Abstract
To test the effectiveness of N2 and CO2 in preventing coal from spontaneously combusting, researchers used an adiabatic oxidation apparatus to conduct an experiment with different temperature starting points. Non-adsorbed helium (He) was used as a reference gas, and coal and oxygen concentration temperature variations were analyzed after inerting. The results showed that He had the best cooling effect, N2 was second, and CO2 was the worst. At 70℃ and 110℃, the impact of different gases on reducing oxygen concentration and the cooling effect was the same. However, at the starting temperature of 150℃, CO2 was less effective in lowering oxygen concentration at the later stage than He and N2. N2 and CO2 can prolong the flame retardation time of inert gas and reduce oxygen displacement with an initial temperature increase. When the starting temperature is the same, N2 injection cools coal samples and replaces oxygen more effectively than CO2 injection. The flame retardancy of inert gas is the combined result of the cooling effect of inert gas and the replacement of oxygen. These findings are essential for using inert flame retardant technology in the goaf.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Most of China’s coal mines belong to underground mining, and after coal is extracted, a goaf will be formed behind the working face. Coal spontaneous combustion in goaf areas is one of the common disasters in coal mines in China, accounting for over 60% of the total number of coal mine fires (Wang et al. 2021a, b, c). The spontaneous combustion of coal in goaf not only generates a large amount of toxic and harmful gases (Song et al. 2014; Wang et al. 2021a, b) but also induces various thermal disasters (Zhao et al. 2022; Xu et al. 2022; Xu et al. 2022; Zeng et al. 2022).
Inert gas fire prevention and extinguishing are used technologies in goaf (Su et al. 2017; Liu et al. 2020; Fang et al. 2022; Ming et al. 2022). Nitrogen (N2) and Carbon dioxide (CO2) have stable chemical properties and are commonly used gases in goaf to suppress the spontaneous combustion of residual coal. The earliest case of using CO2 to prevent and extinguish fires was in the Clackmanan coal mine in Scotland in 1851, while N2 was first used to extinguish mine fires in 1949 (Fang 2021). After injecting inert gas into the goaf, it can significantly reduce the oxygen concentration and carry away heat, effectively reducing the range of coal spontaneous combustion danger areas in the goaf (Yuan and Smith. 2014; Liu et al. 2016; Zhang et al., 2019). However, effectiveness is the primary consideration for decision-makers in determining which gas to use.
In recent years, some scholars have extensively studied the flame retardancy of N2 and CO2. Wu et al. (2018) and Lei et al. (2020) conducted experiments on inert gas suppression during open-flame coal combustion in confined spaces. They found that CO2 has a better ability to extinguish open-flame coal combustion. Jiang et al. (2014) studied the apparent activation energy after introducing air, N2-air mixture gas, and CO2-air mixture gas and found that CO2 has a better inhibitory effect on coal spontaneous combustion. Smith and Glasser (2005) studied the impact of various factors on the low-temperature oxidation rate of coal, and the results showed that N2 concentration can reduce the oxidation reaction rate of coal. Zhu et al. (2014a) carefully studied the changes in oxygen consumption rate under different CO2-N2-O2 mixed gas conditions through temperature-programmed experiments. They found that as the concentration of N2 and CO2 increased, the oxygen consumption rate of coal samples gradually decreased. These studies have shown that N2 and CO2 have good flame retardancy, and almost all believe CO2 has better flame retardancy. However, there is still a process of environmental heat dissipation during comparing the two.
Adiabatic oxidation experiment is an essential means to study the spontaneous combustion characteristics of coal (Beamish et al. 2000; Beamish 2005; Tan et al. 2013; Zhu et al. 2014b; Arisoy et al. 2017; Wang et. 2018; Zhang et al. 2020). When the adiabatic oxidation method is used to study the spontaneous combustion process of coal, a certain amount of coal sample is first supplied with oxygen to heat itself. Then the tiny heat generated by the oxidation of the coal sample in the coal sample tank is retained through the adiabatic devices such as Cryogenic storage dewar, insulating asbestos, and thermostat to realize the self-heating process of the coal sample until the temperature rises. Spontaneous combustion occurs (Tan et al. 2013). The adiabatic oxidation method can reflect the oxidation heat release characteristics of coal itself and avoid the influence of external heat sources (Li et al. 2019). Therefore, using adiabatic conditions to cool the oxidized crushed coal can accurately compare the flame retardancy of inert gas, as heat exchange only occurs between inert gas and coal.
This article compares the flame retardancy of N2 and CO2 on oxidized and heated crushed coal under adiabatic oxidation conditions to eliminate the influence of external heat exchange. The experiment involves heating the coal sample to a specific temperature through self-heating under adiabatic conditions and then injecting it with the same inert gas flow rate to observe the difference in flame retardancy between the two gases during the low-temperature oxidation stage of coal. The aim is to provide theoretical guidance for using inert gas to prevent spontaneous coal combustion.
2 Experimental equipment and process
2.1 Experimental setup
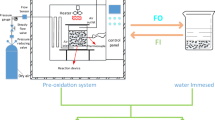
The experimental device adopts a coal low-temperature adiabatic oxidation experimental device, which mainly includes a gas supply system, a vacuum pumping system, a temperature and flow control system, a reaction system, and a constant temperature box, as shown in Fig. 1. The gas supply system can provide four types of high-pressure gases (O2, N2, CO2, and He) with constant flow rate and temperature for the experiment; The vacuum system can pre extract oxygen from the coal sample tank to avoid oxidation of the coal sample during the drying process; The temperature and flow control system mainly controls temperature and flow; The reaction system primarily consists of a coal sample tank, which can accommodate approximately 200 g of coal samples; The incubator is mainly composed of a constant temperature chamber and copper coils. During the adiabatic oxidation experiment, the temperature in the constant temperature chamber follows the temperature in the coal sample tank and remains consistent with the temperature in the coal sample tank, with an error of ± 0.1 ~ 0.2 ℃; The copper coil is about 6 m long and can ensure that the imported gas is preheated in the constant temperature chamber. After entering the coal sample tank, the gas temperature is consistent with the coal temperature and will not take away the coal temperature.
Experimental device for low-temperature adiabatic oxidation of coal
Note 1- Pressure reducing valve; 2- Pressure stabilizing valve; 3- Pressure gauge; 4-6-way valve; 5- Inlet mixing chamber; 6- Temperature and flow control system; 7- Thermostat; 8-Copper coil; 9- Heater; 10- Fan; 11- Air outlet pipeline; 12- Thermocouple; 13- Temperature measurement line; 14- Coal sample; 15- Coal sample tank; 16- Gas outlet mixing chamber
2.2 Experimental process
After the experimental coal samples were removed from the coal seam, they were wrapped with cling film and transported to the laboratory. Coal samples with particle sizes of 3 ~ 5 mm were prepared according to (GB474-2008 Preparation Methods of Coal Samples). Nine sets of parallel experiments were conducted with the coal low-temperature adiabatic oxidation experimental apparatus, and the experimental steps were:
(1) Weigh the prepared 200 g coal sample into the jar and then load the coal sample jar into the experimental system.
(2) Close the inlet valve after checking the gas tightness of the device, turn on the vacuum pump, and carry out vacuuming. The time of evacuation is 2 h.
(3) After turning off the vacuum pump, open the valve of the high-pressure N2 bottle, and pass N2 into the coal sample tank at the maximum flow rate (300ml/ min) on the adiabatic oxidation device, and after a certain period when the coal sample tank is no longer under negative pressure, open the valve of the outlet and monitor the oxygen concentration.
(4) When the oxygen concentration at the outlet is zero, set the temperature rise program to warm up the coal sample. After heating up, gradually reduce the N2 flow rate to 150ml/min and dry the coal sample at 105℃ for 15 h (Tan et al. 2013; Zhu et al. 2014b).
(5) After the drying is completed, the temperature is set to manual mode to keep the temperature of the coal sample tank at 40℃ for 20 min. The N2 is replaced with oxygen, the oxygen flow rate is 60ml/min, the temperature is set to automatic tracking mode, and the coal sample tank coal sample starts to undergo a self-heating reaction.
(6) After the temperature of the coal body in the coal sample tank increases, when the temperature reaches 70 °C, 110 and 150 °C, respectively, immediately inject N2 for flame retardation until the temperature drops to 30 °C;
(7) Repeat steps (1) ~ (6) for the flame retardant experiments of CO2 and He, respectively.
3 Results and discussion
3.1 Temperature changes of coal samples after inert gas injection
The temperature changes of each group of experiments are shown in Fig. 2. From Fig. 2, and it can be observed that the maximum heating time for each group of experiments does not exceed 70 h. During the self-heating phase, there were some differences in the warming curves for different target temperatures, but the overall error was small, which indicates that the experiments were reproducible. In the cooling stage, with the injection of inert gas, the temperature of the coal sample tank showed a trend of first briefly increasing and then continuously decreasing. Overall, the cooling curves of the inert gas flame retardant experiment at different temperature stages were different, while the higher the target temperature, the longer the flame retardant time required under the same inert gas injection flow rate.
3.2 Changes in oxygen concentration after inert gas injection
The curve of the outlet oxygen concentration with time during the cooling stage after injecting three types of inert gas is shown in Fig. 3.
It is easy to analyze from Fig. 3:
(1) Regardless of the gas type, the outlet oxygen change curve at an initial temperature of 150 ℃ was always below 110 ℃, and the oxygen change curve at an initial temperature of 70 ℃ was at the top. However, during the initial cooling stage in Fig. 3a, the oxygen concentration change curve with an initial temperature of 70 ℃ was located below 110 ℃, which the discreteness of the instrument may cause. The position relationship of the oxygen concentration change curve at the outlet after inert injection at three initial temperatures was jointly determined by the coal oxidation rate of coal and the residual amount of oxygen in the coal sample tank. According to the Arrhenius formula, the higher the temperature, the faster the reaction rate of coal, which means that the oxygen consumption of coal is greater simultaneously. After insertion, the remaining oxygen in the coal sample tank was the highest at an initial temperature of 70 ℃, followed by 110 ℃, and the lowest at 150 ℃. These two factors determine the positional relationship of the oxygen concentration curve with time at three different initial temperatures.
(2) Comparing the temperature changes of the same gas at different initial temperatures, it was found that the positional relationship of the curves corresponding to He, N2, and CO2 is different. Among them, after He injection (Fig. 3a), the position relationship of the three curves was almost equidistant during the approximately vertical decrease in oxygen concentration. The three-position relationships of the other two gases were relatively similar: the curves corresponding to the initial temperature of 70 ℃ and 110 ℃ almost overlap, and the curves corresponding to the initial temperature of 150 ℃ had noticeable spacing. The corresponding relationship between the three curves indicated that as the initial temperature increases, the concentration of outlet oxygen is determined by the rate of oxygen consumption, which means that the rate at which N2 and CO2 displace oxygen from the coal is lower than the rate at which oxygen is consumed during the high-temperature stage. Therefore, under high-temperature conditions, increasing the flow rate of N2 or CO2 accelerates the dilution of oxygen concentration in coal pores and replaces adsorbed oxygen. Only by reducing the oxygen concentration in the coal at a rate more significant than the oxygen consumption rate can further heating the coal be avoided.
3.3 Flame retardant efficiency
3.3.1 Temperature and oxygen concentration decreases at the same initial temperature
The cooling and outlet oxygen concentrations of different inert gases under the same initial temperature conditions are shown in Fig. 4.
From Fig. 4, it can be observed that:
(1) The longest time required for each group of experiments to cool down to 30℃ is inconsistent with the longest time for oxygen to drop to 1%. The cooling time and the Fall time of oxygen concentration are shown in Table 1. For He, when the temperature drops from 70℃ to 30℃, the concentration of oxygen at the outlet was 34%, indicating that He has a more robust cooling ability than the ability to replace oxygen; For the other two gases, when the temperature drops to 30℃, the outlet concentration was already less than 3%, indicating that at 70℃, the cooling ability of N2 and CO2 was equivalent to the ability to replace oxygen. When the initial temperature was 110℃, the temperature decrease was the same as the time of oxygen concentration decrease, indicating that the cooling and oxygen displacement abilities of these three inert gases were equivalent. When the initial temperature was 150℃, the time when the oxygen concentration dropped to 3% was longer than the time when the temperature dropped. This was because after a certain degree of coal oxidation, the coal structure became looser (Zhu et al. 2023), and the difficulty of oxygen replacement increased.
(2) The main reason for the temperature drop was the convective heat dissipation of the gas. In the temperature curve variation chart, the CO2 cooling curve was above N2, while He was below N2. This indicated that during the same period of temperature decrease, He had the best cooling effect, followed by N2, and CO2 was the weakest.
(3) The adsorption of N2 and CO2 by coal occurs at low temperatures. The cooling curves of N2 and CO2 were close to each other (Fig. 4a, c, and 4 f). This was caused by the fact that the specific heat capacity of He was the largest, and the difference between N2 and CO2 was not much, so the convective heat dissipation ability was strong under the condition of the same flow rate. He is a nonabsorbable gas; N2 and CO2 are adsorbable gases, and the adsorption of N2 and CO2 produced by coal increased after the coal temperature was lowered. When the temperature dropped to about 50℃, the slope of the cooling curves for N2 and CO2 significantly decreased due to the decrease in outlet flow caused by adsorption, resulting in a reduction of the convective heat dissipation intensity of N2 and CO2. While reducing the initial temperature to about 50 ℃, the cooling curves of N2 and CO2 became closer, indicating that the higher the initial temperature, the smaller the difference in the extinguishing ability of N2 and CO2 gases with the same flow rate. However, in the later cooling stage, the convective heat dissipation intensity differed due to differences in gas adsorption performance, and the positions of the two cooling curves were separated. For He, the inflection point in the temperature decrease curve was due to the displacement of a large amount of free oxygen, and the adsorbed state oxygen maintains coal self-heating.
(4) There were two reasons for the decrease in oxygen concentration in coal: firstly, it participated in the coal oxygen reaction; Secondly, it was the replacement of the injected gas. Under the same initial temperature, the position relationship of the oxygen decline curve corresponding to He, N2, and CO2 is different. At initial temperatures of 70 and 110 °C (Figs. 4a, b, c, and d), the rate of decrease of oxygen after injection of He was the fastest, followed by N2, while CO2 was the smallest. When the initial temperature was at 150℃ and dropped from 150℃ to about 50℃, the corresponding oxygen concentration decrease curves of the three coincide; however, in Figs. 4e f, the carbon dioxide was lower than the oxygen concentration in the nitrogen group at temperatures down to about 50 °C. Still, the carbon dioxide group was higher than the oxygen concentration in the nitrogen group. It was likely that some of the carbon dioxide was absorbed by the coal body, resulting in less oxygen being displaced and the coal body sustaining oxygen heat production.
3.3.2 Cooling rate
The time required for the initial temperature to drop to 50℃ was relatively short, and the time necessary for 50℃ to drop to 30℃was rather long. There was a significant difference in the cooling rate between the two. Therefore, the cooling rates of different inert gases should be studied in stages. The cooling rate refers to the amount of temperature decrease per unit time. The curves of the cooling rates of the three inert gases as a function of temperature are shown in Fig. 5, calculated at a boundary temperature of 50℃.
In Fig. 5, during the cooling period, He showed the highest cooling rate, followed by N2, and CO2 was the smallest., which indicated that He works best in the inert gas flame retardant process. From the initial temperature down to the 50℃ process (Fig. 5a), the cooling rate of N2 and CO2 changed similarly. With the initial temperature increases, the cooling rate increases, which was mainly inert gas at high temperatures is not easy to be adsorbed by the coal body, the gas flow to carry out the heat. In Fig. 5b, the specific heat capacity of N2 was slightly larger than that of CO2, and the amount of CO2 adsorbed by the coal body exceeded that of nitrogen at a lower temperature. Not only that, in the adsorption process, the heat of the adsorption of CO2 by the coal body was greater than the heat of the adsorption of nitrogen (Zhou et al. 2020). Therefore, the cooling rate of N2 was faster than that of CO2.
3.3.3 Relative displacement efficiency
The process of injecting inert gas into the coal sample tank is accompanied by the outflow of oxygen, i.e., the replacement of oxygen. The oxygen replacement process is an exchange process of substances. Therefore, to measure the ability of different inert gases to replace oxygen, the relative replacement rate is defined as follows:
Where: \({{V}_{\text{O}}}_{2}\)is the amount of oxygen replaced by inert gas per unit time, L; Vg is the amount of inert gas injected per unit time, L. Assuming a constant outlet gas flow rate, the amount of inert gas injected per unit of time and the amount of oxygen replaced by inert gas can be solved according to the defined Eq. (2):
Where: t is the time, s; Vd is the replacement volume of gas, L; Qt is the flow rate of outlet gas at moment t, L/s; ct is the volume fraction of outlet gas, %. The amount of inert gas injected and the amount of oxygen replaced by inert gas during inert injection calculated according to Eq. (2) are shown in Table 2.
The replacement efficiencies of the three inert gases with increasing temperature are shown in Fig. 6. In Fig. 6, the replacement rate curves of He, N2, and CO2 are “convex”, and the replacement efficiency of the two gases decreases as the initial temperature increases. Therefore, the overall trend shows a better displacement effect, followed by N2, and CO2 is the worst.
3.3.4 Flame retardant efficiency
Inert gas in the flame retardant process has two characteristics, one is the use of convection heat dissipation cooling, and the other is to displace the oxygen in the coal body to reduce the oxygen concentration, to achieve the purpose of flame retardant. It can be seen that the flame retardant efficiency of the inert gas is the result of the coupling of the two efficiencies. Therefore, the flame retardant efficiency in the process of defining the flame retardant of inert gas is
Where: ηz is the flame retardant efficiency, %; ηT is the cooling efficiency, which is approximately equal to the normalized value of the average cooling rate; ηr is the relative replacement rate; %; α is the weighting factor.
Since the flame retardant effect of inert gas is dependent on the amount of cooling and the decrease in oxygen concentration per unit of time, the weighting factor of the cooling efficiency is defined as
Where: tT is the total cooling time, min; tr is the oxygen replacement time, min. whereupon from Table 2, the weighting factor α is
Since the average cooling rate change is nonlinear, increases with the initial temperature, and is not comparable, the nonlinear increasing function normalization model (6) is considered to normalize the average cooling rate to the [0,1] interval.
Where: i denotes the gas, as He, N2, CO2; jdenotes different temperature starting points, as 70 °C, 110 °C, 150 °C; xij is the average cooling rate of i at this temperature starting point of j, °C/min. Thus, the cooling efficiency is
The trends of flame retardant efficiency of He, N2, and CO2 at different initial temperatures with the initial temperature are shown in Fig. 7.
Figure 7 shows that the flame retardant efficiency of He is the best, followed by N2, while CO2 is the weakest. With the increase of the initial temperature, the flame retardant efficiency of He decreases almost linearly. At the same time, N2 and CO2 show a trend of increasing and then decreasing due to the adsorption effect. This outcome suggests that during the flame retardant process, helium, a non-adsorbed gas with a high specific heat capacity of 5.1930kJ/g K at 20℃, primarily contributes to convective heat dissipation to enhance flame retardancy. In comparison, nitrogen (with a specific heat capacity of 1.04kJ/g K at 20℃) and carbon dioxide (with a particular heat capacity of 0.84kJ/g K at 20℃) have lower specific heat capacities. The sizes are close to each other, so the difference in their flame and the difference in displacement capacity mainly causes retardant efficiencies.
4 Conclusions
(1) Injecting He, N2, and CO2 gases at different initial temperatures resulted in three cooling curves. Higher initial temperatures required longer cooling time to reach 30 °C. He had the best cooling effect, followed by N2 and CO2.
(2) The trend of oxygen at the outlet is determined by the consumption of oxygen and the replacement of oxygen by inert gas. By analyzing the oxygen replacement, it was found that the relative replacement rate of N2 and CO2 increased with the increase in temperature, and the relative replacement rate of N2 was larger than that of CO2.
(3) The flame retardant efficiency of different inert gases was obtained by combining the cooling rate and displacement efficiency of inert gases at different initial temperature stages. Comparing the flame retardant efficiencies of different inert gases, the results showed that the flame retardant efficiency of inert gases is dominated by the convective heat dissipation ability of inert gases and the ability to displace the amount of oxygen in the coal body.
Data Availability
Data will be made available on request.
References
Arisoy A, Beamish BB, Yoruk b (2017) Moisture moderation during coal self-heating. Fuel 210:352–358
Beamish BB (2005) Comparison of the R70 self-heating rate of New Zealand and australian coals to suggate rank parameter. Int J Coal Geol 64:139–144
Beamish BB, Modher AB, John D (2000) Adiabatic testing procedures for determining the self-heating propensity of coal and sample ageing effects. Thermochimica acta 362(1–2):79–87
Fang X (2021) Study on the flame retardancy difference of inert gases based on gas displacement. Dissertation, China Coal Research Institute
Fang X, Wang H, Tan B, Wang F, Shao Z, Cheng G, Yao H (2022) Experimental comparison study of CO2 and N2 inerted loose coal based on atmospheric pressure gas replacement. Fuel 328:125347
Lei B, He B, Xiao B, Du P, Wu B (2020) Comparative study of single inert gas in confined space inhibiting open flame coal combustion. Fuel 265:116976
Li J, Lu W, Liang Y, Qi G, Kong B, Hu X (2019) Variation of CO2/CO ratio during pure-oxidation of feed coal. Fuel 262:116588
Liu M, Shi G, Guo Z, Wang Y, Ma L (2016) 3-D simulation of gases transport under condition of inert gas injection into goaf. Heat Mass Transfer 52:2723–2734
Liu Y, Wen H, Guo J, Jin Y, Wei G, Yang Z (2020) Coal spontaneous combustion and N2 suppression in triple goafs: a numerical simulation and experimental study. Fuel 271:117625
Ming Q, Ren T, Roberts J, Yang X, Li Z, Wu J (2022) New insight into proactive goaf inertisation for spontaneous combustion management and control. Process Saf Environ Prot 161:739–757
Smith M, Glasser D (2005) Spontaneous combustion of carbonaceous stockpiles. Part II. Factors affecting the rate of the low-temperature oxidation reaction. Fuel 84(9):1161–1170
Song Z, Kuenzer C (2014) Coal fires in China over the last decade: a comprehensive review. Int J Coal Geol 133:72–99
Su H, Zhou F, Li Ji, Qi H (2017) Effects of oxygen supply on low-temperature oxidation of coal: a case study of jurassic coal in Yima, China. Fuel 202:446–454
Tan B, Zhu H, Wang H, Hao Y, Ja G (2013) Prediction model of coal spontaneous combustion critical point and the characteristics of adiabatic oxidation phase. J China Coal Soc 38(1):38–43
Wang X, Luo Y, Vieirab B (2018) Experimental technique and modeling for evaluating heat of rewetting effect on coals’ propensity of spontaneous combustion based on adiabatic oxidation method. Int J Coal Geol 187:1–10
Wang H, Fang X, Du F, Tan B, Zhang L, Li Y, Xu C (2021a) Three-dimensional distribution and oxidation degree analysis of coal gangue dump fire area: a case study. Sci Total Environ 772:145606
Wang H, Fang X, Li Y, Zheng Z (2021b) Research and application of the underground fire detection technology based on multi-dimensional data fusion. Tunn Undergr Space Technol 109:103753
Wang D, Shao Z, Zhu Y (2021c) Several scientific issues on major thermodynamic disasters in coal mines. J China Coal Soc 46(1):57–64
Wu B, Guo Z, Chen J, Ma X (2018) Comparative research on the fire-extinguishing effects of N2 CO2 on the whole process of coal combustion. J China Univ Min Technol 47(02):247–256
Xu Y, Liu Z, Wen X, Wang L, Lv Z, Wu J, Li M (2022) Effect mechanism of nitrogen injection into fire-sealed-zone on residual-coal re-ignition under stress in goaf. Int J Coal Sci Technol 9(1):74
Yuan L, Smith A (2014) CFD modelling of nitrogen injection in a longwall gob area. Int J of Mining and Mineral Engineering 5:164–180
Zeng Q, Shen L (2022) Experimental study on the oxidation kinetics of coal in typical coal mining areas of the Southern Junggar coalfield, Xinjiang, China. Int J Coal Sci Technol 9(1):78
Zhang D, Yang X, Deng J, Wen H, Xiao Y, Jia H (2020) Research on coal spontaneous combustion period based on pure oxygen adiabatic oxidation experiment. Fuel 288:119651
Zhang J, An J, Wen Z, Zhang K, Pan R, Mamun N (2019) Numerical investigation of coal self-heating in longwall goaf considering airflow leakage from mining induced crack. Process Saf Environ Prot 2019;134
Zhao J, Ming H, Guo T, Zhang Y, Deng J, Song J, Zeng Q, Shu C (2022) Semi-enclosed experimental system for coal spontaneous combustion for determining regional distribution of high-temperature zone of coal fire. Int J Coal Sci Technol 9(1):62
Zhou W, Gao K, Xue S, Han Y, Shu C, Huang P (2020) Experimental study of the effects of gas adsorption on the mechanical properties of coal. Fuel 281:118745
Zhu H, Wang H, Yang C, Xu J (2014a) Characteristics and model of loose coal low-temperature oxygen consumption rate by multiple factors. J Cent South Univ (Science Technology) 45(8):2845–2850
Zhu H, Wang H, Song Z, He C (2014b) The relationship between oxidation kinetics characteristic parameters of coal adiabatic progress and metamorphic degree. J China Coal Soc 39(3):498–503
Zhu H, Wang H, Xie L, Du (2023) Experimental study on the effects of pre-oxidation on the pore structure and fractal characteristics of coal. Energy sources, part A: recovery, utilization, and Environmental Effects. 45:7648–7660
Acknowledgements
The financial support was received from the National Natural Science Foundation of China (52074156).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Credit author statement
Xiyang Fang: Conceptualization, Formal analysis, Methodology, Writing-original draft; Bo Tan: Writing-review and editing, Supervision; Haiyan Wang: Funding acquisition, Writing-review, and editing.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fang, X., Tan, B. & Wang, H. Comparative study on the flame retardancy of CO2 and N2 during coal adiabatic oxidation process. Int J Coal Sci Technol 10, 79 (2023). https://doi.org/10.1007/s40789-023-00652-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40789-023-00652-y