Abstract
Background and Objective
Iron deficiency is the most common cause of anemia. We compared the effect of ferric carboxymaltose (FCM), low-dose intravenous (IV) iron (LDI), and iron sucrose on total cost of care in patients with iron-deficiency anemia (IDA) from a US health plan perspective.
Methods
We conducted a retrospective claims analysis using the IQVIA PharMetrics Plus database. Patients with index (first) claims of FCM and LDI and a medical claim associated with IDA between 1 January 2017 and 31 December 2019 were included. Monthly total healthcare and inpatient and outpatient costs after receiving index IV iron for patients in the treatment cohorts were compared using a generalized linear model with gamma distribution and log-link.
Results
The overall study cohort included 37,655 FCM, 44,237 LDI, and 27,461 iron sucrose patients. Mean per-patient-per-month numbers of IV iron infusions for FCM, LDI, and iron sucrose were 0.20, 0.34, and 0.37, respectively. Compared with baseline, the FCM group had greater reductions in the number of hospital admissions and smaller increases in the number of outpatient visits in the 12 months post-IV iron therapy than LDI and iron sucrose, translating to significantly lower total healthcare cost (post-index adjusted cost ratio for total cost: 0.96 and 0.92, respectively; both P < 0.0001).
Conclusions
Higher drug acquisition cost of FCM relative to LDI and iron sucrose was offset by significantly lower inpatient and outpatient costs in the 12 months post-IV iron therapy. These results support the economic value of FCM for patients with IDA receiving IV iron therapy.
Plain Language Summary
Iron deficiency is one of the most common causes of anemia. Patients with iron deficiency anemia (IDA) may require IV iron replacement therapy. This study was a retrospective claims analysis that utilized medical and pharmacy claims from the IQVIA PharMetrics Plus database. We found that ferric carboxymaltose (FCM), a high-dose formulation of IV iron that delivers up to 1500 mg per course of treatment, was associated with lower inpatient and outpatient costs than low-dose IV iron formulations (LDI) in the 12 months following treatment, offsetting its higher drug acquisition cost relative to LDI. Analysis of subgroups with chronic conditions (cancer, chronic kidney disease, and heart failure) showed greater levels of cost reductions with use of FCM than in the overall study cohort. Findings from this real-world analysis are consistent with previous studies, indicating that FCM was a cost-effective treatment option for IDA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Iron deficiency anemia is often undertreated and associated with high economic burden. |
This study showed ferric carboxymaltose (FCM) was associated with lower inpatient and outpatient cost than low-dose iron formulations (LDI) and iron sucrose, offsetting its higher drug acquisition cost and resulted in lower total healthcare resource utilization in the 12 months after treatment. |
This study demonstrates the economic value of FCM and supports its coverage for the treatment of IDA. |
1 Introduction
Iron deficiency is one of the most common causes of anemia. Iron is needed for many cellular functions, including erythropoiesis, and the resulting reduction in hemoglobin (Hgb) concentration due to the deficiency of iron may lead to symptoms such as fatigue, dyspnea, headache, vertigo, and worsening of comorbid diseases that are commonly associated with iron-deficiency anemia (IDA) [1, 2]. IDA may affect a patient’s quality of life (QOL) if left untreated [3]. There are several chronic diseases, including cancer, heart failure (HF), and chronic kidney disease (CKD), in which IDA may present as a comorbidity [4].
In patients with cancer, iron deficiency with and without anemia occurs in 42% of patients with solid or hematologic malignancies [5]. IDA is a frequent complication at both diagnosis and during treatment [2]. The bioavailability of iron is often reduced due to the inflammatory process of cancer or treatment with chemotherapy [2]. Iron deficiency in patients with cancer leads to the onset or exacerbation of preexisting anemia with an array of clinical consequences as well as a worsening of QOL [2]. Current treatment guidelines recommend monitoring iron and iron replacement in patients with cancer [2]. The goal of treatment in these patients is to increase iron while reducing the need for blood transfusions, which are associated with an increased risk of infections [2].
In patients with HF, the prevalence of IDA is approximately 50% and it is associated with an increased risk of hospitalization and mortality [6]. Cardiac tissue is sensitive to the reduced iron availability, and IDA is hypothesized to impact HF pathophysiology [7,8,9]. The 2022 American Heart Association/American College of Cardiology/Heart Failure Society of America (AHA/ACC/HFSA) guidelines recommend IV iron replacement for patients with HF and iron deficiency, with or without anemia, to improve QOL and exercise capacity [10]. Due to poor absorption and inadequate repletion of iron stores, oral iron supplements are not adequate to treat IDA in patients with HF [10].
IDA is also a frequent complication in patients with CKD [3, 11]. With their deteriorating kidney function, medications, and dietary restrictions, patients may develop iron deficiency, which reduces iron supply to the bone marrow [3]. The prevalence of IDA increases with the severity of kidney disease, with approximately 50% of patients with CKD diagnosed with anemia. Many patients with CKD, especially those receiving hemodialysis, may require additional iron, usually administered by infusion [3]. If left untreated, IDA in patients with CKD affects their responsiveness to erythropoiesis-stimulating agents.
IDA is associated with an increased economic burden [12]. The goal for treating the underlying physiological cause of IDA with supplemental iron is to normalize Hgb concentrations, replenish iron stores, and improve QOL, symptoms, and prognosis of chronic diseases [4]. In addition to addressing the underlying causes of IDA, intravenous (IV) iron therapy is often required for patients with IDA who have chronic diseases and those who are not responsive to or who cannot tolerate oral iron [4, 5, 8, 10]. Current IV iron therapies available in the USA include high-dose IV iron formulations such as ferric carboxymaltose (FCM), ferumoxytol, and ferric derisomaltose, and low-dose IV iron (LDI) formulations such as iron sucrose, iron dextran, and sodium ferric gluconate complex in sucrose [13]. High-dose IV iron formulations allow patients to receive more iron with fewer infusions than LDI and have been shown to allow more patients to achieve normal serum Hgb levels than LDI [1].
Due to the price differential between high-dose IV iron formulations and LDI, data on the cost consequences of IV iron therapy would be informative to population health decision-makers as they manage healthcare budgets and make formulary decisions. The objective of this study was to compare the effect of FCM and low-dose IV iron therapy on the total cost of care in patients with IDA from a US commercial health plan perspective.
The choice of using FCM as the main product was made because FCM was the most commonly used high-dose IV iron in the USA during the study design and data collection period. The prevalence of FCM use in real-world clinical practices made the study findings more applicable to a broader patient population and made inferences about specific subpopulations of patients.
2 Methods
2.1 Study Design and Participants
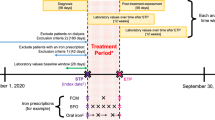
This retrospective claims analysis utilized medical and pharmacy claims from the IQVIA PharMetrics Plus database (IQVIA, Durham, NC, USA), from 01/01/2017 to 31/12/2019. IQVIA PharMetrics Plus is a longitudinal health plan database comprising deidentified medical and pharmacy claims data among commercially insured patients and a small portion of Medicare and Medicaid patients in the USA [14]. Data in the database are deidentified and compliant with the Health Insurance Portability and Accountability Act. Patients with an index claim of FCM or LDI (including iron sucrose, iron dextran, sodium ferric gluconate complex in sucrose) between 1 January 2017 and 31 December 2019 were included (Online Resource Supplementary Fig. 1). The index date was the date of their first claim for IV iron. Patients were required to have continuous enrollment for ≥ 6 months before the index date and ≥ 12 months after the index date and at least one medical claim associated with IDA (ICD10: D50.x) during the study observation period. Patients who had a medical claim for CKD stage 5 (ICD10, N18.5) and end-stage renal disease (N18.6) or dialysis [Healthcare Common Procedure Coding System Current Procedural Terminology (HCPCS CPT) 90935–90999] during the 6-month period before the index date were excluded.
Patients treated with FCM were compared with a cohort of patients treated with LDI, which included iron sucrose, iron dextran, and sodium ferric gluconate complex in sucrose. Additionally, a subanalysis was completed in which patients treated with iron sucrose were extracted from the LDI cohort for a comparison with FCM. Patients in the FCM cohort were required to receive a second dose of FCM within 21 days of the index date. Patients were excluded in the FCM cohort if they had received other IV iron infusions during the 6-month period before their index date. Patients were excluded in the LDI and iron sucrose cohorts if they had received FCM, ferumoxytol, or other IV iron infusions during the 6-month period before their index date. Ferric derisomaltose was not available in the USA during the study period.
Analyses were performed in the overall population and in subgroups of patients with cancer, HF, and CKD. In the cancer subgroup, patients were required to have a medical claim associated with a cancer diagnosis (ICD10: C00.X–C96.X) in the 6-month period before their index date, respectively. Similarly, in the HF subgroup, patients were required to have a medical claim associated with a HF diagnosis (ICD10: I50.1–I50.9) in the 6-month period before their index date, respectively. The CKD subgroup included patients with a medical claim associated with a CKD diagnosis (ICD10: N18.1–N18.5) in the 6 months prior to index IV infusion. These patient subgroups were not mutually exclusive.
2.2 Outcomes and Statistical Analysis
Healthcare resource use data were summarized on a per-patient-per-month (PPPM) basis for the FCM group and compared with the LDI and iron sucrose groups using t-tests and logistic regression analysis. Descriptive statistics were used to summarize the differences in monthly total healthcare cost before and after index IV iron therapy. Because cost data are skewed, monthly healthcare costs after receiving index IV iron were compared using a generalized linear model with gamma distribution and log-link function [15]. The patient costs were divided into the following five mutually exclusive groups: inpatient, emergency room (ER), outpatient (IV iron infusion and related costs), outpatient other costs (excluding IV iron infusion and related costs), and pharmacy oral. The adjusted cost ratio post-index analysis was conducted for total cost, inpatient cost, and outpatient other cost. Covariates included in the monthly total cost regression models were the pre-index monthly total healthcare cost, patient age and gender, year of treatment index, and Charlson Comorbidity Index (CCI). In the cancer subgroup, the National Cancer Institute (NCI) Comorbidity Index was used instead of the CCI. Covariates included in the monthly inpatient cost and monthly outpatient cost regression models were similar except pre-index monthly total healthcare cost was replaced by pre-index monthly inpatient cost and pre-index monthly outpatient cost, respectively. To assess the robustness of the study results, three sensitivity analyses were performed to compare the costs for FCM versus LDI and iron sucrose. In the propensity score–weighting sensitivity analysis, monthly total healthcare cost before index, patient age and gender, year of treatment index date, and CCI (or NCI Comorbidity Index for the cancer subgroup) were used to estimate propensity scores, and generalized linear model with inverse probability of treatment weights of propensity scores were fitted for cost ratios. In the matched baseline total cost sensitivity analysis, only FCM and LDI/iron sucrose patients with similar baseline total cost were included in the analysis. The pre-index period was defined as the 6 months prior to the patient’s index date. The third sensitivity analysis used a generalized linear model with gamma distribution and log-link function comparing monthly total healthcare cost, inpatient cost, and outpatient cost of patients who had received at least one dose of FCM compared with the singular dose that patients received among the LDI group. Each sensitivity analysis aimed to serve different purposes, with the goal of enhancing the validity of the study findings. Propensity score weighting was used to help control for selection bias [16]. The generalized linear model with gamma distribution and log-link function was used to help address non-normality of cost data and provide more accurate estimates among skewed distributions. Analyses were conducted using SAS 9.4 software.
3 Results
The overall study cohort included 37,655 patients treated with FCM and 44,237 treated with LDI, of which 27,461 were treated with iron sucrose (Online Resource Supplementary Tables 1 and 2). Patients in the FCM cohort were slightly older, with a mean age of 51.3 years compared with 48.4 and 47.9 years, respectively, for the LDI and iron sucrose cohorts. Most patients were female, making up 81, 82, and 82% for FCM, LDI, and iron sucrose, respectively. Mean (SD) CCI was highest in the FCM cohort at 0.42 (1.21) and similar for the LDI and iron sucrose cohorts [0.36 (1.15) and 0.36 (1.18), respectively]. In general, the overall study cohort included younger patients and a higher proportion of female patients than the cancer, HF, and CKD subgroups. Patients in the overall study cohort also had lower comorbidities burden (as measured by CCI) compared with patients in the HF and CKD subgroups (Online Resource Supplementary Table 2).
Healthcare resource utilization is summarized on a PPPM basis in Online Resource Supplementary Table 3. Post-index mean number of IV iron infusions PPPM were lowest for FCM (0.20) compared with LDI (0.34) and iron sucrose (0.37). Compared with the pre-index period, the number of outpatient visits PPPM increased slightly in the post-index period for all cohorts, with FCM having the smallest increase in number of outpatient visits (+ 0.02) versus LDI (+ 0.16) and iron sucrose (+ 0.18). The proportion of patients having hospital admissions increased in the post-index period in all treatment groups compared with the pre-index period, with FCM having the smallest increase among all treatment groups. There was a greater reduction in the mean number of hospital admission days in the post-index period from baseline in the FCM group (− 0.06) than in the LDI (− 0.01) and iron sucrose (− 0.01) groups. Utilization of emergency room visits was low in both the pre-index and post-index period in all treatment groups, with marginal differences between treatment groups (Online Resource Supplementary Table 2).
Unadjusted mean monthly total cost post-index was lowest for those in the FCM cohort at $2684 PPPM compared with $2840 PPPM and $3016 PPPM for LDI and iron sucrose, respectively (Fig. 1). Iron sucrose had the largest increase in costs from pre- to post-index ($371 PPPM), while FCM had the smallest increase in costs ($314 PPPM). After controlling for covariates, post-index total cost (including IV iron) was lower for FCM versus LDI (adjusted cost ratio, 0.96; P < 0.0001) and lower for FCM versus iron sucrose (adjusted cost ratio, 0.92; P < 0.0001). Adjusted cost ratio results showed that post-index inpatient and outpatient costs for FCM were 20 and 5% lower than LDI, respectively (P < 0.0001). Adjusted post-index inpatient and outpatient costs were also 20 and 8% lower for FCM versus iron sucrose, respectively (P < 0.0001) (Fig. 1).
PPPM cost categories 6 months before and 12 months after index IV iron, FCM versus LDI and iron sucrose for all patients. Statistical comparison using generalized linear model adjusting for pre-index cost, age, gender, Charlson Comorbidity Index, and treatment index year. The bars at the far right represent the difference between the first and second set of bars, for overall cost, and by cost category. ACR adjusted cost ratio, ER emergency room, FCM ferric carboxymaltose, IV intravenous, LDI low-dose IV iron (iron sucrose, iron dextran, sodium ferric gluconate complex in sucrose), PPPM per-patient-per-month
3.1 Cancer Subgroup
The subgroup of patients with cancer included data from 7829 patients treated with FCM and 6899 patients treated with LDI, with 4086 of those patients having been treated with iron sucrose (Online Resource Supplementary Table 1). Gender distribution (female: 64% for FCM and LDI and 63% for iron sucrose) and mean age were similar among the three cohorts. Mean (SD) NCI Comorbidity Index was similar among the FCM cohort at 0.52 (0.6), LDI cohort at 0.56 (0.6), and iron sucrose cohort at 0.58 (0.6) (Online Resource Supplementary Table 1). At 12 months post-index, FCM had the lowest PPPM number of IV iron infusions (0.21) versus LDI (0.35) and iron sucrose (0.39), respectively (Online Resource Supplementary Table 2). FCM had a greater decrease in the PPPM number of inpatient admission days versus LDI and iron sucrose from the pre- to the post-index period (mean difference, pre- versus post-index, − 0.11 versus − 0.04 and − 0.04, respectively). There was a smaller increase in the mean number of outpatient visits from the pre- to the post-index period in the FCM group versus LDI and iron sucrose (Online Resource Supplementary Table 2). Unadjusted mean monthly total cost post-index was lowest for FCM at $5596 PPPM versus LDI and iron sucrose at $6367 PPPM and $6820 PPPM, respectively (Fig. 2). FCM had the smallest increase in costs from pre- to post-index ($684 PPPM) and iron sucrose had the largest increase in costs ($999 PPPM). The adjusted cost ratio for post-index total cost was significantly lower for FCM versus both LDI (0.89) and iron sucrose (0.84) (P < 0.0001). Adjusted post-index inpatient and outpatient costs were 21 and 12% lower for FCM versus LDI, respectively (P < 0.0001). Similar levels of reduction were observed for FCM compared with iron sucrose (Fig. 2).
PPPM cost categories 6 months before and 12 months after index IV iron, FCM versus LDI and iron sucrose for patients with cancer. Statistical comparison using generalized linear model adjusting for pre-index cost, age, gender, NCI Comorbidity Index, and treatment index year. The bars at the far right represent the difference between the first and second set of bars, for overall cost, and by cost category. ACR adjusted cost ratio, ER emergency room, FCM ferric carboxymaltose, IV intravenous, LDI low-dose IV iron (iron sucrose, iron dextran, sodium ferric gluconate complex in sucrose), NCI National Cancer Institute, PPPM per-patient-per-month
3.2 Heart Failure Subgroup
Data were analyzed from 3002 patients with HF treated with FCM and 3641 treated with LDI, with 2403 of those patients having been treated with iron sucrose (Online Resource Supplementary Table 1). Most patients were female (57, 56, and 55% for FCM, LDI, and iron sucrose, respectively). Mean age was slightly older for the FCM than the LDI and iron sucrose cohorts (69.4, 67.0, and 67.2 years, respectively). Mean (SD) CCI was highest for FCM at 1.9 (2.5), followed by LDI at 1.7 (2.5) and iron sucrose at 1.6 (2.5) (Online Resource Supplementary Table 1). Mean PPPM number of IV iron infusions was lowest for those treated with FCM (0.25) versus those treated with LDI (0.40) and iron sucrose (0.44) (Online Resource Supplementary Table 2). In patients treated with FCM, there was a decrease in number of outpatient visits versus those treated with LDI and iron sucrose from the pre-index to the post-index period (mean difference PPPM, pre- versus post-index, 0.03 versus 0.54 and 0.66, respectively). Similarly, the FCM cohort had a decrease in number of inpatient admission days versus LDI and iron sucrose during the post-index period (mean difference PPPM, pre- versus post-index, − 0.12 versus 0.05 and 0.05, respectively) (Online Resource Supplementary Table 2). Unadjusted mean monthly total cost post-index was lowest for FCM at $4081 PPPM versus LDI and iron sucrose ($5804 PPPM and $5691, respectively) (Fig. 3). Unadjusted mean monthly inpatient and outpatient costs were lowest for FCM as well. The adjusted cost ratio post-index for total cost was 0.78 for FCM versus LDI (P < 0.0001) and 0.75 for FCM versus iron sucrose (P < 0.0001). FCM had the smallest increase in costs from pre- to post-index ($40 PPPM), while LDI and iron sucrose had similar increases ($404 and $415 PPPM, respectively). The adjusted cost ratios for inpatient and outpatient costs revealed a 34% reduction in inpatient cost and a 23% reduction in outpatient cost for FCM versus LDI, and a 31% reduction in inpatient cost and a 27% reduction in outpatient cost for FCM versus iron sucrose (all P < 0.0001) (Fig. 3).
PPPM cost categories 6 months before and 12 months after index IV iron, FCM versus LDI and iron sucrose in patients with HF. Statistical comparison using generalized linear model adjusting for pre-index cost, age, gender, Charlson Comorbidity Index, and treatment index year. The bars at the far right represent the difference between the first and second set of bars, for overall cost, and by cost category. ACR adjusted cost ratio, ER emergency room, FCM ferric carboxymaltose, IV intravenous, LDI low-dose IV iron (iron sucrose, iron dextran, sodium ferric gluconate complex in sucrose), PPPM per-patient-per-month
3.3 Chronic Kidney Disease Subgroup
Data were analyzed from 3727 patients with CKD treated with FCM and 5068 treated with LDI, with 3489 of those patients having been treated with iron sucrose (Online Resource Supplementary Table 1). Most patients were female (61% in the FCM and 60% in the LDI and iron sucrose cohorts). Patients in the FCM cohort were slightly older [67.9 versus 64.8 (LDI) and 64.6 (iron sucrose) years]. Mean (SD) CCI was highest for those patients treated with FCM at 1.9 (2.5), followed by both LDI and iron sucrose [1.6 (2.4) and 1.6 (2.4), respectively] (Online Resource Supplementary Table 1). Mean PPPM number of IV iron infusions was lowest for the FCM cohort versus the LDI and iron sucrose cohorts post-index (0.24 versus 0.43 and 0.46, respectively) (Online Resource Supplementary Table 2). Unadjusted mean monthly total cost post-index was lowest for those in the FCM cohort at $3660 PPPM, followed by $4804 PPPM for LDI and $4916 for iron sucrose (Fig. 4). FCM had the lowest increase in costs from pre- to post-index ($478 PPPM) compared with LDI ($763 PPPM) and iron sucrose ($725 PPPM). Similarly, unadjusted costs were lowest for the FCM cohort for inpatient and outpatient costs. Adjusted post-index cost ratios showed significantly lower total costs for the FCM cohort versus LDI (0.81; P < 0.0001) and iron sucrose (0.79, P < 0.0001). The adjusted cost ratio was significantly lower for the FCM cohort for inpatient and outpatient costs as well, with a 27% reduction for both inpatient and outpatient costs versus LDI, and a 26% reduction in inpatient cost and a 30% reduction in outpatient cost versus iron sucrose (all P < 0.0001) (Fig. 4).
PPPM cost categories 6 months before and 12 months after index IV iron, FCM versus LDI and iron sucrose in patients with CKD. Statistical comparison using generalized linear model adjusting for pre-index cost, age, gender, Charlson Comorbidity Index, and treatment index year. The bars at the far right represent the difference between the first and second set of bars, for overall cost, and by cost category. ACR adjusted cost ratio, ER emergency room, FCM ferric carboxymaltose, IV intravenous, LDI low-dose IV iron (iron sucrose, iron dextran, sodium ferric gluconate complex in sucrose), PPPM per-patient-per-month
3.4 Sensitivity Analysis
The results of the three sensitivity analyses were generally consistent with the main analyses (Table 1), confirming a generally significant reduction in monthly post-index healthcare costs in the FCM group versus the LDI and iron sucrose groups. The results of the three sensitivity analyses were similar to the main analyses for the cancer, HF, and CKD subgroups, confirming statistically significant lower monthly total, inpatient, and outpatient costs in the FCM group versus the LDI and iron sucrose groups during the 12 months after initiating IV iron therapy (Table 1).
When patients receiving at least one dose of FCM were included in the overall study cohort analysis, post-index monthly total cost in the FCM group was similar to LDI, with the upper bound of the 95% confidence interval of the adjusted cost ratio reaching 1.0 (Table 1). However, post-index monthly inpatient and outpatient costs remained significantly lower in the FCM group versus LDI and iron sucrose. Adjusted cost ratios derived from the propensity score weighting method provided more conservative estimates of the level of cost reduction, while results from the matched baseline cost analysis provided similar results as the main analysis. Using the propensity score weighting method, post-index monthly inpatient cost of FCM was similar to LDI and iron sucrose in the overall study cohort analysis. Monthly outpatient cost during the 12 months after index IV iron treatment for FCM was also similar to LDI in the overall study cohort analysis under the propensity score weighting method (Table 1). Distribution of propensity score weights for each comparison are presented in Online Resource Supplementary Fig. 2.
4 Discussion
This retrospective claims analysis compared healthcare resource utilization and cost of care in patients treated with FCM with those treated with LDI or iron sucrose in the 12 months after initiating IV iron therapy. This analysis showed that patients receiving FCM had a lower total monthly cost compared with patients treated with LDI and those treated with iron sucrose. Greater levels of cost reductions were observed in the subgroup analyses of specific chronic conditions than in the overall study cohort. Patients with HF receiving FCM had the highest level of reduction in post-index monthly total cost (22%) followed by the CKD subgroup (19%), cancer subgroup (11%), and the overall study cohort (4%) versus LDI. Similar levels of reduction in the FCM group were found when compared with iron sucrose. These results suggest that despite the higher drug acquisition cost of FCM compared with LDI, the price differential is offset by reductions in inpatient and outpatient costs in the 12 months after receiving IV iron therapy.
The economic benefit of iron supplementation to reduce the economic burden of IDA is well documented in the literature [17]. Several studies have also demonstrated the incremental economic benefit of IV iron therapy over oral iron therapy in patients with specific medical conditions [18, 19]. However, few studies have compared the economic benefits between IV iron formulations. IV iron formulations that provide more iron per administration may help to address potential adherence issues in real-world settings by requiring fewer return visits to receive sufficient iron repletion. This hypothesis is supported by the results of two real-world analyses that showed that higher-dose iron formulations can be more effective in managing anemia. LaVallee et al found that use of high-dose IV iron formulations for the initial course of treatment resulted in fewer outpatient visits and fewer retreatments [13]. In a retrospective analysis of integrated electronic health records and claims data in the USA, 77% of patients had an iron deficit of > 750 mg. Patients receiving FCM were found to be 5 times and 17 times as likely to receive full iron repletion compared with ferumoxytol and LDI formulations, respectively [20]. While the choice of iron therapy did not vary by iron deficit levels prior to treatment, FCM was associated with a reduced number of outpatient visits in the 12 months following treatment compared with ferumoxytol and LDI formulations. The current study corroborates these findings to support reduced healthcare resource utilization from IV iron formulations that provide more iron per administration.
IDA is common in patients with chronic diseases. Patients with chronic diseases are older, have more comorbidities, and utilize more healthcare resources than most patients receiving IV iron therapy. Previous studies have shown that IV iron therapy reduced the need for blood transfusions in patients with cancer, reduced cardiovascular-related hospitalizations in patients with HF, and reduced the need for erythropoietin-stimulating agent (ESA) by improving response to ESA in patients with CKD [21,22,23,24,25]. Previous assessments of the economic implications of IV iron therapy in these patient populations were limited to economic modeling or studies with small sample sizes outside of the USA [18, 26,27,28,29]. These model studies have limited generalizability to US payers, given the major differences in delivery and financing of healthcare between the USA and other countries. The current study filled this gap by analyzing claims data on the cost consequences of IV iron therapy in a large sample of patients from a US payer perspective. Within this analysis, we found that patients receiving FCM had lower inpatient cost and lower outpatient cost than patients receiving LDI. The magnitude of reductions in total cost of care was greater in patients with cancer, HF, and CKD receiving FCM versus LDI compared with the overall IV iron patient population, suggesting a higher unmet need for iron repletion in patients with chronic diseases. Patients with chronic diseases also have a greater challenge in achieving target Hgb levels than otherwise healthy patients [1, 30,31,32]. High-dose IV iron formulations may increase the likelihood of receiving full iron replacement in patients with chronic disease and reduce subsequent healthcare resource use. Further research in patients with chronic diseases is warranted.
This study had several limitations. During the development of the study, certain factors such as patient region and insurance type were not taken into consideration as possible covariates. Both region and insurance type can affect choice of treatment and utilization [33]. However, the exclusion of insurance type as a variable is justified given that PharMetrics data comprise mostly commercially insured patients. Therefore, we did not believe it would be a significant factor when explaining the cost differences in the analysis. Claims data are also subject to coding errors, missing data, and variations in reporting across clinical practices. The use of concomitant over-the-counter iron replacement therapies is not captured within claims data and cannot be accounted for. This study used a retrospective cohort design to indirectly compare the economic outcomes of patients treated with IV iron formulations. We tried to improve the robustness of our study findings by performing sensitivity analyses to complement results from the main analyses; however, potential bias resulting from unobservable differences between study cohorts cannot be ruled out. Without accompanying medical information, we are unable to definitively confirm whether all healthcare expenditures and resource utilization are attributable to a specific drug or diagnosis. Generalizability of the study may be limited given the time at which the study was conducted. Additionally, continuous enrollment was an inclusion criterion, which is a common approach to database studies to improve internal validity; it is conceivable that excluding those patients without continuous enrollment impacted the external validity of the study. Follow-up for patients who initiated their index treatment in 2019 included claims from 2020, which may have been impacted by the coronavirus disease 2019 (COVID-19) pandemic. Due to the reduction in healthcare resource utilization during that time, costs and resourcing from 2019 index patients may be lower than expected. Finally, in addition to inpatient and outpatient categories, we also looked into the ER and oral pharmacy categories. Adjusted cost ratio results showed post-index oral pharmacy costs for FCM were lower than LDI, but not statistically significant for cancer, HF, and CKD subgroups. Costs incurred at the ER made up only 1% of all categories. Due to these reasons, we decided to only focus on analyses of cost accrued in the inpatient and outpatient settings, as they were the highest cost categories.
5 Conclusions
Patients receiving FCM had significantly lower monthly total healthcare cost versus patients receiving LDI or iron sucrose in the 12 months following IV iron treatment. The higher drug acquisition cost for FCM versus LDI was offset by lower inpatient and outpatient costs following IV iron therapy. This real-world study mirrors findings from previous studies and supports the economic value of FCM for patients receiving IV iron therapy.
References
LaVallee C, Cronin P, Bansal I, Kwong WJ, Boccia R. Importance of initial complete parenteral iron repletion on hemoglobin level normalization and health care resource utilization: a retrospective analysis. Pharmacotherapy. 2019;39:983–93.
Naoum FA. Iron deficiency in cancer patients. Rev Bras Hematol Hemoter. 2016;38:325–30.
Mikhail A, Brown C, Williams JA, Mathrani V, Shrivastava R, Evans J, et al. Renal association clinical practice guideline on anaemia of chronic kidney disease. BMC Nephrol. 2017;18:345.
Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet. 2016;387:907–16.
Ludwig H, Muldur E, Endler G, Hubl W. Prevalence of iron deficiency across different tumors and its association with poor performance status, disease status and anemia. Ann Oncol. 2013;24:1886–92.
McEwan P, Ponikowski P, Davis JA, Rosano G, Coats AJS, Dorigotti F, et al. Ferric carboxymaltose for the treatment of iron deficiency in heart failure: a multinational cost-effectiveness analysis utilising AFFIRM-AHF. Eur J Heart Fail. 2021;23:1687–97.
Maeder MT, Khammy O, dos Remedios C, Kaye DM. Myocardial and systemic iron depletion in heart failure implications for anemia accompanying heart failure. J Am Coll Cardiol. 2011;58:474–80.
Haddad S, Wang Y, Galy B, Korf-Klingebiel M, Hirsch V, Baru AM, et al. Iron-regulatory proteins secure iron availability in cardiomyocytes to prevent heart failure. Eur Heart J. 2017;38:362–72.
Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J. 2013;34:816–29.
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. J Am Coll Cardiol. 2022;79:1757–80.
Pergola PE, Fishbane S, Ganz T. Novel oral iron therapies for iron deficiency anemia in chronic kidney disease. Adv Chronic Kidney Dis. 2019;26:272–91.
Park YJ, Lim HS, Kim TH. Annual prevalence, health expenditures, and co-morbidities trend of iron deficiency anemia in Korea: National Health Insurance Service Data from 2002 to 2013. Int J Environ Res Public Health. 2020;17:4433.
LaVallee C, Bansal I, Kamdar S, Kwong WJ, Boccia RV. Relationship between initial parenteral iron therapy dosing and treatment effectiveness: a real-world retrospective analysis. J Blood Med. 2022;13:133–42.
IQVIA PharMetrics(R) Plus fact sheet. 2022 [cited 26 January 2024]; Available from: https://www.iqvia.com/locations/united-states/library/fact-sheets/iqvia-pharmetrics-plus.
Diehr P, Yanez D, Ash A, Hornbrook M, Lin DY. Methods for analyzing health care utilization and costs. Annu Rev Public Health. 1999;20:125–44.
Olmos A, Govindasamy P. A practical guide for using propensity score weighting in R. Pract Assess Res Eval. 2019;20:13.
Rakanita Y, Syamsunarno M, Sinuraya RK, Suradji EW, Abdulah R, Suwantika AA. Cost-effectiveness of ferrous fumarate-folic acid and ferrous gluconate-multivitamins in a high prevalence area of iron deficiency anemia in Indonesia. Ther Clin Risk Manag. 2021;17:1075–81.
Wong G, Howard K, Hodson E, Irving M, Craig JC. An economic evaluation of intravenous versus oral iron supplementation in people on haemodialysis. Nephrol Dial Transplant. 2013;28:413–20.
Ray S, Neogi SB, Singh R, Devasenapathy N, Zodpey S. Is IV iron sucrose a cost-effective option for treatment of severe anaemia in pregnancy as compared with oral iron? Health Policy Plan. 2021;35:1339–46.
LaVallee C, Cronin P, Bansal I, Kwong WJ, Boccia RV. Effectiveness of parenteral iron therapy in the real-world setting: a retrospective analysis. J Clin Haematol. 2020;1:16–25.
Gafter-Gvili A, Rozen-Zvi B, Vidal L, Leibovici L, Vansteenkiste J, Gafter U, et al. Intravenous iron supplementation for the treatment of chemotherapy-induced anaemia—systematic review and meta-analysis of randomised controlled trials. Acta Oncol. 2013;52:18–29.
Dangsuwan P, Manchana T. Blood transfusion reduction with intravenous iron in gynecologic cancer patients receiving chemotherapy. Gynecol Oncol. 2010;116:522–5.
Anker SD, Kirwan BA, van Veldhuisen DJ, Filippatos G, Comin-Colet J, Ruschitzka F, et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis. Eur J Heart Fail. 2018;20:125–33.
Macdougall IC, White C, Anker SD, Bhandari S, Farrington K, Kalra PA, et al. Intravenous iron in patients undergoing maintenance hemodialysis. N Engl J Med. 2019;380:447–58.
Macdougall IC. Intravenous iron therapy in patients with chronic kidney disease: recent evidence and future directions. Clin Kidney J. 2017;10:i16–24.
Rognoni C, Ortalda V, Biasi C, Gambaro G. Economic evaluation of ferric carboxymaltose for the management of hemodialysis patients with iron deficiency anemia in Italy. Adv Ther. 2019;36:3253–64.
Aiello A, Berto P, Conti P, Panichi V, Rosati A. Economic impact of ferric carboxymaltose in haemodialysis patients. Gior Ital Nefrol. 2020;37(Suppl 75):2020-S75.
Calvet X, Gené E, ÀngelRuíz M, Figuerola A, Villoria A, Cucala M, et al. Cost-minimization analysis favours intravenous ferric carboxymaltose over ferric sucrose or oral iron as preoperative treatment in patients with colon cancer and iron deficiency anaemia. Technol Health Care. 2016;24:111–20.
Cirillo L, Somma C, Allinovi M, Bagalà A, Ferro G, Di Marcantonio E, et al. Ferric carboxymaltose vs. ferrous sulfate for the treatment of anemia in advanced chronic kidney disease: an observational retrospective study and cost analysis. Sci Rep. 2021;11:7463.
Joosten E, Lioen P. Iron deficiency anemia and anemia of chronic disease in geriatric hospitalized patients: how frequent are comorbidities as an additional explanation for the anemia? Geriatr Gerontol Int. 2015;15:931–5.
Clark SF. Iron deficiency anemia. Nutr Clin Pract. 2008;23:128–41.
Zaninetti C, Klersy C, Scavariello C, Bastia R, Balduini CL, Invernizzi R. Prevalence of anemia in hospitalized internal medicine patients: Correlations with comorbidities and length of hospital stay. Eur J Intern Med. 2018;51:11–7.
Institute of Medicine. America’s uninsured crisis: consequences for health and health care. Washington, DC: The National Academies Press; 2009.
Acknowledgements
Medical writing assistance, funded by Daiichi Sankyo, was provided by Peloton Advantage, LLC, an OPEN Health company, under the direction of the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Daiichi Sankyo.
Conflict of interest
Kevin Wang is an employee of Daiichi Sankyo, Inc., Basking Ridge, NJ. Winghan Jacqueline Kwong and Peng Wang are former employees of Daiichi Sankyo, Inc. Ralph Boccia has been a consultant to AbbVie, Daiichi Sankyo/American Regent, and Bristol Myers Squibb and has served on speakers bureaus for AbbVie, Daiichi Sankyo/American Regent, Bristol Myers Squibb, Amgen, Incyte, Genentech, AstraZeneca, Regeneron, and Sanofi. Medical writing assistance, funded by Daiichi Sankyo, was provided by Peloton Advantage, LLC, an OPEN Health company, under the direction of the authors.
Availability of data and material
The database used to conduct this study is available from IQVIA with a licensing agreement (https://www.iqvia.com/solutions/real-world-evidence/real-world-data-and-insights). Sharing of data supporting the results reported in this article may be considered upon request by contacting kwang@dsi.com.
Ethics approval
This retrospective claims analysis utilized medical and pharmacy claims from the IQVIA PharMetrics Plus database (IQVIA, Durham, NC, USA), from 01/01/2017 to 31/12/2019. Data in the database are deidentified and compliant with the Health Insurance Portability and Accountability Act.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
Jackie Kwong, Kevin Wang, and Peng Wang designed the study. Jackie Kwong and Ralph Boccia served as study investigators. Kevin Wang collected, assembled, and analyzed the data. All authors interpreted the data, prepared the manuscript, reviewed and revised the manuscript, and provided final approval for publication.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kwong, W.J., Wang, K., Wang, P. et al. Effect of Ferric Carboxymaltose Versus Low-Dose Intravenous Iron Therapy and Iron Sucrose on the Total Cost of Care in Patients with Iron Deficiency Anemia: A US Claims Database Analysis. Drugs - Real World Outcomes 11, 251–261 (2024). https://doi.org/10.1007/s40801-024-00418-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40801-024-00418-1