Abstract
Background
CT-P13 is an infliximab biosimilar that was granted market authorization in Switzerland in 2016. Despite the growing literature supporting the equivalence of CT-P13 compared with originator infliximab regarding the efficacy, safety, and immunogenicity and the undeniable cost-saving opportunities, CT-P13 remains widely underused in Switzerland.
Objective
Leaving aside the phenomenon of a low initiation rate, this study aimed to explore the reasons behind the high discontinuation rate observed among the patients taking CT-P13 in a large tertiary hospital in Western Switzerland.
Methods
We performed a retrospective cohort study using routinely collected data. Patients were eligible if they received originator infliximab or CT-P13 between September 2017 and December 2020. They were included if they had received at least two CT-P13 infusions during the same period. Patients were excluded if the follow-up was incomplete prior to or 6 months after their first CT-P13 infusion and if they had an oncological main diagnosis. Primary outcomes were the reasons for treatment discontinuation.
Results
One hundred and fifty-six patients were included and classified into two groups: switchers who were treated with originator infliximab and were switched to CT-P13 (n = 85, 54%) and initiators who did not receive originator infliximab prior to CT-P13 treatment (n = 71, 46%). Included patients belonged to three different groups of diagnosis: gastroenterological (67, 43%), rheumatological (61, 39%), and immunological (28, 18%). Twenty-three (27%) switchers and 35 (49%) initiators discontinued CT-P13 after 12 months. Main reasons for CT-P13 discontinuation were lack of efficacy (n = 21, 36%) and secondary loss of response (n = 16, 28%); however, objective assessments were not available. Initiators’ probability to discontinue CT-P13 at 12 months was significantly higher than switchers’ (p < 0.01).
Conclusions
Lack of efficacy and secondary loss of response were the main reasons for the high CT-P13 discontinuation rate observed in a large tertiary hospital in Western Switzerland. Lack of active training and coordination among healthcare professionals and little education in patients may have exacerbated patients’ subjective complaints and increased the CT-P13 discontinuation rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In the hospital, coordination between the various healthcare professionals involved with the patient is a prerequisite for biosimilars to achieve their maximum potential. |
The lack of formal guidelines regarding biosimilars and the lack of education for healthcare professionals and patients may exacerbate patient-reported adverse events and/or lack of efficacy following biosimilar transition. |
1 Introduction
Biological drugs (i.e., biologics) include a multitude of specialties with a wide range of active agents. They are created by biotechnology from a living system such as a bacterium, a plant, or an animal cell [1]. Biologics have revolutionized the treatment of a wide range of diseases, including oncology and disabling autoimmune diseases such as rheumatoid arthritis (RA) and inflammatory bowel disease (IBD) [2,3,4]. Because of their effectiveness, the new opportunities for patients’ management and their considerable development costs, pharmaceutical companies have been charging higher prices for biologics than for “traditional” drugs [5, 6].
The global biologics market in 2020 was estimated at ~ US$302 billion, which represented around 24% of the world’s pharmaceutical sales [7,8,9]. In Switzerland, biologics were also responsible for ~ 23% of overall drug sales [10]. In both cases, the market is projected to grow exponentially within the 5 following years [11,12,13].
This craze for biologics has been putting pressure on government health systems and has limited the accessibility of these innovative products to a large number of patients, particularly in emerging countries [3, 5]. The arrival of biosimilars has made it possible to considerably increase the number of patients benefiting from these innovative therapies while sparing the paying parties [12,13,14].
Biosimilars are biologics that are highly similar to a reference biologic (i.e., originator) already on the market, with whom they share no clinically significant differences [1, 15, 16]. Just like generics, biosimilars can only be registered on the market after the expiration of the originator’s patent [1, 17]. In Switzerland, the price of a biosimilar has to be at least 25% lower than the originator’s to be considered economical and therefore reimbursed by basic health insurance [18].
Infliximab is a murine chimeric monoclonal antibody that acts as a tumor necrosis factor-α (TNFα) inhibitor. Infliximab has been one of the first biologics approved for reimbursement for the treatment of RA, psoriatic arthritis, ankylosing spondylitis (AS), and IBD in Switzerland. It is administered via intravenous infusions, mainly in a hospital setting [19, 20]. As with other biologics in Switzerland, the initiation of treatment with infliximab is subject to a second opinion from the patient’s insurance physician.
In 2016, CT-P13 entered the Swiss market as the first infliximab biosimilar, offering promising cost-saving opportunities [21]. Despite an average price per treatment day that is 33% lower, TNFα inhibitor biosimilars occupied only 16% of the TNFα inhibitor market in Switzerland in 2020, half the European market average [22] .
To date, the exact reasons for the low market share of TNFα inhibitor biosimilars n Switzerland are still uncertain. On the one hand, there is a low initiation rate where new patients start their treatment with originator infliximab (OI) instead of CT-P13 and do not want to switch for CT-P13. On the other hand, patients who started with CT-P13, or were switched from OI, may decide to discontinue their treatment because of various reasons [11, 13, 22]. Both phenomena could be explained by: residual doubts regarding the equivalence of CT-P13 in terms of efficacy, security, and immunogenicity compared to OI; negatively biased information that may deter providers and patients from biosimilar use; and the lack of Swiss economic incentives aimed at promoting biosimilar use [2, 13, 23]. In the literature, there have been no post-marketing surveillance trials that reported any safety issues with CT-P13 and a multitude of studies have been ensuring equity of CT-P13 for all indications in a multitude of patient populations, compared to OI [4, 24,25,26,27,28,29]. A recent systematic review with 90 studies and over 14,000 patients suggested that secondary loss of response and adverse events (AEs) linked to CT-P13 use were the main reasons for its discontinuation [4].
Differences regarding the safety, efficacy or immunogenicity between CT-P13 and OI will not be addressed in the present study as their equivalence was extensively demonstrated for all indications in rigorous randomized clinical trials [25,26,27, 30,31,32], assessed in a plethora of real-world studies [24, 33], and is supported by the US Food and Drug Administration and the European Medicines Agency [34, 35]. Hence, this retrospective cohort study aims to explore the reasons for CT-P13 discontinuation in patients who were treated with OI and were switched to CT-P13 (switchers), and in patients who did not receive OI prior to CT-P13 treatment (initiators) in a large tertiary hospital of western Switzerland. To our knowledge, this is the first retrospective study exploring the reasons for CT-P13 discontinuation in a large tertiary hospital of western Switzerland after a transition from OI to CT-P13.
2 Methods
This study was written in accordance with the REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement [36].
2.1 Study Design and Setting
CT-P13 was introduced into the drug formulary of a large tertiary care hospital in the canton of Vaud, Switzerland, at the end of September 2017. The introduction of CT-P13 into the hospital formulary was supervised by the hospital’s Pharmaceutical and Therapeutic Committee. The Pharmaceutical and Therapeutic Committee oversaw the supply of CT-P13 through the hospital’s pharmacy department, while instructing senior healthcare professionals (HCPs) to pass on information to their respective teams regarding the transition from OI to CT-P13 in daily care. Communication regarding CT-P13 took place orally during departmental colloquia without a formal and uniform protocol being established. To our knowledge, no active education has been provided to the HCPs before, during, or after the introduction of CT-P13 and no official follow-up was implemented, whether patients were switched from OI to CT-P13 or initiated with CT-P13. In this context, we performed a retrospective cohort study using routinely collected medical data from inpatients and outpatients who received OI and/or CT-P13 between 1 September, 2017 and 31 December, 2020. Data of interest were extracted in January 2021 by the hospital’s DataScience team after validation of our request by the hospital’s good research practice department. Information strictly necessary for the study was then collected anonymously.
2.2 Participants
Eligible patients received at least one infusion of OI or CT-P13 between 1 September, 2017 and 31 December, 2020. Two authors independently included the patients in the study following the inclusion and exclusion criteria listed in Table 1. There were no age-based criteria as OI and CT-P13 treatments cover both juvenile and senior diseases. Patients from the oncology department were excluded because OI was administered primarily for autoimmune symptoms caused by their underlying oncology treatment.
2.3 Variables and Data Sources/Measurements
Each variable of interest was manually retrieved using a protocol for data collection available in Electronic Supplementary Material (ESM). Classifications of patients who initiated treatment with OI and were switched to CT-P13 (switchers) and patients who did not receive OI prior to CT-P13 treatment (initiators) were performed by two different sets of authors. When both authors could not reach an agreement for a particular patient, a third author acted as an arbitrator. Primary outcomes were the reasons for treatment discontinuation (RTDs). When the RTD was not explicit, or not objectively assessed, time of exposure (TOE) to a treatment was used to decide between lack of efficacy [LOE] (TOE < 180 days) and secondary loss of response (TOE > 180 days). Other RTDs involved AEs, ambulatory relay, acute systemic reaction, pregnancy, and remission. When no information was available, the RTD was marked as unknown. The rest of the variables’ definitions and methods of assessment are available in Table 1 of the ESM.
2.4 Statistical Methods
Median patient age and time to CT-P13 discontinuation were analyzed using non-parametric methods (i.e., Wilcoxon rank sum and Log-rank tests, respectively). Additional analyses, available in the ESM, assessed the association between the number of biologics tried prior to CT-P13 treatment and CT-P13 discontinuation, by diagnosis groups and patient status (i.e., initiator or switcher). This association was modeled using logistic regression analyses and analyzed using the non-parametric likelihood ratio, chi-square test, and Fisher’s exact test. All analyses were performed using R [37].
Missing data occurred when the reasons for stopping CT-P13 treatment were not explicitly available. This problem was addressed by using the TOE to CT-P13 as explained above.
3 Results
3.1 Participants
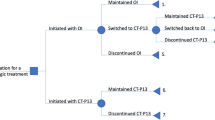
A total of 308 patients either received an OI or CT-P13 infusion between 1 September, 2017 and 31 December, 2020 and were thus eligible for the study. One hundred and fifty-two patients (49%) were excluded following the inclusion and exclusion criteria listed in Table 1. Details regarding the excluded patients are listed in the flow diagram in Fig. 1. Among the 156 remaining patients who were included, 85 (55%) started their treatment with OI and switched to CT-P13 (switchers) and 71 (45%) were naive to OI before their treatment with CT-P13 (initiators). Their primary conditions are detailed in Table 2 below, by diagnosis group. Initiators were younger than switchers (age 36 vs 51 years, p < 0.01) and a majority of the patients were female (51%). Additional analyses by diagnosis group are available in Chapter III of the ESM.
3.2 Main Results
All diagnoses included, a significant proportion of switchers (n = 23, 27%) and initiators (n = 35, 49%) discontinued CT-P13 at 12 months. Among them, switchers mainly reverted to OI (n = 15, 65%) while initiators switched for a different treatment (n = 32, 91%). Initiators’ had a higher probability to discontinue CT-P13 at 12 months (p < 0.01, Fig. 2). The main reasons for discontinuing CT-P13 were similar in both groups: LOE (n = 8, 35% and n = 13, 37%), secondary loss of response (n = 8, 35% and n = 8, 23%), and AEs (n = 3, 23% and n = 5, 14%), respectively. There were also five (14%) initiators who discontinued CT-P13 because of an acute systemic reaction occurring during one of the first CT-P13 infusions (Table 3). As expected, the types of AEs and their frequency were consistent with the manufacturer’s official information [38].
Kaplan–Meier plot showing the proportion of patients who discontinued CT-P13 over 360 days, by status. Both continuous and dotted heavy lines represent the median function curves. Both shaded areas represent the interquartile range. The black dotted line at 0.50 indicates when 50% of the initiators discontinued CT-P13 (~ 330 days)
3.2.1 Switchers
Among the 15 switchers who reverted to OI, three experienced AEs while the rest felt a LOE or a loss of response after the switch to CT-P13. Regarding the three patients who experienced AEs, there was one gastroenterological (GAS) patient who had a highly itchy dermatitis after his first CT-P13 infusion, one rheumatological (RHE) patient who experienced migraine, asthenia, and dizziness after his third infusion, and one immunological (IMM) patient who had a chill syndrome, burning sensations on the skin, and asthenia after his fourth infusion. However, none of these patients experienced any of the symptoms caused by their respective disease after the switch to CT-P13. The 12 remaining switchers reverted to OI because they experienced a LOE or a secondary loss of response, which resulted in a flare, or a marked worsening of the symptoms linked to their disease after the switch to CT-P13. These changes in disease activity were not accompanied by any objective clinical assessment (e.g., diagnostic test results).
Eight other switchers discontinued CT-P13 but were switched to a different treatment. Two GAS patients became pregnant and were switched to certolizumab, one RHE patient had to suspend CT-P13 because of dental procedures and resumed his treatment with golimumab. Another RHE patient had a very strong immune response after the first CT-P13 infusion (hot flashes, difficulty in breathing, drop in systolic blood pressure, and anti-CT-P13 antibodies over 200 ng/mL) and was also switched to golimumab. The last four patients changed treatment because they expressed an early LOE after starting CT-P13 or a resurgence of their disease-related symptoms.
3.2.2 Initiators
Out of the 32 initiators who switched for a different treatment, one RHE and one GAS patient experienced AEs after the fourth and fifth CT-P13 infusions, respectively. The RHE patient had to switch to golimumab because of a CT-P13-induced hepatitis and the GAS patient stopped biologics altogether to remain only taking 5-aminosalicylic acid because of an uncontrolled dermatitis. Three additional patients (one from each diagnosis group) later discontinued CT-P13 after their sixth and eighth infusions because of non-specific allergic symptoms such as urticaria.
Five other patients discontinued CT-P13 because they experienced immunological reactions of various severity: one GAS and one RHE patient had facial edema, erythema, and pruritus after the second CT-P13 infusion; one GAS patient had a cutaneous flush-type reaction with respiratory distress after the third CT-P13 infusion; and one GAS and one RHE patient experienced an anaphylactic reaction with oxygen desaturation (down to 88%) after the fourth CT-P13 infusion.
4 Discussion
Lack of efficacy, secondary loss of response, and AEs were the three main reasons for CT-P13 discontinuation in switchers and initiators in this tertiary hospital of Western Switzerland. As stated before, differences regarding the safety, efficacy, or immunogenicity between CT-P13 and OI will not be addressed in the discussion.
4.1 Treatment Discontinuation Rate
Real-world observational studies and open-label extension studies tend to report increased discontinuation rates in both switchers and initiators compared with randomized clinical trials [24,25,26,27, 30,31,32,33]. In our study, discontinuation rates were even higher than most of the real-world observational studies reported in the literature.
Switchers’ discontinuation rate in our study (27%) was above the values reported in the NOR-SWITCH trial, as well as in the PLANETAS and PLANETRA open-label extension studies, whose methodologies have been described elsewhere [25,26,27]. The trial and the open-label extension studies investigated the switch from OI to CT-P13 over 48–52 weeks among patients with AS, chronic plaque psoriasis, Crohn’s disease, ulcerative colitis, psoriatic arthritis, RA, and spondyloarthritis. The reported discontinuation rates for switchers were 7.5%, 10.4%, and 11.1%, respectively [25,26,27]. Our switchers’ discontinuation rate was also higher than almost every value reported in a systematic review of over 100 real-world observational studies assessing the switch from OI to CT-P13, across all above indications [33]. Overall, 3–260 switchers were included in the review, with a follow-up of 12–54 weeks. In a selection of IBD studies with 33–101 patients and 26–56 weeks of follow-up, only one study reported a switchers’ discontinuation rate that reached 25% [39]. The remaining studies reported rates from 5.4 to 15% [40,41,42,43,44,45,46]. In another selection from the same systematic review, studies regarding AS, psoriatic arthritis, RA, and spondyloarthritis with 59–768 switchers and a follow-up between 26 and 56 weeks, reported discontinuation rates that ranged from 6.7% to 28% for patients switching from OI to CT-P13 [24, 47,48,49,50,51,52].
Regarding initiators, their discontinuation rate (49%) was well above the values reported for the initiators in the aforementioned trials and in most of the retrieved real-world observational studies found in the literature, but also when compared with the values reported for OI initiators. First, the PLANETAS and PLANETRA original trials assessed CT-P13 treatment in 125 AS and 302 RA initiators over 54 weeks and reported discontinuation rates of 15.2% and 22.8%, respectively [30, 31]. Then, regarding observational studies, only a handful of studies with disparate designs could be retrieved. Hence, the use of CT-P13 in initiators was assessed in 22–204 patients with AS, IBD (Crohn’s disease and/or ulcerative colitis), RA, and spondyloarthritis with follow-ups that ranged from 33 to 156 weeks. Reported discontinuation rates for initiators ranged from 4.2 to 62.8% [42, 49, 53,54,55,56,57]. As for a comparison with OI initiators, five major systematic reviews could be retrieved in the literature [58,59,60,61,62]. The reviews included tens to thousands of patients with AS, RA, psoriasis, and IBD in various settings, with a 12- to 60-month follow-up. The discontinuation rate of the initiators of the present study fell into the upper range of the reviews’ reported results (from ~ 20% for clinical trials to ~ 50% for observational studies, at 12 months).
4.2 Reasons for Treatment Discontinuation
In the aforementioned randomized clinical trials and observational studies, AEs and LOE were the main reasons for switchers and initiators to discontinue CT-P13. Interestingly, the authors who reported the highest CT-P13 discontinuation rates in switchers and/or initiators suggested that the patients’ reluctance towards the switch to CT-P13 and/or their negative perception toward biosimilars might have contributed to their high discontinuation rate and an overall nocebo effect [24, 47, 49, 63]. These authors noted that a part of the AEs and LOE reported by these patients matched no objective disease activity assessment (e.g., serum inflammatory markers, tender joint count, anti-drug antibodies) and therefore suggested that a part of the patients’ reported AEs and LOE were purely subjective [39, 49, 64,65,66]. Furthermore, the authors of a prospective cohort study assessing the switch from OI to CT-P13 in RHE patients, reported that once the subjective outcomes were factored in the studies’ analyses, there were no remaining statistical significance between the switchers and the control cohort [49].
Objective evaluations regarding AEs and LOE were not available in the present study, thus we could not differentiate subjective patient-reported outcomes from objective clinical assessments. However, we noticed that some patients have inexplicably changed their treatment; perhaps owing to a lack of confidence by HCPs in the biosimilar and certainly because of the absence of formal guidelines regarding the use of biosimilars in the hospital. For example, a GAS switcher who was not responding to OI, but was switched to CT-P13 nonetheless, only to later discontinue CT-P13 because of a LOE. Another example is an RHE initiator who discontinued CT-P13 after the second infusion to start OI because of an apparent LOE. Interestingly, this patient was taking golimumab prior to his first CT-P13 infusion and had been already complaining about lower limb pain. The patient’s serum analyses returned a high concentration of anti-golimumab antibodies, explaining the marginal effect of golimumab and an incorrect inefficacy attribution to CT-P13. Hence, even though it was probably a sound decision to switch for CT-P13, there seemed to have been a misinterpretation regarding which biologic was truly inefficient and a “wait-and-see” strategy could have prevented CT-P13 discontinuation. Furthermore, the patient should not have started OI if CT-P13 had been truly deemed ineffective. A third patient was reverted to OI after reporting a dermatitis-like skin reaction with CT-P13. However, not only did the dermatitis not improve under OI, but the patient was still taking OI at month 12. This same observation was reported in another study where some of the CT-P13 initiators and switchers were still treated with OI despite the lack of improvement in the patients’ conditions after they reverted back to OI [47]. In the same study, the patients agreed to a “wait-and-see” strategy with OI, but not with CT-P13, suggesting that despite similar disease activity scores with OI and its biosimilar, the patients and the physicians were prone to choose different treatment decisions [47].
4.3 Address Subjective Complaints Through Proper Training, Education, and Coordination
In light of all the aforementioned literature, and leaving aside patients who discontinued CT-P13 because of an acute systemic reaction, a remission, a request for ambulatory relay, or a pregnancy, an important portion of the discontinuers in our study reported subjective complaints (i.e., LOE or secondary loss of response), as suggested in similar observational studies [39, 49, 64,65,66]. Discontinuers might have either experienced a nocebo effect or falsely attributed their AEs and LOE to CT-P13 [24, 47, 49, 63]. In the present study, this mainly led switchers to return to OI and initiators to change their treatment. To address these subjective complaints, the authors of an open-label prospective cohort study that reported a 24% CT-P13 discontinuation rate suggested that communication was a key determinant for a successful biosimilar implementation in daily practice. Thus, improving the communication surrounding the transition to a biosimilar might temper the nocebo effect as well as an incorrect causal attribution [47]. Additional studies would be needed to confirm the same hypothesis in initiators.
The negative perceptions of patients and HCPs toward the safety and efficacy of biosimilars need to be actively addressed in order to optimize the communication surrounding biosimilars [47, 67,68,69]. Previous studies reported that HCPs were unsure about how they should explain biosimilars to the patients, which could lead to an increased nocebo effect in patients or an incorrect causal attribution in both patients and HCPs because of subjective perceptions [70,71,72,73,74]. In the case of the present study, the unease around biosimilars can be partly attributed to the lack of an institutional implementation strategy and the lack of communication/education.
In order to properly educate patients, HCPs must first receive adequate training to tackle disparagement and misinformation in biosimilar use and be able to inform patients in an evidence-based manner [23, 35]. Organized, consistent, and positive information regarding biosimilar use has been reported to be a contributive approach to enhance initiators and switchers acceptance of biosimilars and mitigate the nocebo effect [23, 67, 75,76,77].
4.4 Limitations and Future Studies
The first limitation of the study is the misclassification bias that is inevitable with retrospective designs. It might have impacted our results even though we took several precautions to minimize it, as explained in the methods section. This might have led to an overestimation of the number of initiators and switchers who discontinued CT-P13 because of a LOE or a secondary loss of response. Hence, CT-P13 discontinuation because of AEs might have been under-represented. However, our results did not differ from the literature as both clinical trials and real-world observation studies reported a LOE as the primary reason for OI and CT-P13 discontinuation.
The second limitation is the number of patients eligible for the study. Even though this tertiary hospital is one of the biggest outpatient centers in Switzerland, only 308 patients were receiving OI or CT-P13 treatment between September 2017 and December 2020. Because of the retrospective design of the study, we had to apply rigorous inclusion and exclusion criteria to avoid further bias that may have impaired our results, which shrunk the number of included patients by half. Nevertheless, this study’s number of included patients and results are consistent with the other real-world observational studies retrieved in the literature.
The third limitation of this retrospective cohort study is the unavailability of objective measurement tools for disease activity assessment (such as the Harvey–Bradshaw Index for Crohn’s disease or the Ankylosing Spondylitis Disease Activity Score for AS). Despite this, we were still able to compare the discontinuation rates in our study with the rest of the literature. Furthermore, we emitted a sound hypothesis regarding the subjective nature of patient-reported AEs or LOE and the crucial role of HCPs coordination and patients communication when transitioning to a biosimilar.
Further research should be undertaken to explore the possible barriers that prevent the biosimilars to reach full effectiveness and the available options to promote their prescription and use. Financial incentives for providers as well as shared-benefit programs for patients should be assessed in Switzerland, as they are in place in other European countries where the market share of biosimilars is much higher. As the expectation of cost savings is the main driver for prescribing biosimilars in clinical practice, further studies should assess the extent to which biosimilars’ implementation in hospital is cost effective compared with no intervention [78]. Given the high discontinuation rates reported in the present study and other real-word observational studies, the need for a monetary outcome becomes necessary to grasp the extent to which the failure of collaboration between patients and HCPs undermines the potential cost savings associated with biosimilars.
5 Conclusions
To our knowledge, this is the first retrospective cohort study that explores the reasons for CT-P13 discontinuation in a large tertiary care hospital in Western Switzerland, in patients initiating CT-P13 and patients who were switched from originator infliximab. The present study questions the potential of biosimilars when their implementation is not planned and carefully coordinated within an institution. With cost savings being the primary driver of biosimilar prescribing in clinical practice, additional efforts are needed to realize the true cost-saving potential of CT-P13 in real life.
References
US Food and Drug Administration. Biological product definitions. 2020. https://www.fda.gov/media/108557/download. Accessed 6 Feb 2020
Liu Y, Yang M, Garg V, Wu EQ, Wang J, Skup M. Economic impact of non-medical switching from originator biologics to biosimilars: a systematic literature review. Adv Ther. 2019;36(8):1851–77.
Braun J, Kudrin A. Switching to biosimilar infliximab (CT-P13): evidence of clinical safety, effectiveness and impact on public health. Biologicals. 2016;44(4):257–66.
Cohen HP, Blauvelt A, Rifkin RM, Danese S, Gokhale SB, Woollett G. Switching reference medicines to biosimilars: a systematic literature review of clinical outcomes. Drugs. 2018;78(4):463–78.
Otto ESA, Schrader U. Rapid growth in biopharma: challenges and opportunities. 2014. https://www.mckinsey.com/~/media/McKinsey/Industries/Healthcare%20Systems%20and%20Services/Our%20Insights/Rapid%20growth%20in%20biopharma/Rapid%20growth%20in%20biopharma%20Challenges%20and%20opportunities.ashx. Accessed 6 Feb 2020
Sensabaugh SM. Biological generics: a business case. J Generic Med. 2007;4(3):186–99.
BCC Research. Biologic therapeutic drugs: technologies and global markets. 2021. https://www.bccresearch.com/market-research/biotechnology/biologic-therapeutic-drugs-technologies-markets-report.html. Accessed 14 Sep 2021
Mikulic M. Global pharmaceutical industry: statistics and facts. 2021. https://www.statista.com/topics/1764/global-pharmaceutical-industry/. Accessed 14 Sep 2021
Mordor Intelligence. Biologics market: growth, trends, COVID-19 impact, and forecasts (2021–2026). 2021. https://www.mordorintelligence.com/industry-reports/biologics-market#faqs. Accessed 14 Sep 2021
Curafutura. Baromètres des biosimilaires. 2020. https://curafutura.ch/uploads/tx_pmxitemlist/210408_Barometre_des_biosimilaires_2020_FR.pdf. Accessed 15 Sep 2021
IQVIA Institute for Human Data Science. Advancing biosimilar sustainability in Europe: a multi-stakeholder assessment. 2018. https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/advancing-biosimilar-sustainability-in-urope.pdf?_=1582559849932. Accessed 24 Feb 2020
Moorkens E, Meuwissen N, Huys I, Declerck P, Vulto AG, Simoens S. The market of biopharmaceutical medicines: a snapshot of a diverse industrial landscape. Front Pharmacol. 2017;8:314.
IQVIA Institute for Human Data Science. The global use of medicine in 2019 and outlook to 2023. https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/the-global-use-of-medicine-in-2019-and-outlook-to-2023.pdf?_=1580989922494. Accessed 6 Feb 2020
QuintilesIMS. The impact of biosimilar competition in Europe. 2017. https://www.medicinesforeurope.com/wp-content/uploads/2017/05/IMS-Biosimilar-2017_V9.pdf. Accessed 24 Feb 2020
Agence Européenne des Médicaments. Similar biological medicinal products (lignes directrices générales). 2015. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-rev1_en.pdf. Accessed 6 Feb 2020
Agence Européenne des Médicaments. Les médicaments biosimilaires dans l’UE: guide d’information destiné aux professionnels de la santé. 2017. https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_fr.pdf. Accessed 6 Feb 2020
Canadian Agency for Drugs and Technologies in Health. Biosimilars: regulatory, health technology assessment, reimbursement trends, and market outlook. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2018.
Ordonnance du 27 juin 1995 sur l'assurance-maladie (OAMal). RS 832.102. In: Conseil fédéral suisse, editor. Art 65b1995
MSD Merck Sharp and Dohme AG. Remicade®. 2021. https://www.swissmedicinfo.ch/default.aspx#section4. Accessed 11 Apr 2021
Federal Office of Public Health. Remicade®. 2021. http://www.listedesspecialites.ch/ShowPreparations.aspx. Accessed 11 Apr 2021
Federal Office of Public Health. Inflectra®. 2021. http://www.listedesspecialites.ch/ShowPreparations.aspx. Accessed 11 Apr 2021
Troein P, Newton M, Scott K. The impact of biosimilar competition in Europe. 2020. https://www.iqvia.com/library/white-papers/the-impact-of-biosimilar-competition-in-europe. Accessed 1 Mar 2021
Cohen HP, McCabe D. The importance of countering biosimilar disparagement and misinformation. BioDrugs. 2020;34(4):407–14.
Glintborg B, Sørensen IJ, Loft AG, Lindegaard H, Linauskas A, Hendricks O, et al. A nationwide non-medical switch from originator infliximab to biosimilar CT-P13 in 802 patients with inflammatory arthritis: 1-year clinical outcomes from the DANBIO registry. Ann Rheum Dis. 2017;76(8):1426–31.
Jørgensen KK, Olsen IC, Goll GL, Lorentzen M, Bolstad N, Haavardsholm EA, et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet. 2017;389(10086):2304–16.
Park W, Yoo DH, Miranda P, Brzosko M, Wiland P, Gutierrez-Ureña S, et al. Efficacy and safety of switching from reference infliximab to CT-P13 compared with maintenance of CT-P13 in ankylosing spondylitis: 102-week data from the PLANETAS extension study. Ann Rheum Dis. 2017;76(2):346–54.
Yoo DH, Prodanovic N, Jaworski J, Miranda P, Ramiterre E, Lanzon A, et al. Efficacy and safety of CT-P13 (biosimilar infliximab) in patients with rheumatoid arthritis: comparison between switching from reference infliximab to CT-P13 and continuing CT-P13 in the PLANETRA extension study. Ann Rheum Dis. 2017;76(2):355–63.
Bernard EJ, Fedorak RN, Jairath V. Systematic review: non-medical switching of infliximab to CT-P13 in inflammatory bowel disease. Dig Dis Sci. 2020;65(8):2354–72.
Gisbert JP, Chaparro M. Switching from an originator anti-TNF to a biosimilar in patients with inflammatory bowel disease: can it be recommended? A systematic review. Gastroenterol Hepatol. 2018;41(6):389–405.
Park W, Hrycaj P, Jeka S, Kovalenko V, Lysenko G, Miranda P, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72(10):1605–12.
Yoo DH, Hrycaj P, Miranda P, Ramiterre E, Piotrowski M, Shevchuk S, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72(10):1613–20.
Ye BD, Kim YH, Pesegova M, Alexeeva O, Osipenko M, Lahat A, et al. Phase III randomized controlled trial to compare biosimilar infliximab (CT-P13) with innovator infliximab in patients with active Crohn’s disease: 1-year maintenance and switching results. Gastroenterology. 2018;154(6):S-167–S-168.
Barbier L, Ebbers HC, Declerck P, Simoens S, Vulto AG, Huys I. The efficacy, safety, and immunogenicity of switching between reference biopharmaceuticals and biosimilars: a systematic review. Clin Pharmacol Ther. 2020;108(4):734–55.
European Medicines Agency. Inflectra. 2013. https://www.ema.europa.eu/en/medicines/human/EPAR/inflectra. Accessed 6 Apr 2021
US Food and Drug Administration. Inflectra. 2016. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125544s000lbl.pdf. Accessed 17 Jun 2021
Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, et al. The REporting of studies conducted using observational routinely-collected health data (RECORD) statement. PLoS Med. 2015;12(10): e1001885.
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2021. https://www.R-project.org/. Accessed 30 Mar 2022
Pfizer AG. Inflectra®: information professionnelle. 2020. https://www.swissmedicinfo.ch/#section11. Accessed 15 Sep 2021
Schmitz EMH, Boekema PJ, Straathof JWA, van Renswouw DC, Brunsveld L, Scharnhorst V, et al. Switching from infliximab innovator to biosimilar in patients with inflammatory bowel disease: a 12-month multicentre observational prospective cohort study. Aliment Pharmacol Ther. 2018;47(3):356–63.
Ratnakumaran R, To N, Gracie DJ, Selinger CP, O’Connor A, Clark T, et al. Efficacy and tolerability of initiating, or switching to, infliximab biosimilar CT-P13 in inflammatory bowel disease (IBD): a large single-centre experience. Scand J Gastroenterol. 2018;53(6):700–7.
Petitdidier N, Tannoury J, de’Angelis N, Gagniere C, Hulin A, Rotkopf H, et al. Patients’ perspectives after switching from infliximab to biosimilar CT-P13 in patients with inflammatory bowel disease: a 12-month prospective cohort study. Dig Liver Dis. 2019;51(12):1652–60.
Kolar M, Duricova D, Bortlik M, Hruba V, Machkova N, Mitrova K, et al. Infliximab biosimilar (Remsima™) in therapy of inflammatory bowel diseases patients: experience from one tertiary inflammatory bowel diseases centre. Dig Dis. 2017;35(1–2):91–100.
Glez GER, Hernández LD, Barrios JAM, González MV, Marín CAT, Romero MMV, et al. P629 Efficacy, safety and economic impact of the switch to biosimilar of infliximab in inflammatory bowel disease patients in clinical practice: results of one year. J Crohns Colitis. 2017;11(Suppl_1):S402.
Jung YS, Park DI, Kim YH, Lee JH, Seo PJ, Cheon JH, et al. Efficacy and safety of CT-P13, a biosimilar of infliximab, in patients with inflammatory bowel disease: a retrospective multicenter study. J Gastroenterol Hepatol. 2015;30(12):1705–12.
Puente LG, Flores EI, Benítez JM, Medina RM, Rodríguez IS, Melero PA, et al. Evolution after switching to biosimilar infliximab in inflammatory bowel disease patients in clinical remission. Gastroenterol Hepatol. 2017;40(9):595–604.
Nugent S, Nugent M, Mullane D, Kelly C. P430 EirSwitch echoes of NorSwitch: switching biosimilar therapy in an IBD cohort an Irish experience. J Crohns Colitis. 2017;11(Suppl_1):S295.
Tweehuysen L, van den Bemt BJF, van Ingen IL, de Jong AJL, van der Laan WH, van den Hoogen FHJ, et al. Subjective complaints as the main reason for biosimilar discontinuation after open-label transition from reference infliximab to biosimilar infliximab. Arthritis Rheumatol. 2018;70(1):60–8.
Akrout W, Bosycot A, Levet-Labry R, Gazaix-Fontaine E, Paul M, Claudepierre P. AB0708 transition from ongoing infliximab reference product to its biosimilar: can we talk about a failure? Ann Rheum Diss. 2017;76(Suppl. 2):1301–2.
Scherlinger M, Germain V, Labadie C, Barnetche T, Truchetet M-E, Bannwarth B, et al. Switching from originator infliximab to biosimilar CT-P13 in real-life: the weight of patient acceptance. Jt Bone Spine. 2018;85(5):561–7.
Presberg, Y. et al. THU0646 Interchangeability from infliximab originator to infliximab biosimilar: efficacy and safety in a prospective observational study on 89 patients. Annals of the Rheumatic Diseases 2017;76:450. https://doi.org/10.1136/annrheumdis-2017-eular.6586.
Valido A, Silva-Dinis J, Saavedra MJ, Iria I, Gonçalves J, Lopes JP, et al. Efficacy, immunogenicity and cost analysis of a systematic switch from originator infliximab to biosimilar CT-P13 of all patients with inflammatory arthritis from a single center. Acta Reumatol Port. 2019;44(4):303–11.
Holroyd CR, Parker L, Bennett S, Zarroug J, Underhill C, Davidson B, et al. Switching to biosimilar infliximab: real world data in patients with severe inflammatory arthritis. Clin Exp Rheumatol. 2018;36(1):171–2.
Argüelles-Arias F, Guerra Veloz MF, Perea Amarillo R, Vilches-Arenas A, Castro Laria L, Maldonado Pérez B, et al. Effectiveness and safety of CT-P13 (biosimilar infliximab) in patients with inflammatory bowel disease in real life at 6 months. Dig Dis Sci. 2017;62(5):1305–12.
Armuzzi A, Fiorino G, Variola A, Manetti N, Fries W, Orlando A, et al. The PROSIT cohort of infliximab biosimilar in IBD: a prolonged follow-up on the effectiveness and safety across Italy. Inflamm Bowel Dis. 2019;25(3):568–79.
Kim HA, Lee E, Lee SK, Park YB, Shin K. Retention rate and efficacy of the biosimilar CT-P13 versus reference infliximab in patients with ankylosing spondylitis: a propensity score-matched analysis from the Korean College of Rheumatology Biologics Registry. BioDrugs. 2020;34(4):529–39.
Kim NH, Lee JH, Hong SN, Yoon H, Kang HW, Lee SH, et al. Long-term efficacy and safety of CT-P13, a biosimilar of infliximab, in patients with inflammatory bowel disease: a retrospective multicenter study. J Gastroenterol Hepatol. 2019;34(9):1523–32.
Kim TH, Lee SS, Park W, Song YW, Suh CH, Kim S, et al. A 5-year retrospective analysis of drug survival, safety, and effectiveness of the infliximab biosimilar CT-P13 in patients with rheumatoid arthritis and ankylosing spondylitis. Clin Drug Investig. 2020;40(6):541–53.
Yu CL, Yang CH, Chi CC. Drug survival of biologics in treating ankylosing spondylitis: a systematic review and meta-analysis of real-world evidence. BioDrugs. 2020;34(5):669–79.
Mourad AI, Gniadecki R. Biologic drug survival in psoriasis: a systematic review and comparative meta-analysis. Front Med (Lausanne). 2020;7: 625755.
Blum MA, Koo D, Doshi JA. Measurement and rates of persistence with and adherence to biologics for rheumatoid arthritis: a systematic review. Clin Ther. 2011;33(7):901–13.
Arora A, Mahajan A, Spurden D, Boyd H, Porter D. Long-term drug survival of TNF inhibitor therapy in RA patients: a systematic review of European national drug registers. Int J Rheumatol. 2013;2013: 764518.
Burri E, Juillerat P, Maillard MH, Manz M, Michetti P, Mottet C, et al. Position statement on the use of biosimilars in inflammatory bowel disease. Swiss Med Wkly. 2019;149: w20148.
Odinet JS, Day CE, Cruz JL, Heindel GA. The biosimilar nocebo effect? A systematic review of double-blinded versus open-label studies. J Manag Care Spec Pharm. 2018;24(10):952–9.
Avouac J, Molto A, Abitbol V, Etcheto A, Salcion A, Gutermann L, et al. Systematic switch from innovator infliximab to biosimilar infliximab in inflammatory chronic diseases in daily clinical practice: the experience of Cochin University Hospital, Paris, France. Semin Arthritis Rheum. 2018;47(5):741–8.
Gentileschi S, Barreca C, Bellisai F, Biasi G, Brizi MG, De Stefano R, et al. Switch from infliximab to infliximab biosimilar: efficacy and safety in a cohort of patients with different rheumatic diseases. Response to: Nikiphorou E, Kautiainen H, Hannonen P, et al. Clinical effectiveness of CT-P13 (Infliximab biosimilar) used as a switch from Remicade (infliximab) in patients with established rheumatic disease. Report of clinical experience based on prospective observational data. Expert Opin Biol Ther. 2015;15:1677–1683. Expert Opin Biol Ther. 2016;16(10):1311–2.
Tweehuysen L vdBB, van Ingen IL, de Jong AJL, van der Laan WH, van den Hoogen FHJ, den Broeder AA. Clinical and immunogenicity outcomes after switching treatment from innovator in rheumatic diseases in daily clinical practice [abstract]. 2016. https://acrabstracts.org/abstract/clinical-and-immunogenicity-outcomes-after-switching-treatment-from-innovator-in. Accessed 17 Jun 2021
Gasteiger C, Jones ASK, Kleinstauber M, Lobo M, Horne R, Dalbeth N, et al. Effects of message framing on patients’ perceptions and willingness to change to a biosimilar in a hypothetical drug switch. Arthritis Care Res (Hoboken). 2020;72(9):1323–30.
Peyrin-Biroulet L, Lönnfors S, Roblin X, Danese S, Avedano L. Patient perspectives on biosimilars: a survey by the European Federation of Crohn’s and ulcerative colitis associations. J Crohns Colitis. 2017;11(1):128–33.
Wilkins AR, Venkat MV, Brown AS, Dong JP, Ran NA, Hirsch JS, et al. Patient perspectives on biosimilar insulin. J Diabetes Sci Technol. 2014;8(1):23–5.
van Overbeeke E, De Beleyr B, de Hoon J, Westhovens R, Huys I. Perception of originator biologics and biosimilars: a survey among Belgian rheumatoid arthritis patients and rheumatologists. BioDrugs. 2017;31(5):447–59.
Dylst P, Vulto A, Simoens S. Barriers to the uptake of biosimilars and possible solutions: a Belgian case study. Pharmacoeconomics. 2014;32(7):681–91.
Beck M, Michel B, Rybarczyk-Vigouret MC, Leveque D, Sordet C, Sibilia J, et al. Rheumatologists’ perceptions of biosimilar medicines prescription: findings from a French web-based survey. BioDrugs. 2016;30(6):585–92.
Hemmington A, Dalbeth N, Jarrett P, Fraser AG, Broom R, Browett P, et al. Medical specialists’ attitudes to prescribing biosimilars. Pharmacoepidemiol Drug Saf. 2017;26(5):570–7.
Danese S, Fiorino G, Michetti P. Viewpoint: knowledge and viewpoints on biosimilar monoclonal antibodies among members of the European Crohn’s and Colitis Organization. J Crohns Colitis. 2014;8(11):1548–50.
Haghnejad V, Le Berre C, Dominique Y, Zallot C, Guillemin F, Peyrin-Biroulet L. Impact of a medical interview on the decision to switch from originator infliximab to its biosimilar in patients with inflammatory bowel disease. Dig Liver Dis. 2020;52(3):281–8.
Bakalos G, Zintzaras E. Drug discontinuation in studies including a switch from an originator to a biosimilar monoclonal antibody: a systematic literature review. Clin Ther. 2019;41(1):155-73.e13.
Kristensen LE, Alten R, Puig L, Philipp S, Kvien TK, Mangues MA, et al. Non-pharmacological effects in switching medication: the nocebo effect in switching from originator to biosimilar agent. BioDrugs. 2018;32(5):397–404.
Rezk MF, Pieper B. Treatment outcomes with biosimilars: be aware of the nocebo effect. Rheumatol Ther. 2017;4(2):209–18.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflicts of Interest/Competing Interests
MK, JCD, JM, and FS declare that they have no conflicts of interest.
Ethics Approval
Use of routinely collected medical data was validated by the hospital’s good research practice department and handled anonymously. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
All data relevant to the study are included in the article and available upon further request to the authors.
Code Availability
Not applicable.
Authors’ Contributions
MK and JCD designed the search strategy. MK and JCD selected the patients for inclusion. When MK and JCD did not agree, FS and JM acted as arbitrators. MK extracted the data. MK analyzed the data and wrote the first draft of the manuscript, which was critically revised for intellectual content by JCD, JM, and FS. MK, JCD, JM, and FS contributed to the analysis and/or interpretation of the data, drafting, and critical revision of the manuscript, and they approved the final version submitted for publication.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Krstic, M., Devaud, JC., Marti, J. et al. Exploring the Reasons Behind the Substantial Discontinuation Rate Among Patients Taking CT-P13 in a Large Tertiary Hospital in Western Switzerland: A Retrospective Cohort Study Using Routinely Collected Medical Data. Drugs - Real World Outcomes 9, 425–436 (2022). https://doi.org/10.1007/s40801-022-00299-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40801-022-00299-2