Abstract
Background
Among patients and rheumatologists, current knowledge and perception of biosimilars in comparison with originator biologics is unknown.
Objectives
The aim of this study was to investigate this knowledge and perception in Belgian rheumatologists and rheumatoid arthritis (RA) patients.
Methods
Anonymous web surveys were conducted in Belgian RA patients (n = 121) and rheumatologists (n = 41) during the period January–March 2016. The surveys covered topics on knowledge, similarity, price, preference, interchangeability, extrapolation and switching. Descriptive and statistical analyses of responses were performed.
Results
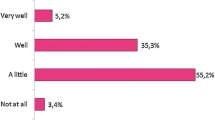
Familiarity with biosimilars was reported by 49% of patients, of whom 77% knew what biosimilars were. RA patients equally questioned the proven efficacy of originators and biosimilars in RA, as well as their side effects and suitability. Furthermore, RA patients questioned the safety of biosimilars more often than that of originators (35 vs. 20%, respectively; p = 0.0094). Rheumatologists, more so than patients, expressed concerns that there might be differences between originators and biosimilars in terms of quality, safety, and price (p = 0.0292, p < 0.0001, p = 0.0129, respectively). The opinions of rheumatologists on interchangeability and extrapolation of indications varied. The price of an originator contributed substantially to the medicine preference of rheumatologists (p = 0.0002), but not patients.
Conclusion
Our study showed that rheumatologists, more so than patients, were convinced that there can be differences between originators and biosimilars. Despite safety being the major concern of patients, patients trusted their physician’s decision to start on or switch to a biosimilar. The evolution of the uptake of biosimilars in Belgium might thus depend mainly on the perception of physicians.







Similar content being viewed by others
References
Yoo DH. The rise of biosimilars: potential benefits and drawbacks in rheumatoid arthritis. Expert Rev Clin Immunol. 2014;10(8):981–3.
Scheinberg MA, Kay J. The advent of biosimilar therapies in rheumatology” “O brave new world”. Nat Rev Rheumatol. 2012;8(7):430–6.
Committee for Medicinal Products for Human Use (CHMP). Guideline on similar biological medicinal products. CHMP/437/04 Rev 1. European Medicines Agency. 2005. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003517.pdf. Accessed 18 Apr 2017.
Jha D, Mishra RK, Pandey R. Biosimilars: current regulatory perspective and challenges. J Pharm Bioallied Sci. 2013;5(1):80–1.
The European Parliament and of the Council. Art. 10(4) of the directive 2001/83/EC on the Community code relating to medicinal products for human use. 2001. https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-1/dir_2001_83_consol_2012/dir_2001_83_cons_2012_en.pdf. Accessed 18 Apr 2017.
The European Parliament and of the Council. Annex I Part II(4) of the directive 2001/83/EC on the Community code relating to medicinal products for human use. 2001. https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-1/dir_2001_83_consol_2012/dir_2001_83_cons_2012_en.pdf. Accessed 18 Apr 2017.
Desanvicente-Celis Z, Gomez-Lopez A, Anaya J-M. Similar biotherapeutic products: overview and reflections. Immunotherapy. 2012;4(12):1841–57.
Expert Committee on biological standardization. Guideline on evaluation of similar biotherapeutic products (SBPs). World Health Organization. 2009. http://www.who.int/biologicals/areas/biological_therapeutics/BIOTHERAPEUTICS_FOR_WEB_22APRIL2010.pdf. Accessed 18 Apr 2017.
Declerck P, Farouk-Rezk M, Rudd PM. Biosimilarity versus manufacturing change: two distinct concepts. Pharm Res. 2016;33(2):261–8.
Goel N, Chance K. Biosimilars in rheumatology: understanding the rigor of their development. Rheumatology (Oxford). 2017;56(2):187–97.
Committee for Medicinal Products for Human Use (CHMP). Assessment report Flixabi. EMA/CHMP/589422/2013. European Medicines Agency. 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002778/WC500151490.pdf. Accessed 18 Apr 2017.
Committee for Medicinal Products for Human Use (CHMP). Assessment report Inflectra. EMA/CHMP/589422/2013. European Medicines Agency. 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002778/WC500151490.pdf. Accessed 18 Apr 2017.
Committee for Medicinal Products for Human Use (CHMP). Assessment report Remsima. EMA/CHMP/589317/2013. European Medicines Agency. 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002576/WC500151486.pdf. Accessed 18 Apr 2017.
Committee for Medicinal Products for Human Use (CHMP). Assessment report Benepali. EMA/CHMP/819219/2015. European Medicines Agency. 2015. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/004007/WC500200380.pdf. Accessed 18 Apr 2017.
Minister of Social Affairs and Public Health Maggie De Block. Circular letter. Subject: Biosimilars Action Plan. MDB/2016/542384. 2016. http://www.riziv.fgov.be/SiteCollectionDocuments/omzendbrief_actieplan_biosimilar.pdf. Accessed 18 Apr 2017.
RIZIV-INAMI. Cheap prescription: focus on “as cheap as possible“. D/2015/0401/8. 2015. http://www.riziv.fgov.be/SiteCollectionDocuments/brochure_goedkoop_voorschrijven.pdf. Accessed 18 Apr 2017.
Minister of Social Affairs and Public Health Maggie De Block. Covenant: startover for biosimilar medicines in Belgium. 2016. http://www.riziv.fgov.be/SiteCollectionDocuments/convenant_biosimilaire_geneesmiddelen_belgie.pdf. Accessed 18 Apr 2017.
Tsiftsoglou AS, Ruiz S, Schneider CK. Development and regulation of biosimilars: current status and future challenges. BioDrugs. 2013;27(3):203–11.
Ruiz S, Calvo G. Similar biological medicinal products: lessons learned and challenges ahead. J Generic Med. 2011;8(1):4–13.
Blackstone EA, Fuhr JP. The economics of biosimilars. Am Health Drug Benefits. 2013;6(8):469–78.
Simoens S, Verbeken G, Huys I. Biosimilars and market access: a question of comparability and costs? Target Oncol. 2012;7(4):227–31.
Social affairs, public health and environment. Royal Decree in establishing procedures, terms and conditions for compensation for the compulsory insurance for medical care and benefits in the costs of pharmaceutical specialties. 2001-12-21/38. 2001. http://www.ejustice.just.fgov.be/cgi_loi/change_lg.pl?language=nl&la=N&cn=2001122138&table_name=wet. Accessed 28 Aug 2017.
Chancery of the First Minister, Internal Affairs, Social Security, Economics. SME, small business and energy, justice, employment, work and social consultation, finance. Art. 30. Law containing various provisions. 2013-07-30/01. 2013. http://www.ejustice.just.fgov.be/cgi_loi/change_lg.pl?language=fr&la=F&table_name=loi&cn=2013073001. Accessed 29 Aug 2017.
Roger SD. Biosimilars: current status and future directions. Expert Opin Biol Ther. 2010;10(7):1011–8.
Committee for Medicinal Products for Human Use (CHMP). Guideline on immunogenicity assessment of biotechnology-derived therapeutic proteins. EMEA/CHMP/BMWP/14327/2006. European Medicines Agency. 2007. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003946.pdf. Accessed 18 Apr 2017.
Lee H. Is extrapolation of the safety and efficacy data in one indication to another appropriate for biosimilars? Am Assoc Pharm Sci J. 2014;16(1):22–6.
Dranitsaris G, Amir E, Dorward K. Biosimilars of biological drug therapies: regulatory. Clinical and commercial considerations. Drugs. 2011;71(12):1527–36.
Belgian Royal Society for Rheumatology (BRSR). Demographics of the Belgian rheumatologist population [online]. E-mail to Eline van Overbeeke (eline.vanoverbeeke@kuleuven.be). Accessed 26 Apr 2016.
Jacobs I, Singh E, Sewell KL, Al-Sabbagh A, Shane LG. Patient attitudes and understanding about biosimilars: an international cross-sectional survey. Patient Prefer Adherence. 2016;10:937–48.
Attara G, Bailey R, Bressler B, Marshall J, Panaccione R, Aumais G. Su1012 canadian patient and caregiver perspectives on subsequent entry biologics/biosimilars for inflammatory bowel disease. Gastroenterology. 2016;150(4 Suppl 1):S443–4.
Peyrin-Biroulet L, Lönnfors S, Danese S, Roblin X, Avedano L, Greco M. P432 Patient perspectives on biosimilars: a European Federation of Crohn’s and Colitis Associations survey. 2016. In: Presented at the 11th Congress of ECCO. https://www.ecco-ibd.eu/index.php/publications/congress-abstract-s/abstracts-2016/item/p432-patient-perspectives-on-biosimilars-a-european-federation-of-crohnx2019s-and-colitis-associationsx00a0survey.html. Accessed 29 Apr 2016.
Wilkins AR, Venkat MV, Brown AS, Dong JP, Ran NA, Hirsch JS, et al. Patient perspectives on biosimilar insulin. J Diabetes Sci Technol. 2014;8(1):23–5.
Lepage-Nefkens I, Gerkens S, Vinck I, Piérart J, Hulstaert F, Farfán-Portet M-I. Barriers and opportunities for the uptake of biosimilar medicines in Belgium. Health Services Research (HSR) Brussels: Belgian Health Care Knowledge Centre (KCE). 2013. KCE Reports 199. D/2013/10.273/13.
Dylst P, Vulto A, Simoens S. Barriers to the uptake of biosimilars and possible solutions: a Belgian case study. PharmacoEconomics. 2014;32(7):681–91.
Beck M, Michel B, Rybarczyk-Vigouret MC, Leveque D, Sordet C, Sibilia J, et al. Rheumatologists’ perceptions of biosimilar medicines prescription: findings from a french web-based survey. BioDrugs. 2016;30(6):585–92.
Cohen H, Beydoun D, Chien D, Lessor T, McCabe D, Muenzberg M, et al. Awareness, knowledge, and perceptions of biosimilars among specialty physicians. Adv Ther. 2017;33(12):2160–72.
Waller J, Sullivan E, Piercy J, Black CM, Kachroo S. Assessing physician and patient acceptance of infliximab biosimilars in rheumatoid arthritis, ankylosing spondyloarthritis and psoriatic arthritis across Germany. Patient Prefer Adherence. 2017;11:519–30.
Hemmington A, Dalbeth N, Jarrett P, Fraser AG, Broom R, Browett P, et al. Medical specialists’ attitudes to prescribing biosimilars. Pharmacoepidemiol Drug Saf. 2017;26(5):570–7.
Berghea F, Popescu C, Ionescu R, Damjanov N, Singh G. AB1052 Biosimilars use in rheumatology: the patient perspective. Ann Rheum Dis. 2014;73(Suppl 2):1148–9.
IMS Health. The impact of biosimilar competition. 2016. https://www.imshealth.com/files/web/MarketInsights/IMS_Health_Impact_of_Biosimilar_Competition_EU_2016.pdf. Accessed 18 Apr 2017.
Kvien TK, Uhlig T, Odegard S, Heiberg MS. Epidemiological aspects of rheumatoid arthritis: the sex ratio. Ann N Y Acad Sci. 2006;1069:212–22.
Mundipharma. Munidpharma anounces the reimbursement of Remsima starting on the 1st of April. 2015. http://www.mediplanet.be/sites/default/files/Mundipharma_TerugbetalingRemsima_NL_0.pdf. Accessed 18 Apr 2017.
IMS Health. Assessing biosimilar uptake and competition in European markets. 2014. https://www.imshealth.com/files/web/IMSHInstitute/HealthcareBriefs/Assessing_biosimilar_uptake_and_competition_in_European_markets.pdf. Accessed 30 Aug 2017.
Park W, Yoo D, Miranda P, Brzosko M, Wiland P, Gutierrez-Ureña S, et al. Efficacy and safety of switching from reference infliximab to CT-P13 compared with maintenance of CT-P13 in ankylosing spondylitis: 102-week data from the PLANETAS extension study. Ann Rheum Dis. 2017;76:346–54.
Sieczkowska J, Jarzębicka D, Banaszkiewicz A, Plocek A, Gawronska A, Toporowska-Kowalska E, et al. Switching between infliximab originator and biosimilar in paediatric patients with inflammatory bowel disease. Preliminary observations. J Crohn Colitis. 2016;10(2):127–32.
Smits LJ, Derikx DL, de Jong DJ, Boshuizen RS, van Esch AAJ, Drenth JPH, et al. Clinical outcomes following a switch from Remicade® to the biosimilar CT-P13 in inflammatory bowel disease patients: a prospective observational cohort study. J Crohn Colitis. 2016;10(11):1287–93.
Nikiphorou E, Kautiainen H, Hannonen P, Asikainen J, Kokko A, Rannio T, et al. Clinical effectiveness of CT-P13 (Infliximab biosimilar) used as a switch from Remicade (infliximab) in patients with established rheumatic disease. Report of clinical experience based on prospective observational data. Expert Opin Biol Ther. 2015;15(12):1677–83.
Acknowledgements
The authors are pleased to acknowledge the patient organizations ReumaNet vzw and RA-Liga vzw, as well as the Belgian Royal Society for Rheumatology, for their contribution to the surveys and for distributing the surveys to participants. Many thanks go to all participants who completed the surveys.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Medical Ethics Committee of UZ KU Leuven, protocol number S58653, on 14 December 2015. The study was performed in accordance with the ethical standards of the Declaration of Helsinki, and informed consent was obtained from all individual participants included in the study.
Funding
This study was not financially supported by any funding agency or grant.
Conflicts of interest
In the interests of transparency, Eline van Overbeeke reports that she did an internship at Pfizer NV during the conduct of the study that was part of her masters’ thesis. She did not experience any undue influence from Pfizer NV on the design, conduct, and publication of the study, and did not receive any financial support from Pfizer NV or any other funding agency or grant. For transparency reasons, Birgit De Beleyr declares she is a former employee of Pfizer NV. At the time of development of this study and writing of the manuscript, she worked at the Medical Affairs Department of Pfizer NV. In her role as co-promotor of the masters’ thesis and supervisor of the internship of Eline van Overbeeke, she did not experience any undue influence from Pfizer NV on the presented work. Pfizer NV did not provide any financial support for this role. Isabelle Huys reports that she is co-founder of the academic research fund MABEL regarding market access of biosimilars; however, the presented work was not financially supported by MABEL. Jan de Hoon and Rene Westhovens declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
van Overbeeke, E., De Beleyr, B., de Hoon, J. et al. Perception of Originator Biologics and Biosimilars: A Survey Among Belgian Rheumatoid Arthritis Patients and Rheumatologists. BioDrugs 31, 447–459 (2017). https://doi.org/10.1007/s40259-017-0244-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-017-0244-3