Abstract
Kaolinite, as a mineral in fine coal, has an important influence on the flotation of coal particles. In this study, the effects of ultrafine kaolinite particles on the flotation recovery of coal particles were investigated. Flotation tests were carried out using a mixture of coal particles and different amounts of ultrafine kaolinite particles. Combined with the Stefan–Reynold theory, the effect of liquid film drainage rate between coal bubbles in a kaolinite suspension was calculated. The yield of flotation clean coal increases quickly with the increasing content of ultrafine kaolinite particles. The ultrafine kaolinite particles can reduce the surface tension of the suspension, weaken the bubble coalescence, and stabilize the structure of the froth layer. In addition, the ultrafine kaolinite particles increase the apparent viscosity of the flotation pulp slightly. It is concluded that the role of ultrafine kaolinite particles on the positive effect of froth properties conceals the negative effect on the liquid film drainage rate between coal particles and bubbles caused by the kaolinite particles, which ultimately leads to an increasing yield of clean coal with an increasing content of kaolinite particles. This study is important for understanding the influence of ultrafine kaolinite on coal particle flotation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Flotation is the most effective method for separating fine coal from gangue particles (Ni et al. 2018a, b; Yoon 2000). There are many ultrafine gangue minerals in the flotation pulp, which are produced by grinding or mudding of the clay minerals. These minerals include kaolinite, illite and montmorillonite. The presence of these ultrafine minerals in coal flotation leads to a major problem: causing a low recovery but a high ash content of clean coal by the entrainment of fine gangues (Wang et al. 2015; Yu et al. 2017a, b; Zheng et al. 2006). Therefore, it is essential to reduce the mineral matter in clean coal to reach the requirements for coal utilization.
In the past 100 years, the negative effects of fine slimes on coal flotation have been extensively studied (Gulsoy 2005; Ni et al. 2015; Zhang et al. 2013). However, in recent years, many scholars have found that in the flotation process, the presence of a small amount of ultrafine particles helps to increase the stability of the froth layer, thereby increasing the recovery rate of the flotation concentrate (Horozov 2008; Ni et al. 2018a, b; Zhang et al. 2008). The essence of coal flotation is that the coal particles collide with the bubbles, and the liquid film between the coal and bubbles gradually thins and ruptures; then, the coal particles adhere to the bubbles (Nguyen and Schulze 2004). Ultrafine kaolinite particles are hydrophilic and exert less inertial force in the flow field environment, as they barely adhere to the bubble surface selectively in a thermodynamic environment. They hinder the coalescence between the particles and stabilize the suspension. During the bubble generation process, these ultrafine kaolinite particles enter the boundary water layer of the bubble non-selectively, with the bubble entering the froth layer.
In previous decades, researchers reviewed and considered the effects of high-ash fine slimes or gangue particles on coal flotation behavior, and most of them focused on water entrainment, mechanical entrainment and slime coating (Warren 1985; Yu et al. 2017a, b; Zhang et al. 2013). However, few studies have revealed the effect of fine slimes, such as kaolinite, on the drainage behavior of liquid films during particle-bubble collision and adhesion. In this paper, a suspension consisting of ultrafine kaolinite powder was analyzed, and the flotation behavior of coal particles under different kaolinite powder contents was studied. Throughout this paper, new insights are provided regarding the effect of ultrafine kaolinite on the coal flotation process.
2 Experiment
2.1 Materials and reagents
The experimental coal sample was selected from low-ash coking coal from the Shanghaimiao Coal Preparation Plant, Inner Mongolia, China. The coal samples were crushed and ground to − 0.5 mm. Then, a final coal sample with a size fraction of 0.25–0.5 mm and a density less than 1500 kg/m3 was obtained via the screening test and float-and-sink test. The proximate analysis of the final coal sample is listed in Table 1.
Ultrafine kaolinite was purchased from the Hongtu Mining Company in Guangdong Province, China. The mineral composition of the kaolinite was determined using an X-ray diffractometer (D8 ADVANCE, Bruker, Germany), and the result is presented in Fig. 1. The XRD result indicates high purity kaolinite in the ultrafine kaolinite powder.
Furthermore, ultrafine kaolinite was determined using laser particle size analysis (Microtrac Inc. S3500, USA) to determine particle size distribution properties, and the result is presented in Fig. 2. The laser particle size result indicates that the kaolinite powder content of less than 10 μm in the sample reached 85%. In mineral/coal flotation, particles below 10 μm are usually referred to as ultrafine particles (Wang et al. 2005).
The static contact angles of coal and kaolinite particles were measured using the dropping method (Zhongchen JC2000D, China). As illustrated in Fig. 3, the static contact angles of coal and kaolinite particles were 96° and 11°, respectively. This means that the coal surface was very hydrophobic, while the kaolinite particles were very hydrophilic. Therefore, the collector was not used in the flotation tests to float coal particles based on its natural hydrophobicity.
Experimental chemical reagents included 2-octanol (analytical grade), NaCl (analytical grade), and Watsons deionized water with a conductivity of 0.0006 s/m. 1 mM/L NaCl solution was prepared using deionized water and sodium chloride.
2.2 Surface tension and froth layer properties tests
According to a test method mentioned in previous studies (Dadashev et al. 2015; Zhou and Dong 2016), the surface tension tests were performed using the Wilhelmy Hoist method. The surface tension of the ultrafine kaolinite suspension was measured with a surface tensiometer (Kruss K100, Germany).
The definition of different ultrafine kaolinite contents in this paper was as follows: the ratio of the mass of kaolinite to coal particles was 0%, 5%, 15%, and 25%, meaning that a mass of 30 g coal particles was fixed in the flotation. According to the mass ratio, the mass of the ultrafine kaolinite was 0 g, 1.5 g, 4.5 g and 7.5 g, respectively. The test procedure was as follows: first, weighing the specified amount of kaolinite into 0.5 L of 1 mM/L NaCl solution, stirring it until the bottom of the beaker has no precipitation, and quickly placing the suspension into the K100 surface tensiometer. Each measurement was repeated 3 times. The experimental tests were performed at a temperature of 298 K.
The three-phase froth was prepared by a modification of the Ross–Mile method. The airflow method was used to inflate the bottom of the acrylic tube to measure the maximum height and half-life of the froth layer produced by the kaolinite suspension and flotation pulp. The equipment structure is shown in Fig. 4. First, suspensions of different ultrafine kaolinite contents or flotation pulp were prepared; the method of preparation was the same as in the surface tension test. Then, they were transferred to the acrylic tube to begin inflating, and the air flow was set at 0.2 m3/h. After 30 s, the froth layer height (which is the maximum froth layer height) was recorded, and the time required to reduce the froth layer height by half (which is the half-life of the froth layer) was recorded.
2.3 Apparent viscosity of suspension tests
The apparent viscosity measurements were measured by an NDJ/SNB digital display viscometer (Shanghai Haozhuang, China). The measurement range was 1–2 kPa s, and the rotation speeds were 6, 12, 30, and 60 r/min. The apparent viscosity of the kaolinite suspension was measured using a No. 0 rotor at 60 r/min, and each measurement was repeated 3 times. Finally, the average value is the apparent viscosity of the kaolinite suspension. Due to the excessive sedimentation rate of coal particles, the apparent viscosity of the pulp cannot be measured. The experimental tests were performed at a temperature of 298 K.
2.4 Tests of bubble size
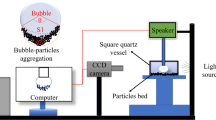
Since coarse particles have less influence on the size of the bubbles, the size of the bubbles in the suspension can represent the true size of the bubbles in the flotation system. The bubble size experiment was observed in 0.5 L RK/FD flotation cells under the same conditions as flotation tests (no collector but with the addition of frother of 30 g/t sec-octyl alcohol). The suspension preparation process was consistent with the surface tension test but with the addition of the frother. A Bailey differential pressure transmitter was located on the flotation cell to determine the gas hold up (Quinn et al. 2007). The equipment structure is shown in Fig. 5. For selected conditions, the bubble size distribution was determined using high-speed video microscopy (I-Speed713, UK). Typically, over 500 bubble images were processed. The BSD data were presented in Image-Pro Plus software. In addition, the Sauter mean diameter (SMD) is a popular representation of the mean bubble size, defined as Eq. (1) (Hosseinzadeh et al. 2018; Pacek et al. 1998):
where Ni is the number of bubbles and di is the individual mean bubble diameter.
2.5 Flotation tests
All flotation experiments were conducted in a 0.5 L flotation cell (RK/FDII, China) with an impeller speed of 1800 r/min. An air flow of 0.2 m3/h was assigned. The flotation water was 1 mM/L NaCl solution. The frother was sec-octyl alcohol at a dosage of 30 g/t to maintain the stability of the froth. No collector was added. The flotation sample is a mixture of 30 g coal particles and different proportions of ultrafine kaolinite consistent with the surface tension tests.
First, the mixture sample was added to the flotation cell with NaCl solution and pre-wetted for 3 min. Then, the frother was added, and the pulp was conditioned for 0.5 min. Finally, the air flow was provided, and the flotation concentrate was collected within 3 min. The clean coal and tailings were collected. To effectively separate the coal particles and kaolinite in the clean coal and tail coal, the recovery rate of coal was checked to minimize the amount of coal particles mixed into the kaolinite during the experiment. This study selected clean coal and tail coal by wetting screening (because the particle size of the coal sample was 0.25–0.5 mm, the products below 0.074 mm were considered to be all kaolinite powder). The product on the screen was filtered and dried at 60 °C for 5 h. The products were then weighed for yield analysis. In addition, the ash content of clean coal was also analyzed using a muffle furnace.
3 Results and discussion
3.1 Analysis of surface tension and froth properties
Figure 6 shows that the maximum froth layer height and froth half-life of the kaolinite particle suspension increase with increasing kaolinite particle content. Figure 7 shows that the surface tension of the ultrafine kaolinite suspension decreases and then increases with increasing content of kaolinite particles. The low surface tension is favorable for the formation of bubbles, prevents the coalescence and breakage of the bubbles, and forms a relatively stabilized froth layer.
The flotation pulp of kaolinite and coal particles were different from kaolinite particle suspensions. Its maximum froth layer height and froth half-life increased rapidly to 6.5 cm from 3 cm, and the half-life of the froth also increased to 8 s from 5 s when the kaolinite content was increased from 0% to 5%. Then, it slowly increases with increasing kaolinite content. According to a previous study, Dadashev et al. (2015) found that the surface tension of a bentonite suspension system showed a minimum between 3% and 5% of bentonite solids. Similarly, in this paper, a similar phenomenon was found with the ultrafine kaolinite particles that were used as the research object.
3.2 Analysis of suspension apparent viscosity and theoretical calculation of liquid film drainage rate
Figure 7 illustrates that the content of ultrafine kaolinite particles in the low-concentration NaCl solution has little effect on the apparent viscosity of the suspension.
Owing to different compositions, structures, and charge properties, clay minerals and nonclay minerals affect pulp rheology quite differently (Chen and Peng 2018). In the literature, the change in pulp rheology can also significantly affect froth stability as reviewed by Farrokhpay (2011). Zhang and Peng (2015) found that clay minerals modified the pulp rheology depending on the type of clay minerals present. High pulp apparent viscosity was related to decreased gold flotation, and slightly increased pulp apparent viscosity by clay minerals enhanced gold flotation Chen et al. (2017). found that amorphous silica caused a significant increase in the apparent viscosity of the pulp. This resulted in a reduction in the recovery of copper and a reduction in the grade of copper concentrate. Ndlovu et al. (2014) found that kaolinite suspensions were characterized by high yield stress and low apparent viscosity, and this behavior was attributed to changes in charge anisotropy, aspect ratio and surface morphology. In addition, Shi and Zheng (2003) believed that high froth viscosity led to an increase in froth residence time that decreased the water holdup in the froth phase and provided a longer time for water and entrained kaolinite to drain back from the froth to the pulp phase.
In this study, the content of ultrafine kaolinite particles had little effect on the apparent viscosity of the suspension. The collision of coal particles with bubbles was a hydrodynamic problem involving the size of the coal particles, the size of the bubbles, and the relative motion between the particles and the bubbles. In the gas–liquid two phase foam, the thermodynamic drainage process in the liquid film mainly comes from two aspects. One is capillary pressure, and the other is the disjoining pressure between gas–liquid interfaces formed by surface forces. Ejtemaei and Nguyen (2017) observed a liquid film between the bubble and particle drainage behavior by high-speed video microscopy. During the liquid film spreading process, the capillary pressure promotes the thinning of the liquid film, and the liquid film will produce the opposite force during the thinning process, which is the disjoining pressure. Ye et al. (2017) demonstrated that with increasing apparent viscosity, the liquid film drainage rate was significantly reduced.
Based on previous studies, the liquid film drainage rate of the colloidal force and disjoining pressure proposed by Nguyen and Schulze in 2004 is shown in Eq. (2) (2004). It is applied to the liquid film of the suspension of different kaolinite particle contents involved in this paper (Ejtemaei and Nguyen 2017).
h is the thickness of the liquid film between the bubble and the particle, taking 0.1 mm (Ejtemaei and Nguyen 2017). μ is the apparent viscosity of the suspension, σ is the surface tension of the suspension, and Rf is the radius of the liquid film between the bubble and the particle, which is approximately 0.19 mm (Rf is determined by the radius of the coal particles. The coal particles used in this paper were 0.25–0.5 mm, so the average value was 0.375 mm. Rb is the radius of the bubble. The size of the bubbles in the kaolinite suspension is shown in Fig. 8. П is the disjoining pressure between the bubble and the coal. In the equation, \(\frac{2\sigma }{{R_{b} }}\) is the capillary pressure. In our system, the disjoining pressure is defined as the sum of electrical double-layer interaction \(\prod_{{{\text{edl}}}}\) and van der Waals interaction \(\prod_{vdw}\), in Eq. (3) (Li and Somasundaran 1993; Zhang et al. 2017).
Here A is the Hamaker constant (Visser 1969), n is the number of electrolyte ions per unit volume in the aqueous film, k is the Boltzmann constant, ze is the charge on the electrolyte ions, T is the absolute temperature, and \(\kappa^{ - 1}\) is the Debye length. φsl and φbl are the surface potentials of the solid–liquid and bubble–liquid interfaces, respectively. The surface potentials of the bubble–liquid interface under different conditions are taken from the literature as − 40 mV (Li and Somasundaran 1993).
Figure 8 shows that the size of the bubbles gradually decreases with increasing kaolinite content. The apparent viscosity of the kaolinite suspension does not change much with increasing kaolinite content, but the surface tension of the kaolinite suspension clearly decreases, which mainly leads to a decrease in the bubble size in the kaolinite suspension with increasing kaolinite content.
Figure 9 shows that when the kaolinite content was increased from 0% to 25%, the liquid film drainage rate between coal particles and bubbles was decreased. The decrease in the drainage rate was mainly caused by the decrease in the surface tension of the kaolinite suspension. The decrease in liquid film drainage rate between coal particles and bubbles reduces the probability of adhesion (Pa), which ultimately affects the flotation yield of clean coal particles.
3.3 Flotation experiment results and discussion
Figure 10 shows that the yield of clean coal increases with increasing content of kaolinite particles, and the change of clean coal ash content is in the range of 3.6%–4.0%. With the increasing content of kaolinite powder in the flotation pulp, the clean coal ash content is slightly changed, while the clean coal yield is significantly changed.
Fine particles contribute to the stability of the froth layer (Horozov 2008). Ni et al. (2018a, b) discovered that the flotation rate of coal particles in the 0.5–0.25 mm size range initially increased and then decreased as the content of slime particles increased. Zhang et al. (2008) believed that by adding suitable nanoparticles to the froth system, a three-dimensional network structure can be formed between the bubble surface and the continuous phase to prevent the aggregation of bubbles.
According to the theory of flotation dynamics, the probability (P) of a particle being collected by an air bubble in the froth phase of a flotation cell can be given by Eq. (4) (Xie 2012):
where Pc is the probability of bubble–particle collision, Pa is the probability of adhesion, Pd is the probability of detachment, and Ps is the stable probability of froth layer formation.
Pc is determined by the hydrodynamics of the system, which is strongly affected by the particle size, bubble size and turbulence. In this paper, it can be considered basically unchanged. Pa is also affected by hydrodynamics but is largely a function of the surface chemistry involved. This paper argues that it is controlled by the coal particle–bubble liquid film drainage rate (Nguyen et al. 1998). Pd can be negligibly small because of the low inertia. Ps is determined by the stability of the froth phase.
Figure 9 shows that as the kaolinite content increases, the liquid particle–bubble liquid film drainage rate decreases when the kaolinite content increases from 0% to 25%. In the kaolinite flotation system, Pa decreases gradually. Additionally, Fig. 6 shows that the maximum froth layer height and half-life of the kaolinite suspension increase with increasing kaolinite content. In addition, when the kaolinite content in the flotation system increases from 0% to 5%, the maximum froth layer height increases by 3.5 cm, and the froth layer half-life increases by 3 s. Hence, Ps is gradually increased relative to the kaolinite content of 0% in the kaolinite system. With increasing kaolinite content, the yield of clean coal increases gradually. The stable froth has a significant positive effect on the coarse coal flotation behavior in the kaolinite suspension. The role of ultrafine kaolinite particles on the positive effect of froth properties conceals the negative effect on the liquid film drainage rate between coal particles and bubbles caused by the kaolinite particles, which ultimately leads to an increasing yield of clean coal with increasing content of kaolinite particles.
4 Conclusions
A surface tension test of the suspension indicates that the surface tension of the suspension can be significantly affected by ultrafine kaolinite, which contributes to the formation of bubbles in the pulp, weakens the coalescence of the bubbles, and stabilizes the froth layer. A test of the apparent viscosity of the suspension proves that the apparent viscosity of the slurry has a slight influence with the increase of the kaolinite particle content. Combined with Stefan-Reynold theory, the effect of kaolinite on the liquid film drainage rate between bubbles and coal particles was negative. This leads to a change in the probability of adhesion. The influence of ultrafine kaolinite particles on the pulp froth layer leads to the enhancement in the stable probability of froth layer formation, which ultimately leads to an improvement in the flotation behavior of coal particles under the addition of ultrafine kaolinite particles.
References
Chen X, Peng Y (2018) Managing clay minerals in froth flotation—a critical review. Miner Process Extr Metall Rev 39(5):289–307
Chen X, Hadde E, Liu S, Peng Y (2017) The effect of amorphous silica on pulp rheology and copper flotation. Miner Eng 113:41–46
Dadashev RK, Dzhambulatov RS, Elimkhanov DZ (2015) Features of the concentration dependences of the surface tension of water suspensions of bentonites. Russ J Phys Chem A 89(8):1504–1506
Ejtemaei M, Nguyen AV (2017) A comparative study of the attachment of air bubbles onto sphalerite and pyrite surfaces activated by copper sulphate. Miner Eng 109:14–20
Farrokhpay S (2011) The significance of froth stability in mineral flotation-a review. Adv Colloid Interface Sci 166(1–2):1–7
Gulsoy OY (2005) A simple model for the calculation of entrainment in flotation. Korean J Chem Eng 22(4):628–634
Horozov TS (2008) Foams and foam films stabilised by solid particles. Curr Opin Colloid Interface Sci 13(3):134–140
Hosseinzadeh M, Shirvani M, Ghaemi A (2018) A study on mean drop size and drop size distribution in an eductor liquid–liquid extractor. Sep Purif Technol 201:205–213
Li C, Somasundaran P (1993) Role of electrical double layer forces and hydrophobicity in coal flotation in NaCl solutions. Energy Fuels 7(2):244–248
Ndlovu B, Forbes E, Farrokhpay S et al (2014) A preliminary rheological classification of phyllosilicate group minerals. Miner Eng 55:190–200
Nguyen AV, Schulze HJ (2004) Colloidal science of flotation, vol 118. Marcel Dekker, New York, pp 1–850
Nguyen AV, Ralston S, Schulze JH (1998) On modelling of bubble–particle attachment probability in flotation. Int J Miner Process 53(4):225–249
Ni C, Xie G, Liu B et al (2015) A design of an inclined froth zone in column flotation device to reduce ash content in clean coal. Int J Coal Prep Util 35(6):281–294
Ni C, Bu X, Xia W et al (2018a) Observing slime-coating of fine minerals on the lump coal surface using particle vision and measurement. Powder Technol 339:434–439
Ni C, Bu X, Xia W, Peng Y, Xie G (2018b) Effect of slimes on the flotation recovery and kinetics of coal particles. Fuel 220:159–166
Pacek AW, Man CC, Nienow AW (1998) On the Sauter mean diameter and size distributions in turbulent liquid/liquid dispersions in a stirred vessel. Chem Eng Sci 53(11):2005–2011
Quinn JJ, Kracht W, Gomez CO, Gagnon C, Finch JA (2007) Comparing the effect of salts and frother (MIBC) on gas dispersion and froth properties. Miner Eng 20(14):1296–1302
Shi FN, Zheng XF (2003) The rheology of flotation froths. Int J Miner Process 69(1):115–128
Visser J (1969) On Hamaker constants: a comparison between Hamaker constants and Lifshitz–van der Waals constants. Adv Colloid Interface Sci 3(4):331–363
Wang D, Qiu G, Hu Y (2005) Resource processing. Science Press, Beijing, pp 1–281
Wang L, Peng Y, Runge K, Bradshaw D (2015) A review of entrainment: mechanisms, contributing factors and modelling in flotation. Miner Eng 70:77–91
Warren LJ (1985) Determination of the contributions of true flotation and entrainment in batch flotation tests. Int J Miner Process 1(14):33–44
Xie G (2012) Mineral processing. China University of Mining and Technology Press, Xuzhou, pp 413–490
Ye X, Yang S, Li C (2017) Synergistic effects of disjoining pressure and surface viscosity on film drainage process. Acta Phys Sin 66(19):178–190
Yoon RH (2000) The role of hydrodynamic and surface forces in bubble–particle interaction. Int J Miner Process 58(1–4):129–143
Yu Y, Ma L, Cao M, Liu Q (2017a) Slime coatings in froth flotation: a review. Miner Eng 114:26–36
Yu Y, Ma L, Wu L, Ye G, Sun X (2017b) The role of surface cleaning in high intensity conditioning. Powder Technol 319:26–33
Zhang M, Peng Y (2015) Effect of clay minerals on pulp rheology and the flotation of copper and gold minerals. Miner Eng 70:8–13
Zhang S, Lan Q, Liu Q, Xu H, Sun D (2008) Aqueous foams stabilized by laponite and CTAB. Colloids Surf A Physicochem Eng Asp 317(1–3):406–413
Zhang Z, Liu J, Xu Z, Ma L (2013) Effects of clay and calcium ions on coal flotation. Int J Min Sci Technol 23(5):689–692
Zhang X, Manica R, Tchoukov P, Liu Q, Xu Z (2017) Effect of approach velocity on thin liquid film drainage between an air bubble and a flat solid surface. J Phys Chem C 121(10):5573–5584
Zheng X, Johnson NW, Franzidis JP (2006) Modelling of entrainment in industrial flotation cells: water recovery and degree of entrainment. Miner Eng 19(11SI):1191–1203
Zhou Y, Dong X (2016) Relationship between flotation result and surface tension of coal slime. China Min Mag 2:105–109
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Y., Xia, W., Peng, Y. et al. Effect of ultrafine kaolinite particles on the flotation behavior of coking coal. Int J Coal Sci Technol 7, 623–632 (2020). https://doi.org/10.1007/s40789-020-00304-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40789-020-00304-5