, Wang F, Dai Y, Lin L, Tong Q, Liao Y, et al. Efficacy and safety of fenofibrate as an add-on in patients with elevated triglyceride despite receiving statin treatment. Int J Cardiol. 2016;221:832–6.
Graham DJ, Staffa JA, Shatin D, Andrade SE, Schech SD, La Grenade L, et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA. 2004;292:2585–90.
Enger C, Gately R, Ming EE, Niemcryk SJ, Williams L, McAfee AT. Pharmacoepidemiology safety study of fibrate and statin concomitant therapy. Am J Cardiol. 2010;106:1594–601.
Gavish D, Leibovitz E, Shapira I, Rubinstein A. Bezafibrate and simvastatin combination therapy for diabetic dyslipidaemia: efficacy and safety. J Intern Med. 2000;247:563–9.
Meas T, Laloi-Michelin M, Virally M, Peynet J, Giraudeaux V, Kévorkian JP, et al. Switching fibrate to statin in type 2 diabetic patients: consequences on lipid profile. Eur J Intern Med. 2009;20:197–200.
Nakatani E, Tabara Y, Sato Y, Tsuchiya A, Miyachi Y. Data Resource Profile of Shizuoka Kokuho Database (SKDB) using integrated health- and care-insurance claims and health checkups: the Shizuoka Study. J Epidemiol. 2022;32:391–400.
Miyakoshi A, Nakatani E, Kaneda H, Hawke P, Sasaki H, Urano T, et al. Administration of tranexamic acid after burr hole craniotomy reduced postoperative recurrence of chronic subdural hematoma in a Japanese regional population. Neurosurgery. 2023;93:1160–7.
Ubukata N, Nakatani E, Hashizume H, Sasaki H, Miyachi Y. Risk factors and drugs that trigger the onset of Stevens-Johnson syndrome and toxic epidermal necrolysis: a population-based cohort study using the Shizuoka Kokuho database. JAAD Int. 2023;11:24–32.
Hashizume H, Nakatani E, Sasaki H, Miyachi Y. Hydrochlorothiazide increases risk of nonmelanoma skin cancer in an elderly Japanese cohort with hypertension: the Shizuoka Study. JAAD Int. 2023;12:49–57.
Saito K, Sato Y, Nakatani E, Kaneda H, Yamamoto S, Miyachi Y, et al. Statin exposure and pancreatic cancer incidence: a Japanese regional population-based cohort study, the Shizuoka study. Cancer Prev Res. 2021;14:863–72.
Funaki D, Kaneda H, Miyakoshi A, Saito K, Sasaki H, Nakatani E. Identification of subgroups within a Japanese older adult population for whom statin therapy is effective in reducing mortality. PLoS One. 2023;18: e0295052.
Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183:758–64.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Bytyçi I, Penson PE, Mikhailidis DP, Wong ND, Hernandez AV, Sahebkar A, et al. Prevalence of statin intolerance: a meta-analysis. Eur Heart J. 2022;43:3213–23.
Gislason GH, Rasmussen JN, Abildstrøm SZ, Gadsbøll N, Buch P, Friberg J, et al. Long-term compliance with beta-blockers, angiotensin-converting enzyme inhibitors, and statins after acute myocardial infarction. Eur Heart J. 2006;27:1153–8.
Tomida J, Yoshida T, Senda S, Sato T, Nakatsuma A, Iihara N. Statin persistence and adherence among older initiators: a nationwide cohort study using the national health insurance claims database in Japan. Pharmacoepidemiol Drug Saf. 2023;32:873–85.
Danaei G, Tavakkoli M, Hernán MA. Bias in observational studies of prevalent users: lessons for comparative effectiveness research from a meta-analysis of statins. Am J Epidemiol. 2012;175:250–62.
Ansquer J-C, Dalton RN, Caussé E, Crimet D, Le Malicot K, Foucher C. Effect of fenofibrate on kidney function: a 6-week randomized crossover trial in healthy people. Am J Kidney Dis. 2008;51:904–13.
Zhao YY, Weir MA, Manno M, Cordy P, Gomes T, Hackam DG, et al. New fibrate use and acute renal outcomes in elderly adults: a population-based study. Ann Intern Med. 2012;156:560–9.
Su X, Zhang L, Lv J, Wang J, Hou W, Xie X, et al. Effect of statins on kidney disease outcomes: a systematic review and meta-analysis. Am J Kidney Dis. 2016;67:881–92.
Haynes R, Lewis D, Emberson J, Reith C, Agodoa L, Cass A, et al. Effects of lowering LDL cholesterol on progression of kidney disease. J Am Soc Nephrol. 2014;25:1825–33.
Hsia J, MacFadyen JG, Monyak J, Ridker PM. Cardiovascular event reduction and adverse events among subjects attaining low-density lipoprotein cholesterol <50 mg/dl with rosuvastatin. J Am Coll Cardiol. 2011;57:1666–75.
Evans M, Rees A. The myotoxicity of statins. Curr Opin Lipidol. 2002;13:415–20.
Backman JT, Kyrklund C, Neuvonen M, Neuvonen PJ. Gemfibrozil greatly increases plasma concentrations of cerivastatin. Clin Pharmacol Ther. 2002;72:685–91.
Juurlink DN. Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA. 2003;289:1652.
Leonard CE, Bilker WB, Brensinger CM, Flockhart DA, Freeman CP, Kasner SE, et al. Comparative risk of ischemic stroke among users of clopidogrel together with individual proton pump inhibitors. Stroke. 2015;46:722–31.
Schelleman H, Bilker WB, Brensinger CM, Han X, Kimmel SE, Hennessy S. Warfarin with fluoroquinolones, sulfonamides, or azole antifungals: interactions and the risk of hospitalization for gastrointestinal bleeding. Clin Pharmacol Ther. 2008;84:581–8.
Bhardwaj SS, Chalasani N. Lipid-lowering agents that cause drug-induced hepatotoxicity. Clin Liver Dis. 2007;11:597–613.
Athyros VG, Papageorgiou AA, Hatzikonstandinou HA, Didangelos TP, Carina MV, Kranitsas DF, et al. Safety and efficacy of long-term statin-fibrate combinations in patients with refractory familial combined hyperlipidemia. Am J Cardiol. 1997;80:608–13.
Prueksaritanont T, Richards KM, Qiu Y, Strong-Basalyga K, Miller A, Li C, et al. Comparative effects of fibrates on drug metabolizing enzymes in human hepatocytes. Pharm Res. 2005;22:71–8.
Bergman AJ, Murphy G, Burke J, Zhao JJ, Valesky R, Liu L, et al. Simvastatin does not have a clinically significant pharmacokinetic interaction with fenofibrate in humans. J Clin Pharmacol. 2004;44:1054–62.
Pan W-J, Gustavson LE, Achari R, Rieser MJ, Ye X, Gutterman C, et al. Lack of a clinically significant pharmacokinetic interaction between fenofibrate and pravastatin in healthy volunteers. J Clin Pharmacol. 2000;40:316–23.
Goosen TC, Bauman JN, Davis JA, Yu C, Hurst SI, Williams JA, et al. Atorvastatin glucuronidation is minimally and nonselectively inhibited by the fibrates gemfibrozil, fenofibrate, and fenofibric acid. Drug Metab Dispos. 2007;35:1315–24.
Martin PD, Dane AL, Schneck DW, Warwick MJ. An open-label, randomized, three-way crossover trial of the effects of coadministration of rosuvastatin and fenofibrate on the pharmacokinetic properties of rosuvastatin and fenofibric acid in healthy male volunteers. Clin Ther. 2003;25:459–71.
Kyrklund C. Plasma concentrations of active lovastatin acid are markedly increased by gemfibrozil but not by bezafibrate. Clin Pharmacol Ther. 2001;69:340–5.
Hounslow N, Suganami H, Nakamura M. Pemafibrate minimally affected the systemic exposure of statins, and vice versa, in healthy male volunteers. Atheroscler Suppl. 2018;32:156–7.
Qu B, Li Q-T, Wong KP, Tan TMC, Halliwell B. Mechanism of clofibrate hepatotoxicity: mitochondrial damage and oxidative stress in hepatocytes. Free Radic Biol Med. 2001;31:659–69.
Stürmer T, Wang T, Golightly YM, Keil A, Lund JL, Jonsson FM. Methodological considerations when analysing and interpreting real-world data. Rheumatology. 2020;59:14–25.
Acknowledgements
We thank Mark Cleasby, PhD from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The funding for this work was provided by Shizuoka Prefecture. The Shizuoka Graduate University of Public Health conducts contract research projects regarding public health in Shizuoka Prefecture, including the current study.
Conflict of Interest
Yohei Sobukawa, Taichi Hatta, Daito Funaki, and Eiji Nakatani have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
The Ethics Committee of the Shizuoka Graduate University of Public Health approved the study protocol (#SGUPH_2021_001_059).
Consent to Participate
We anonymized the data from the SKDB. Japanese medical ethics guidelines do not require informed consent be obtained for this type of research.
Consent for Publication
Not applicable.
Data Availability
According to the terms of Shizuoka Prefecture’s data use agreement with local insurers, the analyzed data cannot be provided to readers by the authors. Researchers interested in accessing this dataset may apply to Shizuoka Prefecture to request access. Please contact the staff of Shizuoka Graduate University of Public Health (e-mail: info@s-sph.ac.jp).
Code Availability
Not applicable.
Author Contributions
YS conceived the study. YS, EN, and TH designed the study. YS performed the formal statistical analysis. YS, EN, and TH interpreted the data. DF prepared the resources, such as the master codes, for this study. YS wrote the first draft of the manuscript. YS, EN, DF, and TH contributed to the critical revision of the manuscript. All the authors have read and approved the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Sobukawa, Y., Hatta, T., Funaki, D. et al. Safety of Combined Statin and Fibrate Therapy: Risks of Liver Injury and Acute Kidney Injury in a Cohort Study from the Shizuoka Kokuho Database. Drugs - Real World Outcomes (2024). https://doi.org/10.1007/s40801-024-00426-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s40801-024-00426-1