Abstract
Coal is the one of foundations of energy and economic structure in China, while the unsealing of coal mine fires would cause a great risk of coal re-ignition. In order to explore the influence of pressure-bearing state on the re-ignition characteristics for residual coal, the uniaxial compression equipped with a temperature-programmed device was built. The scanning electron microscope, synchronous thermal analyzer and Fourier transform infrared absorption spectrometer was applied to investigate the microscopic structure and thermal effect of the coal samples. Moreover, the microscopic effect of uniaxial stress on coal re-ignition is revealed, and the re-ignition mechanism is also obtained. As the uniaxial stress increasing, the number, depth and length of the fractures of the pre-treated coal increases. The application of uniaxial stress causes the thermal conductivity to change periodically, enhances the inhibition of injecting nitrogen on heat transfer and prolonges the duration of oxidation exothermic. The content of oxygen-containing functional groups has a high correlation with apparent activation energy, and coal samples at 6 MPa is more probability to re-ignite while the fire zone is unsealed. Uniaxial stress could control the re-ignition mechanism by changing the structure of fractures and pores. The side chains and functional groups of coal structure are easier to be broken by thermal-stress coupling. The higher the ·OH content, the more difficult coal samples would be re-ignited. The research results would lay a solid theoretical foundation for the safe unsealing of closed fire-areas underground, tighten the common bond between the actual industry and the experimental theory in closed fire-areas underground, and provide the theoretical guidance for coal re-ignition preventing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Underground coal fires are mainly fires caused by the re-ignition of coal seams (Plakunov et al. 2020). The closed fire extinguishing method is the main measure to solve coal fire disasters in major coal producing countries such as European countries, and Australia, China, and the United States (Zhou et al. 2009). Even though the unsealed fire zone reduces economic losses, the re-ignition of coal seriously threatens the safe production of coal mines. Because the leftover coal is subjected to the concentrated stress caused by the burning and collapse of the roof and the stratum collapse, it will affect the spontaneous combustion characteristics of the coal (Xu et al. 2021a, b, c). Therefore, it is of great significance to prevent and control coal re-ignition through studying the oxidation mechanism of coal re-ignition and exploring the occurrence and oxidation characteristics of coal re-ignition after nitrogen injection.
So far, the research on the re-ignition of coal with the unsealed fire zone is unsealed is mainly related to the disaster caused by gas explosion. Through numerical simulation software, scholars have obtained the gas explosion areas and locations that are most likely to be caused by coal re-ignition during unsealing, and determined the influence of atmospheric pressure on the interior of the fire area (Shi et al. 2017; Zhou et al. 2015; Duan et al. 2020a, b; Duan et al. 2020a, b). The plenty of numerical simulation results have been made outstanding contributions to the prevent of fires in goaf of coal mines. The experimental methods mainly focus on the oxidation and spontaneous combustion of the coal itself. The researchers analyzed the production law of indicator gases, changes in absorbance, development trends of fire areas, methods of predicting coal re-ignition, and measures to reduce gas explosion disasters caused by re-ignition. (Lu et al. 2018; Tan et al. 2014; Zhou et al. 2010; Cheng et al. 2012; Cheng et al. 2013; Zheng et al. 2020; Shang et al. 2019). The research on the mechanics of the residual coal in goaf focuses on the mechanical response of the coal briquette and the influence of the fractures and pores on the seepage characteristics (Gu et al. 2020; Wang et al.2020). In the field of fire, the research on broken coal focuses on the oxidation characteristics of coal with different levels of metamorphism, and the spontaneous combustion characteristics of coal after pre-oxidation at different temperatures (Deng et al. 2019; Xu et al. 2021a, b, c). On the other hand, scholars have also done a lot of work on the stress field and compaction state of the goaf. They pointed out that the residual coal in the mined-out area would go through three stages: natural breaking and expansion zone, stress recovery zone and original rock stress zone (Qian et al. 1998). Moreover, the stress recovery distance of the remnant coal is closely related to the buried depth (King et al. 1971; Conroy et al. 1992; Yavuz 2004). These results are of great significance for mastering the attributes, oxidation characteristics and occurrence of the remaining coal in goaf. However, the residual coal in goaf is a frequent place for re-ignition when the fire area is unsealed. The stress state of the residual coal and the tar produced by pyrolysis will cause changes in the spontaneous combustion of the coal (Chao et al. 2021; Hasan et al. 2015). Their researches shows that the flow field and heat transfer during closed and unsealed coalfield fire areas are very important to the safety production and economic benefits of coal mines. The above researches shows that it is feasible to analyze the molecular structure changes and heat and mass transfer during the coal re-ignition process from the perspective of numerical analysis, gas atmosphere, stress conditions, or micro-scale analysis.
Coal re-ignition is a complex physical and chemical reaction process involving the interaction and transformation of multiple chain reaction mechanisms. Therefore, it is difficult to reflect the state of the coal at the time of unsealing only by investigating its re-ignition characteristics of secondary oxidized coal. The stress caused by the collapse of coal and rock in the unsealed fire zone is redistributed, and the remaining coal in the fire area heats up in a nitrogen atmosphere. Moreover, the internal residual coal in goaf is irregular and inaccessible, which leads to relatively limited understanding of the sealed fire zone and the unsealed fire zone. However, the re-ignition of coal during unsealing is closely related to its accumulation state and environmental conditions. During the occurrence and development of the re-ignition, the coal is in a broken state. After they undergo the action of the stress field, the flow field and the temperature field, the heat and mass transfer during the re-ignition have undergone major changes. Therefore, the fluid–solid–thermal–stress full coupling function of the Uniaxial Compression equipped with a Temperature-Programmed (UTCP) device is applied to better realize the pretreatment of coal. Combined with the synchronous thermal analyzer and the Fourier Transform infrared spectrometer (FTIR), the microscopic characterization of the coal after re-ignition could be revealed.
2 Experimental materials and instruments
2.1 Coal sample preparation
The experimental coal samples were taken from the Liuhuanggou mining area in Xinjiang Province, where large-scale coal field fires had occurred in China. The coal type is long flame coal with low metamorphism. The fresh coal sample was wrapped with multi-layer plastic wrap. After being transported to the ground, the coal sample was put into the sample bag and filled with nitrogen. After the fresh coal sample arrived in the laboratory, the outer shell was stripped, and the internal coal sample was crushed and the coal sample with a particle size of 0.20–0.45 mm was selected. The selected coal sample was quickly placed in a vacuum drying oven for 72 h, and then squeeze out the air to seal for storage. The proximate analysis and ultimate analysis results of the experimental coal sample is given in Table 1.
2.2 Experimental instrument
2.2.1 UCTP device
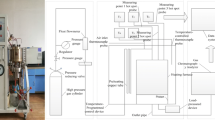
Combined with the environment of the coal in the closed fire zone, the atmosphere environment of the experimental coal sample during the heating process should be the heating in a nitrogen environment. Using the self-built UCTP device, the axial stress test of broken coal can be carried out. After setting different axial loading intensities, the flow rate of different kinds of gases can be adjusted to pass into the reactor to perform heating-up oxidation experiment and percolation experiment. The reactor equipped with the device has a maximum height of 15 cm and a diameter of 10 cm. It is surrounded by a heating jacket that can control the heating rate. The maximum temperature of the device can be from room temperature to 450 °C. Simultaneously, the upper cover is equipped with a temperature probe to measure the core temperature of the coal sample. The accuracy of uniaxial stress is 0.1 MPa, the accuracy of gas pressure is 0.01 MPa, and the flow accuracy can reach 0.001 L/min. The air inlet pipeline passes through the reaction kettle after winding the heating jacket several times, and an air outlet is provided under the reaction kettle.
2.2.2 Fracture and pore structure analysis
The Scanning electron microscope (SEM) produced by Japan JEOL, model JSM-IT500HR was used to observe the morphological and structural changes of the pretreated coal sample. The natural cross-section of the central coal sample in the reactor under different uniaxial stresses was observed by scanning electron microscope, and the distribution characteristics of the meso-structure morphology at different positions of the sample were clearly presented. The coal sample was magnified 1000 times in a vacuum environment. In order to improve the conductivity, the coal sample was sprayed with gold.
2.2.3 DSC and FTIR
The thermal analysis experiment used STA449C synchronous thermal analyzer (NETZSCH company), the coal sample was taken from the inner center of the reactor, the mass was 20 ± 0.1 mg, and the carrier gas was set in the air environment ((\(V\left({\mathrm{O}}_{2}\right)\): \(V\left({\mathrm{N}}_{2}\right)\)) = 1:4). The total gas flow rate was 50 mL/min, the heating rate was 10 °C/min, the start temperature of the test was 30 °C, and the end temperature was 800 °C. Using TENSOR-37 Fourier Transform Infrared Spectrometer (Bruker), the dried KBr and the coal sample in the center of the reactor were mixed according to the mass ratio of 100:1 and then pressed into tablets, and tested by infrared spectroscopy. The wave number ranged from 400 to 4000 cm−1, the resolution was 4 cm−1, and the cumulative number of scans was 32. The test result used the professional infrared analysis software OMNIC, the peak fitting method of Gaussian function, and the baseline was linear. The measurement errors were less than 2% and each experiment was repeated three times having good reproducibility (Fig. 1).
3 Results and analyses
3.1 Micro structure
It was obtained that the SEM results of 4 kinds of pretreated coal samples under uniaxial stress were obtained at a magnification of 1000 times, as shown in Fig. 2.
The surface of the coal sample a is relatively dense at 0 MPa, while the pore structure is very small. The coal is distributed in strips with a layered edge and no obvious fracture structure.
The coal sample under 2 MPa has three obvious fractures. The connectivity between the fractures was poor, which mostly exist on the unevenly distributed surface of the coal, and the fractures are stopped at the intersection of the fissures and the mineral particles. Obviously relaxed fractures appeared at 4 MPa, there are fewer small particles of minerals and coal filling into the fractures, obvious vertical fractures appearing on the surface, and obvious pore structures appearing. At 6 MPa, there are more coal particles and mineral particles filling into the fractures, which also be found that these fillings have occurred secondary fractures, and the coal surface has an obvious lamella structure.
3.2 Basic parameters of coal samples
In order to obtain the effect of uniaxial stress on the microstructure of coal, infrared spectroscopy experiments were carried out. The infrared spectrum of the pretreated coal sample in the center of the reactor is displayed in Fig. 3a, and the type and position of the absorption peak of each functional group are marked on the spectrum. Through the peak fitting method of Gaussian function, the relative content of each functional group is obtained according to the relatively stable C=C functional group strength, as shown in Eq. (1) (Xu et al. 2020a, b). As calculated in Eqs. (2)–(4), the deformation and porosity during the experiment is obtained, while as calculated in Eq. (5), the apparent activation energy (Xu et al. 2021a, b, c) is obtained. The different microstructure and spontaneous combustion tendency of coal are reflected.
From the functional group spectrum of the coal sample after pretreatment in Fig. 3a, it could be seen that under the influence of uniaxial stress, the relative content of ·OH showed a clear trend of first increasing and then decreasing.
The content of C=C and substituted benzene peaks in aromatic hydrocarbons is relatively high, but under nitrogen pretreatment with different uniaxial stresses, the content doesn’t not appear to change significantly with the uniaxial stress. As illustrated in Fig. 3b, the relationship between strain and porosity of coal under uniaxial stress is that with the increase of uniaxial stress, the strain of coal increases gradually, while the growth rate of strain gradually declines, and the porosity is reversed. As the uniaxial stress continues to rise, due to the accumulation of stress energy and energy, the compressibility of the coal is changed, and new fractures are generated in the compacted crushed coal, resulting in a smaller deformation. It could be seen that in the coal structure, the uniaxial stress mainly changes the pore and fracture structure of the coal, resulting in the difference between the coal-oxygen reaction during the re-ignition. It reveals that uniaxial stress mainly changes the fractures and pore structure of coal in the structure of coal body, and the pyrolysis process mainly changes the content and types of functional groups, both of which make coal exhibit different re-ignition characteristics. It is concluded from Fig. 3c that according to the relative content of different functional groups obtained in Fig. 3a, aromatic hydrocarbons account for a large proportion. Because when coal is pyrolyzed under uniaxial stress, liquid tar mainly composes of chain hydrocarbons and aromatic hydrocarbons, and solid products mainly composes of semi-coke mixed in the coal sample, which causes the aromatic hydrocarbon content in the FTIR test to be higher than that of the raw coal. However, the oxygen-containing functional groups (–OH, –COOH, etc.) change significantly under different uniaxial stresses, which is the same result for the carboxyl and hydroxyl groups that directly affect the coal-oxygen reaction (Song et al. 2017; Agarwal et al. 2021).
In order to further analyze the relationship between coal re-ignition and microscopic groups under uniaxial stress, the relative content of hydroxyl and carboxyl groups is summed and compared with the apparent activation energy. Among them, the apparent activation energy can be obtained that \(\mathrm{ln}\frac{C({\text{O}}_{2}{)}^{0}-C\left({\text{O}}_{2}\right)}{C\left({\text{O}}_{2}\right)}\) and \(\frac{1}{T}\) have a linear function relationship. After substituting the experimental data, the slope of the linear function, namely \(-\frac{{E}_{\text{a}}}{R}\), can be obtained. Thus, the apparent activation energy of the experimental coal samples under different uniaxial stresses is calculated. As illustrated in Fig. 3c, the higher the content of oxygen-containing functional groups, the greater the apparent activation energy is, and the more difficult it is for the coal-oxygen reaction to occur. In addition, from the maximum value of the curve, the coal-oxygen reaction is the most difficult when the uniaxial stress is around 2.25 MPa, and the possibility of re-ignition is the lowest when the fire zone was unsealed.
3.3 Heating characteristics of coal samples
To investigate the heating characteristics broken coal after fire extinguishing by nitrogen injection, a heating experiment of broken coal in nitrogen atmosphere under uniaxial stress was carried out. As indicated in Fig. 4, the changes occurred in the heat transfer and thermal conductivity of the coal in the reactor during the uniaxial stress loading process. Since coal does not undergo oxidation reaction, the thermal conductivity of the coal sample is estimated according to the law of conservation of energy (Xu et al. 2020a, b), as calculated in Eq. (6):
It can be concluded from Fig. 4 that the coal under nitrogen atmosphere doesn't have a crossing temperature point during the heating process, and the core temperature of the coal under uniaxial stress is much lower than the temperature control. The main reason is that nitrogen could inhibited the coal-oxygen reaction, reduce the heat of the chain reaction of the active groups. Moreover, the uniaxial stress also could produce fractures of coal, leading to the contact thermal resistance increasing. Therefore, the coal center temperature under uniaxial stress is relatively low. As illustrated in Fig. 4a, the heating trend of coal loaded at 4 MPa is the same as the one at 6 MPa, because the fractures and pore structures of the samples are very similar after being broken under uniaxial stress at the time. Compared with other coal samples under uniaxial stress, the inner temperature of the coal sample loaded at 2 MPa rises faster before 13,580 s, due to more gas injecting into the fractures and pore structure at low temperature stage. Hereafter (after 13,580 s), the trend of the temperature rising drops relatively, since that the broken coal is pyrolyzed, collapsed, collided and fricted, resulting in unstable components such as semi-coke leading to the heat transfer efficiency reducing.
As illustrated in Fig. 4b, the thermal conductivity of coal under uniaxial stress shows periodic changes. \(\lambda\) increases with the increase of temperature before 50 °C, and decreases when the temperature reaches to 50–100 °C. It is because the water in the coal sample evaporates during the initial stage as heating at 50–100 °C, and the thermal conductivity of water is much higher than that of coal, so λ decreases. λ continues to increase after 100 °C, and decreases again at 170 °C. The reason is that the moisture evaporates completely after 100 °C, and the activity of coal macromolecular structure is enhanced, resulting in the thermal conductivity increasing rapidly. After 170 °C, it is the physical adsorption occurred in the coal surface, and a small amount of water vapor is found at the outlet of the airflow in the tests. The thermal conductivity of water vapor is much smaller than that of coal, so that the thermal conductivity begins to decrease again. Moreover, violent decomposition, depolymerization and decomposition reactions are launched at the same time, leading to the temperature differences between the temperature of the coal sample and the furnace wall of the reactor increasing, and the thermal conductivity reducing.
The average thermal conductivity of 0 MPa is the highest, which dues to be affected by the uniaxial stress among the crushed coal particles. As the uniaxial stress is loaded at 2–6 MPa, the average thermal conductivity decreases respectively by 0.459, 0.400, 0.368 W/m2 K, which shows that the smaller the uniaxial stress, the better the heat transfer of the broken coal. The reason is that the broken coal exceeds the compaction limit as the uniaxial stress increasing, resulting in the secondary crushing of coal and even more fractures and pore structures. Meanwhile, under the influence of temperature, the formation of pyrolysis products would also indirectly affect the thermal conductivity.
3.4 Re-ignition characteristic temperature and thermal effect
In order to explore the thermal effect in the process of coal re-ignition under different uniaxial stresses, the heat release, heat absorption and characteristic temperature points were analyzed, where the DSC curve is illustrated in Fig. 5. According to the characteristics of the DSC and DDSC curves, three characteristic temperatures for re-ignition process are obtained, namely the initial heat release temperature T1, the maximum heat release temperature T2 and the end heat release temperature T3.
3.4.1 Re-ignition characteristic temperature analysis
As illustrated in Fig. 5, the re-ignition process is divided into 3 stages. The Stage I is from the beginning to the low-temperature endothermic stage of coal (T1), where the heat absorbed by the evaporation of the original gas and moisture of the coal is greater than the heat released by the coal-oxygen reaction (an endothermic state). During Stage II (T1–T3), the exothermic heat of the coal-oxygen reaction is much higher than the endothermic heat. This stage presents an exothermic reaction, which is called the oxidation exothermic stage. While the temperature reaches to T2 (DSCmax), the heat release reaches the maximum value. After the DSC curve reaches the maximum value, the coal mass is quickly consumed. Simultaneously, the DSC curve shows a rapid downward trend, and the coal-oxygen reaction rate has a rapid drop trend. After the temperature reached T3 (DDSC = 0), until the end of the experiment, the coal mass is consumed and enters the burnout phase, which namely Stage III. The characteristic temperatures under different uniaxial stresses are given in Table 2.
The results in Fig. 5 and Table 2 demonstrate that the curve changes show an increase with the uniaxial stress, and the coal re-ignition curve first moves to the high temperature zone and then gradually downs to the low temperature zone. It is because coal sample at 2 MPa is suppressed during the re-ignition process, which causes the curve to ascent to the high temperature zone and the characteristic temperature point rise. However, as the uniaxial stress is exceeded 2 MPa, it shows a tendency to promote. It is concluded that the re-ignition characteristics of the residual coal in goaf under different uniaxial stress conditions are different when the fire zone is unsealed.
It can also be seen from Fig. 5 that in the low-temperature endothermic Stage I, there is no significant difference among the DSC and the DDSC curves of the coal sample under various uniaxial stresses. In Stage II, the curve separation is larger. It can be further seen that the uniaxial stress has a significantly stronger influence on the exothermic stage. Because the change of heat flux in stage I mainly depends on the moisture evaporation phenomenon and gas desorption behavior in coal that is susceptible to temperature, rather than the stress state of the coal. Further analysis of the coal re-ignition phenomenon, it can be seen that the uniaxial stress has a significantly stronger influence on the heat release stage of coal re-ignition than on the low temperature endothermic stage, and the characteristic temperature of the coal sample under uniaxial stress is higher. As displayed in Table 2, the characteristic temperature under different uniaxial stress is consistent with the heat flow curve, obviously. Compared with loose coal, T1 at a uniaxial stress of 2, 4, 6 MPa is higher than 65.24 °C, 50.22 °C, and 40.23 °C. T2 is 4.27 °C higher on average, while T3 is 0.4 °C, 5.2 °C, and 1.26 °C lower than loose coal. In stage III, it is mainly the pyrolysis and exotherm of residual minerals and ash. Based on the above analysis, it can be seen that uniaxial stress has a greater impact on the heat release stage of coal re-ignition.
3.4.2 Thermal effect analysis
The equilibrium temperature is obtained through NETZSCH software analysis, and combines with the curve area, the heat change of the coal sample under different uniaxial stresses is obtained, as revealed in Fig. 6.
Figure 6 illustrates the heat change of coal samples under different uniaxial stresses. As concluding the results, with the increase of uniaxial stress, it shows a trend of first decreasing and then increasing. According to our previous research (Liu et al. 2021), compared with the heat release, nitrogen injection plays a key role in suppressing the spontaneous combustion of coal in the unsealed fire zone. However, judging from the heat release of coal re-ignition under different uniaxial stresses indicates that the heat generated during re-ignition of coal under uniaxial stress is quite different from the heat released by residual coal. The pressure-bearing coal pillars and residual coal should be intensively prevented and controlled as in the fire area unsealed.
In particular, relative to the coal sample of 0 MPa, as the uniaxial stress is at 2 MPa and 4 MPa respectively, there is a significant decrease and increase, which proves that when the stress at 2 MPa inhibits the oxidation exothermic process, the heat release is reduced by 1196 MJ. However, the heat release at 4 MPa is significantly increased by 642 MJ, which significantly promotes the heat release process of the coal-oxygen reaction. The heat release at 8 MPa is reduced by 187 MJ, and the lower heat release difference also indicates that the heat release characteristics of coal under higher uniaxial stress conditions are very close to that of loose coal. It means that the coal pillars and residual coal with a high compaction has the risk of re-ignition as contacting the injected oxygen when the fire zone is unsealed. This is also the reason causing repeated spontaneous combustion of coal while the fire zone has been unsealed. Therefore, it is necessary to explore the reaction characteristics in heat release stage of coal re-ignition under uniaxial stress.
3.4.3 The relationship between characteristic temperature and group content
As indicated in the DSC curve, T1 is the critical temperature of stage I and stage II. The low-temperature adsorption stage before T1 determines the severity of the oxidation reaction after T1. Moreover, the hydroxyl content in the oxygen-containing functional group has a greater impact on stage I. In order to further analyze the impact of the hydroxyl group on the re-ignition temperature point, the temperature T1 and the hydroxyl content is compared and analyzed, as shown in Fig. 7.
It can be founded that when the uniaxial stress is at 2 MPa, the low-temperature adsorption stage lasts the longest temperature stage. After 226.25 °C, it ends endothermic and starts the oxidation exothermic stage. Similarly, it can be seen that as the uniaxial stress increasing, the hydroxyl content increases firstly and then decreases. The reason could be that as the uniaxial stress is lower than 2 MPa, the degree of compaction turns to higher, the flow rate of nitrogen between the coal particles turns slowly, and then it leads to the hydroxyl group more difficult to decompose ·H and the content of hydroxyl groups increasing. As the uniaxial stress is higher than 2 MPa, the coal particles would be fractured twice, the fractures would increase again, and the ability to decompose ·H would go up. As the coal sample heating up in a nitrogen environment, the carbon radicals in the coal cannot continue to be oxidized to generate –C–O–O·, thus cannot be decomposed into hydroxyl groups, which could affect its content. Therefore, it can be inferred that the uniaxial stress causes the coal to emerge the mechanochemical effects during the heating process. It not only reduces the ·H decompose ability, but also reduces the bond energy between the hydroxyl group and other structure, which causes the hydroxyl group easier to fall off under conditions of higher compaction, resulting in a higher content of hydroxyl groups.
When the uniaxial stress is loaded at 2 MPa, the low temperature adsorption stage lasts the longest temperature stage, because the final oxidation product of hydroxyl is water, and its higher content would prolong the water evaporation and desorption stage. The coal sample ends the endothermic after 226.25 °C and starts the oxidation exothermic stage. Similarly, it can be seen that the coal sample has the highest hydroxyl content under the uniaxial stress, reaching to 26.20%. The reason is that the coal sample at 2 MPa has a higher degree of compaction after pretreatment with UCTP. During re-ignition, oxygen flows more slowly in the cracks and pore structure of the coal, the force between coal particles is stronger, and the hydroxyl content is higher. It is more prone to violent oxidize after exposure to oxygen.
3.5 Mechanism analysis
The morphology and structure of coal samples are under different uniaxial stresses, respectively, as displayed in Fig. 8. The stress state would directly cause different changes to the fractures and pore structure of coal, thereby changing the active sites of coal-oxygen contact and changing the characteristics of re-ignition. As a result, the microscopic mechanism of coal re-ignition under uniaxial stress (shown in Fig. 9) is obtained. Coal is composed of condensed nucleated aromatic layers with branched chains and various functional groups. Under stress, part of the branched chains and functional groups are broken, and the macromolecular structure of coal changes (Dong et al. 2017). Moreover, for the pretreated coal sample, the energy of the bond breakage caused by the thermal-stress coupling state and the conversion of functional groups during re-ignition dominates the exothermic change.
During the re-ignition process, ·OH could continue to undergo oxidation reactions during the exothermic stage of oxidation, continuously generating aliphatic hydrocarbons and oxygen-containing functional groups. The content of hydroxyl groups is different under different uniaxial stresses, resulting in different heat absorption and heat release of the coal-oxygen reaction. The coal sample at 2 MPa has the highest degree of compaction, which also causes a large amount of hydroxyl groups to fail to be fully reacted, and leading to the lower heat release in stage II. Compared with loose coal, coal samples under uniaxial stress required higher re-ignition conditions, especially the one at 2 MPa. However, the re-ignition characteristics of the coal sample at 6 MPa are very close. Meanwhile, the number of pores and fractures appears to decrease and then increase as the uniaxial stress rising. Thus, the oxygen supply channels for re-ignition turn to change, resulting in significant variety in Stage I and II. Besides, due to the different degree of coal pyrolysis under stress, the content of oxygen-containing functional groups would change. Therefore, before the fire zone is unsealed, measuring the stress state of the residual coal and testing the FTIR of the coal sample can effectively prevent the risk of re-ignition as the fire zone is unsealing.
4 Conclusions
The re-ignition characteristics of coal samples under different uniaxial stresses are discussed. Applying with the UCTP device, SEM, thermal analysis and FTIR analysis, the effect of uniaxial stress on the fracture structure, functional groups and exothermic stage of the re-ignition of confined coal is also explored. The structure evolution and re-ignition mechanism of confined coal is put forward. The following conclusions are obtained as follows:
-
(1)
Uniaxial stress increases the diversity of fractures and pore structures and reduces the risk of coal re-ignition. Coal particles are rearranged, combined and filled under stress, leading to the change rate of the strain and porosity decreasing gradually. The average thermal conductivity decreases and then increases, while the apparent activation energy does the opposite. The content of oxygen in fractures and pores plays the key role in changing the heat and mass transfer affection between coal particles.
-
(2)
The oxidation exothermic stage of coal re-ignition oxidation becomes longer under stress, and the initial exothermic temperature and maximum heat release temperature first increase and then decrease. The heat and mass transfer efficiency of the coal sample before 2 MPa is obviously suppressed, and then promoted. The thermal effect of the coal samples at 6 and 0 MPa shows very similar characteristics, and has a high tendency to re-ignition.
-
(3)
The key groups of the thermal characteristics of coal re-ignition are dominated by the hydroxyl content, and the difficulty of bonds breaking is affected by the mechanochemistry. The bond energy and the coal-oxygen contact site dominate the heat release rate of the coal re-ignition process.
Abbreviations
- \(W\) :
-
The relative content of functional groups (%)
- \({A}_{\text{i}}\) :
-
Functional group area
- \({f}_{\text{i}}\) :
-
Vibration strength of the functional group
- \(\varepsilon\) :
-
The uniaxial strain
- \(\Delta h\) :
-
The amount of deformation, m
- \({h}_{0}\) :
-
The initial height of the coal sample filled into the reactor (m)
- \({\varphi }_{0}\) :
-
The initial void ratio
- \(\varphi\) :
-
The porosity of a broken coal sample under uniaxial strain
- \(d\) :
-
The inner diameter of the reactor (m)
- \(m\) :
-
The mass of the coal sample (kg)
- \(\rho\) :
-
The density of the coal sample (1.4 g/m3)
- \(\lambda\) :
-
Thermal conductivity (W/m2 K)
- \({C}_{{\text{O}}_{2}}^{0}\) :
-
The oxygen concentration at the inlet (mol/cm3)
- \({C}_{{\text{O}}_{2}}^{\text{L}}\) :
-
The oxygen concentration at the outlet (mol/cm3)
- \({E}_{\text{a}}\) :
-
Apparent activation energy (J/mol)
- \(\theta\) :
-
The core temperature of the coal sample (°C)
- \({A}_{0}\) :
-
Pre-exponential factors (1/s)
- \(Q\) :
-
The flow rate of the supplied air (mol/s)
- \({C}_{\text{e}}\) :
-
The heat capacity, J/(kg K)
- \(\lambda\) :
-
The thermal conductivity (W/m2 K)
- \(\Delta X\) :
-
The distance between the core temperature and the wall of the reactor (m)
- \({T}_{\text{OV}}\) :
-
The control temperature of heating
- \(T\) :
-
The core temperature of the coal sample (K)
References
Agarwal M, Kudapa VK, Sudharsan J (2021) Analytical study of structural characteristics of South Eastern coal field by FTIR spectroscopy and X-ray diffraction. Mater Today Proceed 47(15):5319–5325
Chao JK, Chu TX, Yu MG, Han XF, Hu DM, Liu W, Yang XL (2021) An experimental study on the oxidation kinetics characterization of broken coal under stress loading. Fuel 287:119515
Cheng J, Zhou F (2013) A systematic approach to assess mine atmospheric status. Fire Saf J 58:142–150
Cheng JW, Wang C, Zhang SS (2012) Methods to determine the mine gas explosibility: an overview. J Loss Prevent Proc 25(3):425–435
Conroy J (1992) Influence of elevated atmospheric CO2 concentrations on plant nutrition. Aust J Bot 40(5):445–456
Deng J, Lv HF, Li DJ, Xiao Y, Wang CP (2019) Thermal behavior effects of 1-butyl-3-methylimidazole tetrafluoroborate on coals spontaneous combustion with different metamorphic levels. J China Coal Soc 44(1):254–262
Dong YJ, Han YZ, Hou QL, Wang J (2017) Mechanochemistry mechanism of gas generation during coal deformation. J China Coal Soc 42(04):942–949
Duan YL, Wang S, Wang WH, Zheng K (2020a) Atmospheric disturbance on the gas explosion in closed fire zone. Int J Coal Sci Technol 7:752–765
Duan YL, Wang S, Wang WH, Jiang XS, Li YB, Yang YL (2020b) Analysis on influence of respiratory effect on oxygen concentration in closed fire zone of mine. J Saf Sci Technol 16(04):101–106
Gu HL, Tao M, Li XB, Cao WZ, Li QY (2020) Dynamic tests and mechanical model for water-saturated soft coal with various particle gradations. Int J Rock Mech Min 132:104386
Hasan T, Gerhard JI, Hadden R, Rein G (2015) Self-sustaining smouldering combustion of coal tar for the remediation of contaminated sand: two-dimensional experiments and computational simulations. Fuel 150:288–297
King H, Whittaker B (1971) A review of current knowledge on roadway behavior, especially the problems on which further information is required. Proc Symp Strata Control Roadway 1971:73–90
Liu ZJ, Xu YL, Wen XL, Lv ZG, Wu JD, Li MJ, Wang LY (2021) Thermal properties and key groups evolution of low-temperature oxidation for bituminous coal under lean-oxygen environment. ACS Omega 6:15115–15125
Lu XX, Zhao HR, Zhu HQ, Han Y, Xue X (2018) Characteristic rule of spontaneous combustion tendency of oxidized coal at recrudescence stage. J China Coal Soc 43(10):2809–2816
Plakunov MM, Yavuzturk CC, Chiasson AD (2020) On the effects of temperature-dependent diffusion of carbon dioxide from underground coal fires. Geothermics 85:101768
Qian MG, Xu JL (1998) Research on the characteristics of “O” ring in the distribution of mining cracks in overlying strata. J China Coal Soc 23(5):466–469
Shang HB, Jin DW, Zhang TJ, Li SG, Wang ZZ, Zhao CH, Zhou ZF, Liu ZX (2019) Permeability evolution of broken coal under triaxial stress. J China Coal Soc 44(04):1066–1075
Shi GQ, Zhou T, Liu MX, Wang YM (2017) Numerical analysis on methane explosion hazard during the process of fire zong sealing in coal mine. J China Univ Min Technol 46(05):997–1006
Song HJ, Liu GR, Zhang JZ, Wu JH (2017) Pyrolysis characteristics and kinetics of low rank coals by TG-FTIR method. Fuel Process Technol 156:454–460
Tan B, Zhu HQ, Wang HY, Xu JY, Zou J, Niu HY, Yang CY, Yu SJ (2014) Combustion state and surface temperature field evolution of closed firing zone in top-coal caving region of coal drift. J Cent South Univ 45(03):946–951
Wang G, Han DY, Jiang CH, Zhang ZY (2020) Seepage characteristics of fracture and dead-end pore structure in coal at micro- and meso-scales. Fuel 266:117058
Xu Q, Yang SQ, Yang WM, Tang ZQ (2021a) Secondary oxidation of crushed coal based on free radicals and active groups. Fuel 290:120051
Xu YL, Liu Y, Xie HL, Chen ML, Wang LY (2020a) Experimental study on organic sulfur removal in bituminous coal by a 1-carboxymethyl-3-methyl imidazolium bisulfate ionic liquid and hydrogen peroxide solution. ACS Omega 5(33):21127–21136
Xu YL, Liu ZJ, Bu YC, Chen ML, Lv ZG, Wang LY (2021b) Catastrophic temperature of oxidation-spontaneous-combustion for bituminous coal under uniaxial stress. Chin J Eng 10:1312–1322
Xu YL, Liu ZJ, Wang LY, Lv ZG, Wu JD, Li MJ (2021c) Hysteresis characteristics of oxidation-thermodynamic for residual coal in goaf under uniaxial stress. Fuel 306:121750
Xu YL, Zuo N, Bu YC, Wang LY (2020b) Experimental study on the characteristics of oxidation kinetics and heat transfer for coal-field fires under axial compression. J Therm Anal Calorim 139(1):597–607
Zheng YN, Li QZ, Zhang GY, Zhao Y, Zhu PF, Ma X, Liu XX (2020) Effect of multi-component gases competitive adsorption on coal spontaneous combustion characteristics under goaf conditions. Fuel Process Technol 208:106510
Zhou FB, Li JH (2010) Prediction model for re-ignition of fire zone after unsealing based on BP neural networks. J Min Saf Eng 27(04):494–498
Zhou FB, Li JH, He S, Liu YS (2009) Experimental modeling study on the re-ignition phenomenon when opening a sealed fire zone. Procedia Earth Planet Sci 1(1):161–168
Zhou XH, Li CY, Zhang LL, Song DP, Meng JL (2015) Numerical simulation of gas transport law in process of sealing fire zone. China J Geol Hazard Control 26(02):116–122
Acknowledgements
The authors wish to acknowledge gratefully the financial support of the research funding provided by the National Natural Science Foundation of China (52074108 and 51874124), the Project supported by Fund for Creative Talents of Henan Colleges in China (22HASTIT012) and the Key Science and Technology Program of Henan Province (212102310007). It also supported by the Scientific Research Foundation of the Higher Education Institutions of Henan Province in China (22A620001). We also appreciate all the reviewers and editors for their professional and constructive comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Conflict of interests
All the authors of this manuscript have approved the article’s submission for publication, and there are no conflicts of interest to declare. This paper has not been published elsewhere and is not under consideration by another journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, Y., Liu, Z., Wen, X. et al. Effect mechanism of nitrogen injection into fire-sealed-zone on residual-coal re-ignition under stress in goaf. Int J Coal Sci Technol 9, 74 (2022). https://doi.org/10.1007/s40789-022-00539-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40789-022-00539-4