Abstract
This study aimed to investigate the effects of ammonia addition on ethylene counter-flow diffusion flames with different diluents on the fuel or oxidizer side, using kinetic analyses. A special emphasis was put on assessing the coupled chemical effects of NH3 and CO2 on C2H4 combustion chemistry. The chemical effects could be evaluated by comparing fictitious inert NH3 or CO2 with normal active NH3 or CO2. The results revealed that the addition of NH3 decreased the mole fractions and production rates of key soot precursors, such as acetylene, propynyl, and benzene. When CO2 was used as the dilution gas, the coupled chemical effects of NH3 and CO2 were affected by the chemical effects of CO2 to varying degrees. With the oxidizer-side CO2 addition, the coupled chemical effects of NH3 and CO2 reduced the mole fractions of H, O, OH radicals, acetylene, propynyl, and benzene, while the effects differed from the fuel-side CO2 addition. The coupled chemical effects of NH3 and CO2 also promoted the formation of aldehyde contaminants, such as acetaldehyde, to some extent, particularly with CO2 addition on the oxidizer side.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The goal of reducing carbon emissions had been pursued by various countries in recent years. However, the energy structure was still dominated by fossil fuels, whose incomplete combustion could inevitably generate the production of pollutants such as NOx, carbon oxides, and harmful soot particles. The soot could reduce the efficiency of combustion, damage the environment, and endanger human health (Wang 2011; Dong et al. 2023). Therefore, it was important to explore technologies that could reduce dependence on fossil fuel sources, such as renewable energy sources and carbon-free fuel combustion.
Ammonia (NH3) could be a viable clean fuel, with a 2018 report in the journal of Science lauding it as "liquid sunlight" that would provide a renewable, carbon-free energy source (Service 2018). NH3 also had potential benefits and technical advantages as a sustainable fuel for power generation on vehicles. In particular, NH3 was more effective than other fuels, had a longer driving range, and was more compact and cost-effective (Zamfirescu and Dincer 2009). Additionally, the octane rate of NH3 was high, reaching about 110–130 (Sonker et al. 2022). The applications of NH3 as fuel in gas turbines, pulverized coal co-combustion and industrial furnaces have been very successful in recent years (Kobayashi et al. 2019).
Given ammonia flame instability and low combustion intensity (Lhuillier et al. 2020; Zhou et al. 2021), researchers have been motivated to study its mixing with hydrocarbon fuels such as methane and ethylene (Chen and Liu 2023). Firstly, the combustion of the methane mixing ammonia (Grcar et al. 2004; Shu et al. 2021; Li et al. 2021) has been explored. It revealed that as the proportion of mixed NH3 increased in the CH4/NH3 turbulent premixed flame, the maximum flame surface density decreased, leading to a decrease in the ratio of the turbulent combustion velocity of NH3 to the unstretched laminar combustion velocity (Ichikawa et al. 2019). The primary way in which ammonia affected the velocity of the CH4/NH3 flame was by altering the concentration of H and OH radicals. The sensitivity of flame to stretch increased with equivalence ratio and NH3 concentration (Okafor et al. 2018). Secondly, ethylene (C2H4), as the simplest alkene, has been established its detailed combustion reaction mechanism. For microgravity ethylene diffusion flames, researchers (Lecoustre et al. 2012) have evaluated experimentally and numerically that soot formation occurred in regions where the C/O atom ratio and temperature exceeded critical values of 0.53 and 1305 K, respectively. Moreover, the local C/O atomic ratio associated with soot incipience was lower in terrestrial gravity, falling within the range of 0.32 to 0.44 (Frolov et al. 2023). The carbon atom number and C/H ratio of the final soot particles in ethylene combustion were approximately twice that of methane combustion (Wang et al. 2021). Therefore, the soot production in ethylene turbulent flames after nitrogen was replaced by hydrogen or ammonia was studied (Boyette et al. 2021). It was found that hydrogen substitution increased soot production, while ammonia addition inhibited soot formation. Additionally, the replacement of some Ar by NH3 in ethylene premixed flame was responsible for the decrease of the maximum mole fractions of C4H2 and C5 to C10 species (Renard et al. 2009). In the C2H4 diffusion flame, it was revealed that NH3 delayed and suppressed the formation of polycyclic aromatic hydrocarbons (PAHs), and NH3 had a greater reduction influence than Ar (Ren et al. 2022). By some kinetic studies, some researchers also found that doping ammonia decreased soot particle size, the volume fraction of soot particles and the mole fractions of soot precursors both in laminar premixed (Shao et al. 2022) and diffusion (Liu et al. 2021; Zhang et al. 2023) ammonia/ethylene flames. Then through the kinetic analyses of the mole fractions of typical soot precursors (Deng et al. 2022), it was found that NH3 addition inhibited the production of important precursor polycyclic aromatic hydrocarbons, which was mainly due to the chemical effects of NH3 in C2H4/NH3 diffusion flame. Furthermore, the soot from ethylene/ammonia laminar flames was experimentally and numerically studied, and found that the reduction of soot formation was mainly due to the chemical effects of NH3 (Bennett et al. 2020). However, it was not completely separate the chemical effect of NH3 from its thermal and dilution effects (Liu et al. 2015a). It could be concluded that mixing ammonia with traditional hydrocarbon fuels improved the combustion properties of ammonia and inhibit the formation of soot precursors. However, it was not clear what factors will affect the chemical effects of NH3 on C2H4 combustion chemistry, such as different diluents.
It had been found that different diluent gas could affect the reaction kinetics and change the thermophysical properties of the mixture such as specific heats, diffusion coefficients, etc. to change the combustion temperature or reduce the generation of pollutants (Vancoillie et al. 2013; Chen et al. 2022). Researchers (Yelverton and Roberts 2008) have measured the soot surface temperature in pure and diluted ethylene co-flow diffusion flames, using helium (He), argon (Ar), nitrogen (N2), or carbon dioxide (CO2) individually. It revealed that the addition of a diluent cooled the soot surface. The He-diluted flames were the warmest and the CO2-diluted flames were the coolest. In addition, the sensitivity of the DME flame velocity to the H-atom production and consumption reactions also decreased with the dilution of CO2. In the lean DME flame, both the inert third-body effect and the kinetic effect of CO2 reduced the H-atom production. For the rich DME flame, the inert third-body effect increased the formation of H-atom by inhibiting the kinetic effect of CO2 (Liu et al. 2013). Focusing on the soot formation in CO2-diluted environments, researchers performed experiments on ethylene fuel pyrolysis with varying levels of CO2 dilution and found that 25% CO2 tended to increase soot production while a higher level of CO2 reduced soot formation (Abián et al. 2012). In laminar co-flow C2H4/air diffusion flames, it concluded that the concentrations of the critical soot formation species, including H, C2H2, benzene, and pyrene were lowered in the CO2-diluted flames due to the additional chemical effects of CO2. CO2 remained more effective than N2 as a diluent to suppress soot formation at elevated pressures (Liu et al. 2015). The chemical effects of adding CO2 to the fuel side or oxidant side were simulated in countercurrent ethylene/air diffusion flame, and it concluded that CO2 could suppress soot formation by lowering both temperature and acetylene concentration and enhancing the concentration of OH radical (Liu et al. 2001).
It may be concluded that the combustion atmosphere had very significant influences on the combustion process. However, most of the available studies have focused on the effects of diluents on the combustion of hydrocarbon fuels. The studies of the effects of diluents on ethylene/ammonia counter-flow diffusion flames were limited. Therefore, the aim of this paper was to investigate the combustion chemistry of C2H4/NH3 with various diluents via chemical kinetics analyses. The chemical effects of NH3 addition on the flame temperature, typical radical species, and important intermediate species were analyzed, with particular attention to the coupled chemical effects of NH3 and CO2 on the combustion process. The present study had the potential to give a better understanding of the fundamental ammonia combustion with different diluents, and to provide a reference for the detailed alteration of soot precursors and the control of other pollutant emissions.
2 Kinetic modeling and analysis method
The counter-flow diffusion flame simulations were performed using the CHEMKIN/OPPDIF module. The detailed chemical mechanism employed here was KM2-G, which involved 202 species and 1351 reactions (Wang et al. 2013). The KM2-G mechanism coupled a comprehensive model for nitrogen chemistry with the KM2 hydrocarbon-PAH mechanism (Zhou et al. 2022). The nitrogen chemistry included the oxidation of NH3 and the formation of nitric oxide species, which have been well validated against experimental data (Glarborg et al. 2018). The KM2 mechanism was also shown to perform excellently in terms of the prediction of the first aromatic ring as well as soot formation (Wang et al. 2020).
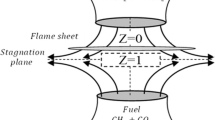
The separation distance between the two nozzles of the counter-flow diffusion flame burner was 8 mm. With the pressure condition of 1 atm, the inlet velocities of the fuel and oxidizer were set at 25 cm/s, and the initial temperature was 300 K. Considering that N2 was unstable at high temperatures, and could produce thermal NOx that affected the combustion chemistry of NH3. Therefore, as the basic working condition, the fuel side consisted of 80% C2H4 + 20% Ar, and the oxidizer side was 21% O2 + 79% Ar. Then He and CO2 replaced the diluent gas Ar on the fuel side or the oxidizer side. Additionally, the coupled chemical effects of NH3 and CO2 were further analyzed by introducing the fictitious inert CO2 and NH3. The specific working conditions were shown in Tables 1 and 2. In Table 1, F1–F5 represented the conditions of the C2H4/NH3 flames with Ar dilution and different NH3 additions. F6–F10 and F11–F15 illustrated the conditions of the C2H4/NH3 flames with He dilution on the fuel side and oxidizer side, respectively, and different NH3 additions. In Table 2, F1–F9 outlined the conditions of the C2H4/NH3 flames with CO2 dilution on the fuel side and various NH3 additions, F10–F18 delineated the conditions of the C2H4/NH3 flames with CO2 dilution on the oxidizer side and various NH3 additions.
As the effects of additives on fuel combustion were divided into three types: (1) dilution effects resulting from the decrease of reactive species mole fractions and collision frequencies, (2) thermal effects as a result of flame temperature variation, and (3) chemical effects caused by the participation of additives in chemical reactions (Du et al. 1990).
These three effects were highly coupled which made it quite difficult to discuss them separately. Therefore, the fictitious species method, which was firstly proposed by Liu et al. (2001) and had been employed by many researchers to analyze the specific effects of additives in different flames (as shown in Table 3), could isolate the chemical effects of additives from the dilution and thermal effects. In the study, in order to identify the coupled chemical effects of NH3 and CO2, normal active CO2 (denoted as CO2) and fictitious inert CO2 (denoted as FCO2) were also introduced for comparisons (Liu et al. 2003, 2015; Gu et al. 2016; Naseri et al. 2017). Similarly, the normal active NH3 was denoted as NH3, and the fictitious inert NH3 was denoted as FNH3. Although the fictitious species could not be directly added to real flames, in the numerical strategy, FCO2 and CO2 had the same thermodynamic parameters, transport parameters, and third-body collision efficiency, and the only difference was that FCO2 did not participate in any relevant chemical reactions. In this way, the dilution and thermal effects, which were similar and commonly both be classified as pure physical effects, were consistent. While the chemical effects of CO2, which not only affected the main combustion products (CO, H2) but also could play an important role in soot evolution, could be isolated (Zhao and Liu 2022). It could be considered that the variation of the flame temperatures and species concentration between the FCO2 and the CO2 flames was purely caused by the chemical effects. The similar method was also used for NH3. The differences between the results from NH3 and FNH3 additions were attributed to the NH3 chemical effects, and the coupled chemical effects of NH3 and CO2 could be suggested from the differences between the CO2/NH3 and FCO2/FNH3 flames (Liu 2014; Li et al. 2015; Ying and Liu 2015; Liu 2015a; Luo and Liu 2017; Pan and Liu 2017; Deng et al. 2022; Zhao and Liu 2022).
3 Results and discussion
3.1 With Ar or He dilution
The effects of NH3 addition on the flame temperature, major species, free radicals and important intermediate species with Ar or He dilution were investigated, with emphasis on distinguishing the detailed effects of dilution, thermal and chemical effects of NH3.
3.1.1 Flame temperature profiles
The effects of NH3 with Ar or He dilution on the flame temperatures were shown in Fig. 1. The dilution and thermal effects of NH3 resulted in temperature differences between 0% NH3 and 20% FNH3 additions, and the differences between 20% NH3 and 20% FNH3 additions were due to the chemical effects of NH3. The combined influences of dilution, thermal, and chemical effects led to the differences between 0% NH3 and 20% NH3 additions. For clarity of exposition, the specific effects were marked in Fig. 1.
Flame temperature with Ar or He dilution and different NH3 additions. Notes: The 20% Ar/79% Ar represented the dilution gas was Ar both on the fuel side and the oxidizer side (F1-F5 conditions in Table 1). The 20% He/79% Ar and the 20% Ar/79% He represented dilution gas He replaced Ar on the fuel side (F6-F10 conditions in Table 1) and the oxidizer side (F11-F15 conditions in Table 1). The annotations for Figs. 2, 3, 4, 5, 6, 7, 8 and 9 have the same meanings, which will not be noted again in the following
In Fig. 1, the chemical effects of NH3 increased the flame temperature, whereas the dilution and thermal effects of NH3 caused the temperature to decrease. Consequently, the flame temperature was reduced due to the dilution and thermal effects of NH3, which had a more pronounced influence on the flame temperature than the chemical effects. The data in Fig. 1c showed that when He replaced Ar on the oxidizer side, the peak flame temperature was slightly lower, which may be due to the higher thermal diffusivity of He (Yelverton and Roberts 2008).
3.1.2 Major species and radicals
The mole fraction profiles of the fuel C2H4 with different NH3 additions were shown in Fig. 2. The differences between the NH3 addition and FNH3 addition revealed that the chemical effects of NH3 could slightly accelerate fuel consumption. In Fig. 2c, as He replaced Ar on the oxidizer side (F11–F15 conditions in Table 1), the profiles shifted toward the oxidizer side. To analyze the detailed consumption of C2H4, the C2H4 rates of production were performed in Fig. 3, it could be found that when the diluent was Ar (F1–F5 conditions in Table 1), C2H4 was mainly consumed by radicals, including C2H4 + H = C2H3 + H2 and C2H4 + OH = C2H3 + H2O reactions. The rates of these reactions responsible for the depletion of C2H4 decreased with the addition of NH3, which dominated the chemical effects. Therefore, it was necessary to analyze the mole fractions of these typical radicals, including H, O, and OH radicals.
Figure 4 gave the mole fractions of the H radical with different NH3 additions. When the dilution gas was Ar (F1–F5 conditions in Table 1) or He (F6–F15 conditions in Table 1), the mole fractions of H radical decreased with increasing concentrations of NH3 additive, and with 20% NH3 addition, the chemical effects of NH3 further reduced H concentration. While the blending ratio was 40%, the chemical effects of NH3 increased the mole fraction of H radical when the dilution gas was only Ar (as shown in Fig. 4a). The results were consistent with Deng et al. (2022) and Zhang et al. (2023).
Oxidation of PAH and soot particles may occur subsequent to formation in diffusion flames and the main oxidation reactants were OH, O, and O2 (Richter and Howard 2000). Therefore, it was necessary to analyze the mole fraction profiles of the O radical, which were shown in Fig. 5. Comparing the O radical mole fractions with NH3 and FNH3 additions, it illustrated that the chemical effects of NH3 could inhibit the formation of O radical regardless of the different dilution gas.
Furthermore, hydroxyl OH played a dominant role in the soot generation area of counter-flow diffusion flame, due to the relatively low concentration of O2. The OH radical was mainly oxidized by reacting with the active sites on the surface of soot, which could inhibit the further generation of soot (Frenklach et al. 2018). The mole fraction profiles were performed in Fig. 6. The addition of NH3 also suppressed the formation of OH radical, but different from the effects on O, H radicals, it was primarily because of the dilution and thermal effects of NH3, which were more pronounced than its chemical effects. Notably, the mole distributions of OH radical with NH3 addition were higher than those with FNH3 addition, indicating that the chemical effect of NH3 promoted the formation of OH radical indeed.
3.1.3 Intermediate hydrocarbon species
The intermediate hydrocarbon species would be oxidized with radicals to inhibit the soot formation to some extent. Therefore, the mole fractions of acetylene (C2H2), propynyl (C3H3), and benzene (A1), which were important soot precursors, in the combustion process of C2H4/NH3 diluted with Ar (F1–F5 conditions in Table 1) or He (F6–F15 conditions in Table 1) were shown in Figs. 7, 8 and 9. It could be found that the combined effects of the NH3 additive reduced the mole fractions of these important intermediate hydrocarbon species from Figs. 7a, 8a and 9a. The similar results had been achieved by Li et al. (2021), Shao et al. (2022) and Ren et al. (2022). In Fig. 7a, the differences between the mole distributions of C2H2 with NH3 addition and FNH3 addition demonstrated that the chemical effects of NH3 promoted the formation of C2H2. Similarly, the chemical effects of NH3 were also observed to increase the mole fractions of C3H3 and A1, as shown in subsequent Figs. 8a and 9a. Therefore, regardless of the dilution environment, the productions of C2H2, C3H3, and A1 were inhibited due to the dilution and thermal effects of NH3, instead of the chemical effects of NH3. It was worth pointing out that, as shown in Figs. 8a and 9a, the mole fractions of C3H3 and A1 were much lower when He replaced Ar on the fuel side or oxidizer side (F6–F15 conditions in Table 1). It revealed that the pathway of A1 generated by small molecule C3H3 in the C2H4/NH3 counter-flow diffusion flame was more easily susceptible to He.
To analyze the detailed formation and consumption of these important intermediate hydrocarbon species, the rates of production were analyzed in Figs. 7b, 8b and 9b. It could be found that the main reaction concerning C2H2 formation was C2H3(+M) = C2H2 + H(+M), although the dilution environment was different, and the reaction rates were greatly reduced with the chemical effects of NH3, as shown in Fig. 7b. Whereas the rate of the main reaction C2H2 + CH2 = C3H3 + H for C3H3 formation was reduced mainly due to the dilution and thermal effects of NH3, as shown in Fig. 8b. Figure 9b illustrated that the reaction A1- + C2H4 = A1 + C2H3 was mainly responsible for A1 formation, and the oxidation reaction A1- + H(+M) = A1(+M) was added when He diluted on the fuel side. Comparing the NH3 and FNH3 additions, it could be found that the chemical effects of NH3 promoted the reaction A1- + C2H4 = A1 + C2H3, whereas it suppressed other reactions.
3.2 With CO2 dilution
When CO2 was used as the diluent, the chemical effects of NH3 may be influenced by the chemical effects of CO2. Therefore, this section would put a special emphasis on the analysis of the coupled chemical effects of NH3 and CO2 on flame temperature, major species, free radicals, important intermediate hydrocarbon species, and oxygenated species.
3.2.1 Flame temperature profiles
As shown in Fig. 10, the addition of NH3 reduced the flame temperature. In Fig. 10a, the differences between CO2/NH3 and CO2/FNH3 revealed that the chemical effects of NH3 increased the temperature. The differences between CO2/NH3 and FCO2/FNH3 additions suggested that the coupled chemical effects of NH3 and CO2 also led to an increase in the flame temperature. However, with CO2 addition on the oxidizer side, as shown in Fig. 10b, the coupled chemical effects decreased the temperature. This was possibly because the chemical effects of CO2 lowered the temperature (Liu et al. 2001) and were dominant with oxidizer-side addition. Meanwhile, it was worth noting that the temperature was greatly lower with the oxidizer-side CO2 addition. Even when the condition was 40% NH3/79% CO2 (F15 condition in Table 2), the fuel could not be ignited. Therefore, the working condition would not be discussed in the following. As the known literature data showed that the threshold local temperature for the onset of soot formation in diffusion flames, Tc, satisfied the condition Tc > 1300–1500 K (Frolov et al. 2023), the analysis of soot precursors concentrations in flame conditions were necessary.
Flame temperature with CO2 dilution and different NH3 additions. Notes: The 20% (F)CO2/79% Ar and the 20% Ar/79% (F)CO2 respectively represented dilution gas CO2 replaced Ar on the fuel side (F1-F9 conditions in Table 2) and the oxidizer side (F10-F18 conditions in Table 2). The (F)CO2 represented the normal CO2 (CO2) or fiction CO2 (FCO2) addition. The annotations for Figs. 11, 12, 13, 14, 15, 16 and 18 have the same meanings, which will not be noted again in the following
3.2.2 Major species and radicals
Figure 11 gave the mole fraction profiles of C2H4. The comparison with the addition of 40% NH3 and 40% FNH3 illustrated that the chemical effects of NH3 reduced the C2H4 mole fraction slightly. However, the coupled effects of NH3 and CO2 on C2H4 were less pronounced when CO2 is added to either the fuel side or the oxidizer side.
The mole fraction profiles of H radical were displayed in Fig. 12. It illustrated that the addition of NH3 decreased the H concentration. The fuel-side CO2 dilution (F1–F9 conditions in Table 2) was similar to the condition of Ar-diluted, the chemical effects of NH3 reduced H concentration with 20% NH3 addition, while increased the mole fraction of H when the blending ratio was 40%, as shown in Fig. 12a. Whereas, Fig. 12b illustrated that the chemical effects of NH3 inhibit H formation with oxidizer-side CO2 dilution (F10–F18 conditions in Table 2). The differences between the H radical of 20% CO2/NH3 and 20% FCO2/NH3 were owing to the chemical effects of CO2, which could decrease the mole fraction of H radical This was the same result as the known literature (Liu 2015a). Moreover, the differences between NH3/CO2 and FNH3/FCO2 additions revealed that the reductions in H concentration were due to the coupled chemical effects of NH3 and CO2, especially with the oxidizer-side CO2 addition. The existing literature (Liu et al. 2001; Mahmoud et al. 2019) indicated that in ethylene counter-flow diffusion flames, the flame sheet front was situated on the oxidizer side of the stagnation plane. Consequently, the addition of oxidizer may result in a more pronounced increase in the CO2 concentrations at the flame sheet. The promotion of the CO2 + H = CO + OH reaction was also more significant for the oxidizer side addition.
Figure 13 illustrated the mole fraction profiles of O radical. Similar to the reduction of the mole fractions of H radical, the coupled chemical effects of NH3 and CO2 reduced O radical concentration, especially with the addition of CO2 on the oxidizing side.
However, the coupled chemical effects of NH3 and CO2 on the mole fraction of OH radical were different. As shown in Fig. 14a, the differences between NH3/CO2 and FNH3/FCO2 additions revealed that the coupled chemical effects of NH3 and CO2 promoted the formation of OH with fuel-side CO2 addition. Nevertheless, the coupled chemical effects had obvious inhibitory effects when CO2 addition on the oxidizer side, which were shown in Fig. 14b. It was possibly because the chemical effects of CO2 also decreased the OH radical mole fractions, which could be found from the differences between NH3/CO2 and NH3/FCO2 additions. And with the oxidizer-side CO2 dilution, the chemical effects of CO2 were more significant.
3.2.3 Intermediate hydrocarbon species
C2H2 was considered to be an important small molecule precursor of soot formation (Ruiz et al. 2007; Chernov et al. 2014; Wang and Chung 2019). C3H3 and A1 also were very vital for the soot production.
Firstly, the chemical effects of CO2 and NH3 on C2H2 concentration were identified in Fig. 15. The differences between CO2/NH3 and FCO2/FNH3 additions represented the coupled chemical effects of NH3 and CO2. Compared with Fig. 15a, b, it could be found that the coupled chemical effects of NH3 and CO2 were weak when CO2 was from the fuel side (F1–F9 conditions in Table 2), while it led to a significant reduction in the mole fractions of C2H2 with CO2 addition on the oxidizer side (F10–F18 conditions in Table 2). To discover C2H2 variation trends with increasing NH3 additions, Fig. 15b gave the C2H2 peak mole fractions. It revealed explicitly that the coupled chemical effects of NH3 and CO2 increased the mole fractions of C2H2 more significantly with the concentration of NH3 increasing, when CO2 was added to the fuel side. Fig. 15c illustrated the rates of production analysis of C2H2, it could be found that the coupled chemical effects of NH3 and CO2 decreased the rate of the reaction C2H3(+M) = C2H2 + H(+M), which was the main reaction for C2H2 formation. Therefore, the participation of C2H2 in the growth reaction of polycyclic aromatic hydrocarbons (PAHs) through the HACA mechanism would be affected (Frenklach 2002), then the surface growth of soot could be inhibited to some extent.
According to the peak mole fractions of C2H2 shown in Fig. 15b and the calculation method shown in Table 4, the chemical effects of NH3 and CO2 could be demonstrated quantitatively (Pan and Liu 2017). In Table 4, Ai (i = 1, 2, 3) represented the chemical effects of NH3 in the combined effects of NH3 and CO2. Similarly, Bi (i = 1, 2, 3) denoted the chemical effects of CO2, Ai + Bi (i = 1, 2, 3) represented the sum of the separate chemical effects of NH3 and CO2, Ci (i = 1, 2, 3) was equal to the coupled chemical effects of NH3 and CO2. In the results of the calculation, a positive value indicated an increase and a negative value represented a decrease. When the condition was 40% NH3/79% CO2 (F15 condition in Table 2), the fuel could not be ignited, so only the coupled chemical effects of adding 20% NH3 were considered with CO2 addition on the oxidizer side. The B1, B2, B3 revealed that the chemical effects of CO2 suppressed the formation of C2H2, which was consistent with the known literature (Liu et al. 2001; Liu 2015a), whereas the chemical effects of NH3 increased the mole fractions of C2H2. The former dominated the latter and the net effects were to reduce the mole fractions of C2H2 with the oxidizer-side CO2 addition, different from CO2 addition on the fuel side. Compared with the (Ai + Bi) and Ci, it could be found that the changes of C2H2 mole fractions in the simultaneous presence of chemical effects of NH3 and CO2 were slightly smaller than the sum of the separate chemical effects of NH3 and CO2. It suggested that chemical interactions between the dopants were negligible, as the results of Mahmoud et al. (2019).
Figure 16 illustrated the mole fraction profiles and the rates of production of C3H3. From Fig. 16a, it could be concluded that the addition of NH3 reduced the mole fractions of C3H3. The differences between NH3/CO2 and FNH3/FCO2 revealed that the coupled chemical effects of NH3 and CO2 increased the concentration of C3H3 with CO2 addition on the fuel side (F1–F9 conditions in Table 2), contrary to the results of the oxidizer-side CO2 addition (F10–F18 conditions in Table 2). Figure 16b illustrated that C3H3 was generated in large quantities by the complex reactions of C2H2 + CH2, which was one of the reasons why C3H3 was considered as the main precursor of A1 generated by small molecules (Jin et al. 2015), then PAHs were easy to form soot after physical or chemical coalesce. When CO2 was added to the oxidizer side, the coupled chemical effects of NH3 and CO2 decreased the rates of all reactions, whereas CO2 was from the fuel side, the rates of reactions PC3H4 = C3H3 + H and 2C3H3 =>A1- + H were decreased mainly due to the coupled dilution and thermal effects of NH3 and CO2. The bar plot of the chemical effects of NH3 and CO2 on the peak mole fractions of C3H3 was shown in Fig. 17. The formation of C3H3 was inhibited mainly because of the chemical effects of CO2, Zhang et al. (2018) also gave the similar results, while the chemical effects of NH3 promoted the formation of C3H3. When CO2 was added to the oxidizer side, the coupled chemical effects of NH3 and CO2 could inhibit the generation of C3H3 due to the dominance of the chemical effects of CO2, different from the fuel-side CO2 addition.
The Difference of peak mole fractions of C3H3. Notes: A represented the fuel side was 60% C2H4 + 20% CO2 + 20% NH3, the oxidizer side was 79% Ar + 21% O2; B represented the fuel side was 40% C2H4 + 20% CO2 + 40% NH3, the oxidizer side was 79% Ar + 21% O2; C represented the fuel side was 60% C2H4 + 20% Ar + 20% NH3, the oxidizer side was 79% CO2 + 21% O2. The positive results represented an increase and the negative results represented a decrease. The letters in Figs. 19 and 20 have the same meanings, which will not be noted again in the following.
It was generally agreed that PAHs were the precursors of soot formation, and the unique chemical structure of benzene ring (A1) was a prominent factor (Dobbins 2007). Therefore, it was necessary to analyze the variations of A1 concentration. The mole fraction profiles of A1 performed in Fig. 18a revealed that the normal NH3 addition could cause the decrease of A1 mole fractions. However, the coupled chemical effects of NH3 and CO2 on the mole fraction of A1 were different due to the diverse sides and concentrations of CO2 addition. With the fuel-side CO2 addition (F1–F9 conditions in Table 2), the differences between CO2/NH3 and FCO2/FNH3 additions suggested that the coupled chemical effects of NH3 and CO2 increased the A1 mole fractions, in contradiction with adding CO2 to the oxidizer side. Meanwhile, comparing the two situations, it could be found that the concentration of A1 was much lower when CO2 was added to the oxidizer side (F10–F18 conditions in Table 2).
To analyze the detailed formation and consumption of A1, Fig. 18b gave the rates of production of A1. It could be found that the main reactions responsible for A1 formation were the closed-loop reactions 2C3H3=>A1 and C2H2 + C4H5-2 = A1 + H. The main reactions responsible for A1 consumption were A1 + H = A1- + H2, A1 + H = C4H5-2 + C2H2, and A1 + OH = A1- + H2O. The pyrolysis consumption paths were mainly through hydrogen extraction reaction attacked by H radical (Yang et al. 2015). Comparing with CO2/NH3 and FCO2/FNH3 additions, it could be concluded that the reduced rates of all reactions were due to the coupled chemical effects of NH3 and CO2 with the oxidizer-side CO2 addition. While adding CO2 to the fuel side, the rate of reactions 2C3H3=>A1 and A1 + H = C4H5-2 + C2H2 were decreased mainly because of the coupled dilution and thermal effects of NH3 and CO2.
By distinguishing the chemical effects of NH3 and CO2, as shown in Fig. 19, it could be found that the chemical effects of NH3 promoted the generation of A1, while the chemical effects of CO2 suppressed it. The results tied well with previous study (Naseri et al. 2017). Therefore, the coupled chemical effects of NH3 and CO2 on A1 mole fractions depended on the dominant effects. With the oxidizer-side CO2 addition, the chemical effects of CO2 dominated the chemical effects of NH3, then the coupled chemical effects of NH3 and CO2 decreased the mole fraction of A1. For fuel-side CO2 addition, the chemical effects of NH3 on the A1 mole fraction were more significant and then the coupled chemical effects of NH3 and CO2 increased A1 concentration.
As the known literature (Liu et al. 2015), the chemical effect of CO2 on soot loading reduction was primarily through reducing the rates of soot formation steps, rather than prompting soot oxidation. Therefore, with the addition of CO2 on the oxidizer side, the coupled chemical effects of NH3 and CO2, which were mainly influenced by the chemical effect of CO2, reduced both the mole fractions of O, H, OH radicals, and C2H2, C3H3, A1 soot precursors.
3.2.4 Intermediate oxygenated species
In addition to intermediate hydrocarbon species, intermediate oxygenated species such as formaldehyde (CH2O) and acetaldehyde (CH3CHO) were also important aldehyde contaminants generated in the combustion of hydrocarbon fuels. Formaldehyde was particularly irritant to the respiratory system and eyes, toxic and carcinogenic and could accelerate the production of the harmful substance ozone (Konnov et al. 2021). Therefore, the mole fraction profiles of CH2O and CH3CHO in C2H4/NH3 flames with diluents were necessary to be revealed in Fig. 20.
Due to the chemical effects of CO2, the chemical effects of NH3 performed diverse impacts on CH2O and CH3CHO concentrations with different side CO2 additions. Figure 20a illustrated that the coupled chemical effects of NH3 and CO2 decreased the mole fractions of CH2O with the fuel-side CO2 addition slightly (F1–F9 conditions in Table 2), whereas for the oxidizer side (F10–F18 conditions in Table 2), the coupled chemical effects promoted CH2O formation. It could be explained that the chemical effects of CO2 promoted the formation of CH2O, and the coupled chemical effects of NH3 and CO2 were mainly affected by the chemical effects of CO2, leading to an increase of the mole fraction of CH2O with the oxidizer-side CO2 addition.
Additionally, the chemical effects of NH3 and CO2 on the mole fraction of CH3CHO were different from those of CH2O. According to Fig. 20b, it could be found that the chemical effects of NH3 promoted the formation of CH3CHO, meanwhile, the coupled chemical effects of NH3 and CO2 further promoted the formation of CH3CHO, especially when CO2 was added to the oxidizer side.
4 Conclusions
The chemical kinetic analyses for the chemical effects of NH3 with different diluents, in particular the coupled chemical effects of NH3 and CO2 in ethylene counter-flow diffusion flames, were investigated in this work. The analyses encompassed several critical aspects, such as flame temperature, major species, typical free radicals, intermediate hydrocarbon products, and oxygenated species. The outcomes of the study led to the following conclusions:
-
(1)
Regardless of the diluents utilized, the flame temperature and the mole fractions of O, H, OH, C2H2, C3H3, A1 were decreased with NH3 addition in C2H4/NH3 counter-flow diffusion flames.
-
(2)
With Ar or He dilution, the chemical effects of NH3 promoted the formation of OH, C2H2, C3H3, and A1. With replacing Ar with He on the oxidizer side, the high thermal diffusivity of He reduced the flame temperature, and the pathway of A1 generated by small molecules C3H3 was more easily susceptible to He, leading to the lower concentrations of C3H3 and A1.
-
(3)
With fuel-side CO2 addition, the coupled chemical effects of NH3 and CO2 increased the flame temperature and the mole fractions of OH, C2H2, C3H3, A1. However, with oxidizer-side CO2 addition, the coupled chemical effects, which were affected by the chemical effects of CO2 significantly, inhibited the flame temperature and the formations of O, H, OH, C2H2, C3H3, A1.
-
(4)
The chemical effects of NH3 resulted in a decrease in the mole fraction of CH2O and an increase in the concentration of CH3CHO. The coupled chemical effects of NH3 and CO2 increased the CH2O concentration with the oxidizer-side CO2 addition.
Availability of data and materials
Data will be available upon the request for authors.
References
Abián M, Millera A, Bilbao R, Alzueta MU (2012) Experimental study on the effect of different CO2 concentrations on soot and gas products from ethylene thermal decomposition. Fuel 91:307–312
Bennett AM, Liu P, Li ZP, Kharbatia NM, Boyette W, Masri AR, Roberts WL (2020) Soot formation in laminar flames of ethylene/ammonia. Combust Flame 220:210–218
Boyette WR, Steinmetz SA, Guiberti TF, Dunn MJ, Roberts WL, Masri AR (2021) Soot formation in turbulent flames of ethylene/hydrogen/ammonia. Combust Flame 226:315–324
Chen C, Liu D (2023) Review of effects of zero-carbon fuel ammonia addition on soot formation in combustion. Renewable Sustainable Energy Rev 185:113640
Chen C, Yang Q, Zhang R, Liu D. (2022) Regulation of organic hydrocarbon pollutants in coal volatiles combustion with CO2 addition. J Cleaner Prod 374:133904.
Chernov V, Thomson MJ, Dworkin SB, Slavinskaya NA, Riedel U (2014) Soot formation with C1 and C2 fuels using an improved chemical mechanism for PAH growth. Combust Flame 161:592–601
Deng QG, Ying YY, Liu D (2022) Detailed chemical effects of ammonia as fuel additive in ethylene counterflow diffusion flames. Int J Hydrogen Energy 47:33498–33516
Dobbins RA (2007) Hydrocarbon nanoparticles formed in flames and diesel engines. Aerosol Sci Technol 41:485–496
Dong WL, Xiang LK, Gao J, Qiu BB, Chu HQ (2023) Effect of CO2 dilution on laminar burning velocities, combustion characteristics and NOx emissions of CH4/air mixtures. Int J Coal Sci Technol 10(1):72. https://doi.org/10.1007/s40789-023-00655-9
Du DX, Axelbaum RL, Law CK (1990) The influence of carbon dioxide and oxygen as additives on soot formation in diffusion flames. Symp (Int) Combust 23:1501–1507
Frenklach M (2002) Reaction mechanism of soot formation in flames. Phys Chem Chem Phys 4:2028–2037
Frenklach M, Liu ZY, Singh RI, Galimova GR, Azyazov VN, Mebel AM (2018) Detailed, sterically-resolved modeling of soot oxidation: role of O atoms, interplay with particle nanostructure, and emergence of inner particle burning. Combust Flame 188:284–306
Frolov SM, Ivanov VS, Frolov FS, Vlasov PA, Axelbaum R, Irace PH, Yablonsky G, Waddell K (2023) Soot formation in spherical diffusion flames. Mathematics 11:261
Glarborg P, Miller JA, Ruscic B, Klippenstein SJ (2018) Modeling nitrogen chemistry in combustion. Prog Energy Combust Sci 67:31–68
Grcar JF, Glarborg P, Bell JB, Day MS, Loren A, Jensen AD (2004) Effects of mixing on ammonia oxidation in combustion environments at intermediate temperatures. Proc Combust Inst 30:1193–1200
Gu MY, Chu HQ, Liu FS (2016) Effects of simultaneous hydrogen enrichment and carbon dioxide dilution of fuel on soot formation in an axisymmetric co-flow laminar ethylene/air diffusion flame. Combust Flame 166:216–228
Guo HS, Smallwood GJ (2008) A numerical study on the influence of CO2 addition on soot formation in an ethylene/air diffusion flame. Combust Sci Technol 180:1695–1708
Ichikawa A, Naito Y, Hayakawa A, Kudo T, Kobayashi H (2019) Burning velocity and flame structure of CH4/NH3/air turbulent premixed flames at high pressure. Int J Hydrogen Energy 44:6991–6999
Jin HF, Frassoldati A, Wang YZ, Zhang XY, Zeng MR, Li YY, Qi F, Cuoci A, Faravelli T (2015) Kinetic modeling study of benzene and PAH formation in laminar methane flames. Combust Flame 162:1692–1711
Kobayashi H, Hayakawa A, Somarathne KDKA, Okafor EC (2019) Science and technology of ammonia combustion. Proc Combust Inst 37:109–133
Konnov AA, Nilsson EJK, Christensen M, Zhou CW (2021) Combustion chemistry of methoxymethanol. Part II: Laminar flames of methanol + formaldehyde fuel mixtures. Combust Flame 229:111411
Lecoustre VR, Sunderland PB, Chao BH, Axelbaum RL (2012) Numerical investigation of spherical diffusion flames at their sooting limits. Combust Flame 159:194–199
Lhuillier C, Brequigny P, Lamoureux N, Contino F, Rousselle CM (2020) Experimental investigation on laminar burning velocities of ammonia/hydrogen/air mixtures at elevated temperatures. Fuel 263:116653
Li ZS, Han W, Liu D, Chen Z (2015) Laminar flame propagation and ignition properties of premixed iso-octane/air with hydrogen addition. Fuel 158:443–450
Li YP, Zhang YR, Zhan R, Huang Z, Lin H (2021) Experimental and kinetic modeling study of ammonia addition on PAH characteristics in premixed n-heptane flames. Fuel Process Technol 214:106682
Liu D (2014) Kinetic analysis of the chemical effects of hydrogen addition on dimethyl ether flames. Int J Hydrogen Energy 39:13014–13019
Liu D (2015a) Chemical effects of carbon dioxide addition on dimethyl ether and ethanol flames: a comparative study. Energy Fuels 29:3385–3393
Liu D (2015b) Detailed influences of ethanol as fuel additive on combustion chemistry of premixed fuel-rich ethylene flames. Sci China Technol Sci 58:1696–1704
Liu FS, Guo HS, Smallwood GJ, Gülder ÖL (2001) The chemical effects of carbon dioxide as an additive in an ethylene diffusion flame: implications for soot and NOx formation. Combust Flame 125:778–787
Liu FS, Guo HS, Smallwood GJ (2003) The chemical effect of CO2 replacement of N2 in air on the burning velocity of CH4 and H2 premixed flames. Combust Flame 133:495–497
Liu D, Santner J, Togbé C, Felsmann D, Koppmann J, Lackner A, Yang XL, Shen XB, Ju YG, Kohse-Höinghaus K (2013) Flame structure and kinetic studies of carbon dioxide-diluted dimethyl ether flames at reduced and elevated pressures. Combust Flame 160:2654–2668
Liu FS, Karatas AE, Gülder ÖL, Gu MY (2015a) Numerical and experimental study of the influence of CO2 and N2 dilution on soot formation in laminar co-flow C2H4/air diffusion flames at pressures between 5 and 20 atm. Combust Flame 162:2231–2247
Liu Y, Cheng XB, Li Y, Qiu L, Wang X, Xu YS (2021) Effects of ammonia addition on soot formation in ethylene laminar diffusion flames. Fuel 292:120416
Luo MY, Liu D (2017) Kinetic analysis of ethanol and dimethyl ether flames with hydrogen addition. Int J Hydrogen Energy 42:3813–3823
Mahmoud NM, Yan FW, Zhou MX, Xu L, Wang Y (2019) Coupled effects of carbon dioxide and water vapor addition on soot formation in ethylene diffusion flames. Energy Fuels 33:5582–5596
Naseri A, Veshkini A, Thomson MJ (2017) Detailed modeling of CO2 addition effects on the evolution of soot particle size distribution functions in premixed laminar ethylene flames. Combust Flame 183:75–87
Okafor EC, Naito Y, Colson S, Ichikawa A, Kudo T, Hayakawa A, Kobayashi H (2018) Experimental and numerical study of the laminar burning velocity of CH4-NH3-air premixed flames. Combust Flame 187:98–185
Park J, Hwang DJ, Choi JG, Lee KM (2003) Chemical effects of CO2 addition to oxidizer and fuel streams on flame structure H2–O2 counterflow diffusion flames. Int J Energy Res 27:1205–1220.
Pan W, Liu D (2017) Coupled chemical effects of carbon dioxide and hydrogen additions on premixed lean dimethyl ether flames. Sci China Technol Sci 60:102–115
Ren F, Cheng XG, Gao Z, Huang Z, Zhu L (2022) Effects of NH3 addition on polycyclic aromatic hydrocarbon and soot formation in C2H4 co-flow diffusion flames. Combust Flame 241:111958
Renard C, Dias V, Tiggelen PJV, Vandooren J (2009) Flame structure studies of rich ethylene-oxygen-argon mixtures doped with CO2, or with NH3, or with H2O. Proc Combust Inst 32:631–637
Richter H, Howard JB (2000) Formation of polycyclic aromatic hydrocarbons and their growth to soot—a review of chemical reaction pathways. Prog Energy Combust Sci 26:565–608
Ruiz MP, Callejas A, Millera A, Alzueta MU, Bilbao R (2007) Soot formation from C2H2 and C2H4 pyrolysis at different temperatures. J Anal Appl Pyrolysis 79:244–251
Service RF (2018) Liquid sunshine. Science 316:120–123
Shao C, Campuzano F, Zhai YT, Wang HY, Zhang W, Sarathy SM (2022) Effects of ammonia addition on soot formation in ethylene laminar premixed flames. Combust Flame 235:111698
Shu B, He X, Ramos CF, Fernandes RX, Costa M (2021) Experimental and modeling study on the auto-ignition properties of ammonia/methane mixtures at elevated pressures. Proc Combust Inst 38:261–268
Sonker M, Tiwary SK, Shreyash N, Bajpai S, Ray M, Kar SK, Balathanigaimani MS (2022) Ammonia as an alternative fuel for vehicular applications: paving the way for adsorbed ammonia and direct ammonia fuel cells. J Cleaner Prod 326:133960
Vancoillie J, Christensen M, Nilsson EJK, Verhelst S, Konnov AA (2013) The effects of dilution with nitrogen and steam on the laminar burning velocity of methanol at room and elevated temperatures. Fuel 105:732–738
Wang H (2011) Formation of nascent soot and other condensed-phase materials in flames. Proc Combust Inst 33:41–67
Wang Y, Chung SH (2019) Soot formation in laminar counterflow flames. Prog Energy Combust 74:152–238
Wang Y, Raj A, Chung SH (2013) A PAH growth mechanism and synergistic effect on PAH formation in counterflow diffusion flames. Combust Flame 160:1667–1676
Wang W, Xu L, Yan J, Wang Y (2020) Temperature dependence of the fuel mixing effect on soot precursor formation in ethylene-based diffusion flames. Fuel 267:117121
Wang Y, Gu MY, Wu JJ, Cao L, Lin YY, Huang XY (2021) Formation of soot particles in methane and ethylene combustion: a reactive molecular dynamics study. Int J Hydrogen Energy 46:36557–36568
Yang JZ, Zhao L, Yuan W, Qi F, Li YY (2015) Experimental and kinetic modeling investigation on laminar premixed benzene flames with various equivalence rations. Proc Combust Inst 35:855–862
Yelverton TLB, Roberts WL (2008) Soot surface temperature measurements in pure and diluted flames at atmospheric and elevated pressures. Exp Therm Fluid Sci 33:17–22
Ying YY, Liu D (2015) Detailed influences of chemical effects of hydrogen as fuel additive on methane flame. Int J Hydrogen Energy 40:3777–3788
Zamfirescu C, Dincer I (2009) Ammonia as a green fuel and hydrogen source for vehicular applications. Fuel Process Technol 90:729–737
Zhang YR, Wang LJ, Liu P, Guan B, Ni H, Huang Z, Lin H (2018) Experimental and kinetic study of the effects of CO2 and H2O addition on PAH formation in laminar premixed C2H4/O2/Ar flames. Combust Flame 192:439–451
Zhang K, Xu YS, Liu Y, Wang HK, Liu YM, Cheng XB (2023) Effects of ammonia addition on soot formation in ethylene laminar diffusion flames. Part 2. Further insights into soot inception, growth and oxidation. Fuel 331:125623
Zhao R, Liu D (2022) Temperature dependence of chemical effects of ethanol and dimethyl ether mixing on benzene and PAHs formation in ethylene counterflow diffusion flames. Energy 257:124809
Zhou SK, Yang WJ, Tan HZ, An QW, Wang JH, Dai HC, Wang XX, Wang XB, Deng SH (2021) Experimental and kinetic modeling study on NH3/syngas/air and NH3/bio-syngas/air premixed laminar flames at elevated temperature. Combust Flame 233:111594
Zhou MX, Yan FW, Ma LH, Jiang P, Wang Y, Chung SH (2022) Chemical speciation and soot measurements in laminar counterflow diffusion flames of ethylene and ammonia mixtures. Fuel 308:122003
Acknowledgements
Authors thank the editor and reviewers’ suggestions for improving the paper.
Funding
This work was supported by the National Natural Science Foundation of China (52076110, 52106160), Jiangsu Provincial Natural Science Foundation of China (BK20200490, BK20220955) and the Fundamental Research Funds for the Central Universities (30923010208 and 30920031103).
Author information
Authors and Affiliations
Contributions
ZMS: investigation, data curation, formal analysis, and writing-original draft; TTX: writing-review and editing; JYX, QGD, XZ, TJL, YYY: investigation; DL: methodology, supervision, writing-review and editing, supervision, project administration, and funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shu, Z., Xu, T., Xiao, J. et al. Comprehensive kinetic study on ammonia/ethylene counter-flow diffusion flames: influences of diluents. Int J Coal Sci Technol 11, 15 (2024). https://doi.org/10.1007/s40789-024-00663-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40789-024-00663-3