Abstract
The distribution and verticals variation of geochemical components in the Kasnau-Matasukh lignites of Nagaur Basin, Rajasthan, were investigated using microscopy, proximate and ultimate analyses, Rock–Eval Pyrolysis, X-ray diffraction and Fourier Transform Infrared analyses, and major/minor/trace element determination. The relationship of elements with ash content and with macerals have also been discussed. These lignites are stratified, black, dominantly composed of huminite group macerals with subordinated amounts of liptinite and inertinite groups. They are classified as type-III kerogen and are mainly gas prone in nature. The concentration (in vol%) of mineral matter is seen to increase towards upper part of seam and so is the concentration (in wt%) of the volatile matter, elemental carbon and sulphur. The common minerals present in these lignitesare mixed clay layer, chlorite, and quartz as identified by X-ray diffraction study. Compared with world average in brown coal, the bulk concentration of Cu is anomalously high in most of the samples while Cd is 2–3 times high and Zn is high in one band. Based on interrelationship, different pyrite forms are noticed to have different preferential enrichment of various elements. The concentration of disseminated pyrite is more than the other pyrite forms and is followed by discrete pyrite grains and massive pyrite.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Coal is an organo-clastic sedimentary rock composed of lithified plant debris. The inorganic constituents occurring in different forms in coal of all rank are collectively known as ‘mineral matter’. Mineral matter may occur as crystalline solids, dissolved salts in pore waters, organo-metallic compounds, discrete and disseminated grains. The type and quantity of mineral matter in coal depends on nature of vegetal matter, mode of accumulation (allochthonous or autochthonous), tectonic framework of the depositional basin, hydrological conditions, climatic conditions and the geomorphology of the hinterland. There are different phases during which the mineral matter gets into coals. These include, (i) inherent in organic matter of plants, (ii) syngenetic minerals added during the stage of peat development, and (iii) secondary or epigenetic minerals deposited through circulating waters during the coalification process. Contributions on the significance of mineral matter and trace elements in coalhave been made by many researchers. Ward et al. (1999) discussed the trace elements and mineral matter of New South Wales, Australia, in the light of quantitative data obtained through X-ray diffraction study. Mineralogical analysis of these Australian coals was further used for the seam correlation (Ward et al. 2001). A detailed account on the significance of mineral matter in coal is provided by Ward (2002). Li et al. (2010) used electron microprobe study to know the occurrence of non-mineral inorganic elements in macerals of low rank coals while Dai et al. (2013) studied the mineralogical and geochemical anomalies of Late Permian coals from southern China and evaluated the influence of hydrothermal fluids and terrigenous materials. They also carried out study on the elements and phosphorus minerals in the Jurassic coals of Tibetan Plateau (Dai et al. 2015a, b). While working on mineral matter in coal, Ren (1996) discussed the significance of trace elements in coal revealing geologic information about coal-bearing sequence formation, depositional condition and regional tectonic history of the basin. Trace elements get accumulated in coal in two ways, through plants and animals, and through geologic processes during peat and post peatification stages (Bouška et al. 2000). The impact of trace elements on environment depends upon their modes of occurrence (mobility), concentration, and toxicity (Finkelman 1995; Dai et al. 2005) and thus, study of trace elements would help in formulating strategies to combat pollution related to coal combustion (Querol et al. 2001; Dai et al. 2005; Wang et al. 2008; Tang et al. 2009). There are certain elements like As, Be, Cd, Cr, Co, Cu, Pb, Mn, Hg, Mo, Ni, Sr, U, V, and Zn which are environmentally more sensitive and have their impact on environment when released into atmosphere especially after their combustion in the thermal power plants (Pickhardt 1989; Turiel et al. 1994; Singh et al. 2011, 2012, 2014; Prachiti et al. 2011; Singh and Singh 2013). The mean abundance of elements in coal is also important for geochemical comparisons (Ketris and Yudovich 2009). Silicates, carbonates and sulphates are the major minerals in coal which consists most of the elements but some elements such as Ge, B, Br, Be, and Cl are associated with the organic matter (Finkelman 1995).
Though, various workers have contributed on the geological aspects of the Kasnau-Matasukh lignites of Nagaur basin, but no work has been carried out on the petrological and geochemical aspects of these lignites. Therefore, the present study has been undertaken to see the distribution and variation of the geochemical constituents, including major/minor/trace elements, vertically along the seam profile of these lignites. Further, the inter-relationships among the geochemical constituents and also with petrographic elements has been discussed. This would help in planning the strategy for their utilization.
2 Geological setting
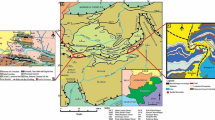
The sedimentary tract of Rajasthan is spread over a large area of 120000 km2 and forms the eastern flank of Indus shelf. The entire sedimentary tract has been sub-divided into four basins which includes- (i) Palana-Nagaur basin, (ii) Jaisalmer basin, (iii) Barmer basin, and (iv) Sanchor basin (Jodha 2008). The present investigation has been carried out on Kasnau-Matasukh lignites. These lignites occur in Palana-Nagaur basin which is an E-W trending elongated basin. It extends for 200 km in length and 50 km in width. Kasnau-Matasukh block is located in Jayal Tehsil of Nagaur district. Nagaur basin has several disconnected small basins of Palana Formation indicating undulating paleotopography. Nagaur basin is linked to a 5–6 m wide channel and hence known as link basin (Lal and Regar 1991). Though the structural features indicate a low tectonic disturbance in the area, gravity survey has indicated the presence of gravity low of 3–4 km width which occurs in NW–SE direction indicating the presence of link channel broadening towards SE. The magnetic survey of the area also substantiates the subsurface structures delineated through gravity survey and show NW–SE elongation of magnetic contour (Lal and Regar 1991).Geological work in and around Nagaur was initially taken up by Blanford (1876) who correlated the Jodhpur set with Vindhyans because of their closer resemblance. The interpretation of exploration data of Nagaur basin has been incorporated in the reports of Mukhopadhyay (1974–1975), Munshi (1975–1977), Faruqi (1978–1979, 1982–1983).
The lignite bearing Lower Tertiary sediments of Palana Formation are deposited unconformably over the Nagaur Formation. Presence of one lignite seam has been identified in the block which is intersected by a number of dirt bands. The Tertiary sequence of rocks comprises of three Formations which include Palana, Marh and Jogira in ascending order (Jodha 2009). The area has scanty outcrops. The lignite occurrences in the Nagaur basin are associated with Palana Formation of Paleocene age. The seams have been reported between 50 m and 150 m depths in the Palana Formation (Jodha 2009). Based on the recovered palynomorphs comprising pteridophytes, angiosperms, algae and fungi, Kulshrestha et al. (1989) and Shah and Kar (1971) have given Paleocene age to this Formation. The general stratigraphic succession of the rocks in the basin is given in Table 1 and the general geological map is shown in Fig. 1. The litholog (after Ghose 1983) and megascopic profile of the Kasnau-Matasukh lignite seam, prepared for this study, is shown in Fig. 2a while geological section is shown in Fig. 2b.
Geological map of Nagaur basin (after Roy and Jakhar 2002) and location of Kasnau-Matasukh lignite
3 Method of study
Lignite samples have been collected from Kasnau-Matasukh mine of Nagaur basin of Rajasthan (Fig. 1) following pillar sampling method (Schopf 1960) so that full lignite seam thickness may be reconstructed in the laboratory. The samples have been crushed and reduced in quantity through quartering and coning to prepare eight composite samples which were subjected to various analyses. The samples were ground to pass 18 mesh size to prepare polished mounts for petrography, while they were further ground to pass 70 mesh size for various chemical analyses like proximate, ultimate, rock–eval pyrolysis and major/minor/trace element analyses. Maceral analysis has been carried out to see the distribution of huminite, liptinite and inertinite group macerals. This is performed under reflected light using a LeitzOrthoplan-Pol Microscope equipped with Wild Photoautomat MPS 45 in the Coal and Organic Petrology Laboratory, Department of Geology, Banaras Hindu University. The line-to-line and point-to-point spacing was maintained at 0.4 mm and more than 600 counts have been taken on each sample following the methodology given by Taylor et al. (1998); huminite macerals have been termed and described as per ICCP-1994 (Sýkorová et al. 2005) while ICCP (2001) has been followed for inertinite macerals. The vitrinite/huminite reflectance (VRo) was measured at National Metallurgical Laboratory, Jamshedpur following ISO 7404-5:2009 (standard used: spinel, yag-yittrium, aluminium garnet, zirconia). On each sample, a minimum of 200 measurements were taken. The proximate analysis has been carried out as per BIS (2003), while the elemental analysis (C, H, N, O, and S) has been performed at CMPDI, Ranchion Elementar Analysensysteme-Vario-III as per ASTM D5373-08.
The Pyrolysis has been carried out on high precision Rock–Eval-6 (make Vinci Technologies, France) at R & D department, Oil India Ltd, Duliajan (Assam) on fourteen lignite samples (represented as four composite samples).This is programmed pyrolysis system and is ultimate to know the source rock potential for hydrocarbon. The significance of this technique is that the coal samples, as such,are analyzed to know the various components. The analysis is performed under controlled temperature and coal samples are heated in absence of oxygen. The produced compounds are quantitatively assessed. During heating the oxygenated compounds released from mineral matter, present in coal, are excluded. The quantitative measurement of various fractions of volatile/non volatile organic compounds, source rock potential and the degree of maturation of lignites samples is obtained through this analysis. The pyrolysis of Kasnau-Matasukh lignite samples has been carried out following the procedures of Espitalié et al. (1977, 1984, 1986). The samples were heated in an open pyrolysis system under non-isothermal condition and the recorded FID signal is divided in two surfaces, S1 and S2, which are expressed in mg HC/g of coal. The method gets completed by combustion (oxidation) of the residual rock recovered after pyrolysis at 850 °C under nitrogen. This is required to avoid incomplete combustion. The released CO and CO2 are monitored online through an infra-red cell. This complementary data acquisition helps in the determination of total organic carbon (TOC) and total mineral or inorganic carbon (TMC or TIC).
The elements Fe, Ca, Mg, Mn, K, Na, Cu, Co, Ni, Cr, Zn, Pb and Ashave been determined on ‘whole coal samples’ in the department of Botany, BHU, Varanasi. For the determination of these elements the coal samples have been digested with 2.5 mL HNO3 andHClO4 in 10:1 ratio on hot water plate following the method ofEaton et al. (1995).The mixture is then filtered usingWhatmanfilter paper (No. 41) and the digested samples are rinsed with 1 % Conc. HNO3.It is then transferred in a separate test tube and the volume is made up to 20 mL. The digested samples have been used for analyzing the concentrations of various elements under Atomic Absorption Spectrophotometer (AAS, Model Perkin Elmer Analyst 800) and the standard used in the analysis were Accu Standard solutions obtained from Merck, KGaA, Darmstadt, Germany. Data of major, minor and trace elements are mean of three independent observations in the present paper. The measured values have shown relative standard deviations less than 5 % for all the elements in the analyzed samples.
Fourier Transform Infrared spectra have been recorded by FTIR spectrophotometer (PerkinElmer Spectrum version 10.03.05) using KBr pellets (transmission mode) in the Department of Chemistry, Banaras Hindu University. Coal: KBr mixture at 1:100 ratio has been used and 20 number of scans have been taken with a spectral resolution of 4 cm−1 at a range of 400–4000 cm−1. X-ray diffraction data have been obtained with the help of computer controlled Xray Diffractometer PanalyticalX’Pert High Score (Plus) v3× database in the Department of Geology, Banaras Hindu University. The operating parameters, in the present study are: start angle- 2°; target- Cu Kα radiation; stop angle- 60°; step size- 0.0250; and 2 theta configuration.
4 Result and discussion
4.1 Petrographic characteristics
These lignites are stratified in nature and are of black color. Huminite is the main component in these lignites (Singh et al. 2015a, b) which is formed due to anaerobic preservation of lignocellulose material in the mire (Sýkorová et al. 2005). Liptinite and inertinitemacerals occur in low concentrations. Huminite (83.9 %–92.5 %; av. 87.3 % mineral matter free basis)is largely contributed by detrohuminite and telohuminite. Detrohuminiteis represented by densinite (19.2 %–42.5 %; av. 31.7 % mineral matter free basis) and attrinite (0 %–13.3 %; av. 5.9 % mineral matter free basis) while telohuminiteis represented by ulminite-A (24.9 %–38.6 %; av. 30.5 % mineral matter free basis),ulminite-B (13.4 %–29.1 %; av. 18.2 % mineral matter free basis) and textinite which occurs in very low amount (<1 %). Liptinite group (5.7 %–13.2 %; av. 10.9 %) and inertinite group (0.2 %–4.0 %; av. 1.9 %) are low in concentrations (Table 2). Mineral matter ranges between 3.5 and 12.0 (av. 7.7 %). The vertical variation of group macerals and mineral matter from base of the seam is shown in Fig. 3. Though, there is no specific trend of variation, yet huminite shows a high concentration at the upper part while liptinite shows a reverse trend. Inertinite is less at the bottom. Mineral matter is more towards the upper part of the seam. The variation has environmental implications. The clastic mineral matter relates directly to water cover in the basin and, therefore, it increases with increase in the water cover during the formation of upper part of the lignite seam. This is also supported by the occurrence of high concentration of huminite group macerals during this period.
4.2 Chemical attributes
Theseligniteshave high volatile matter content (52.6 %–67.0 % daf basis; av. 58.3 %) with moderate ash yield (3.0 %–18.2 %; av. 8.4 %). The ultimate analysis (av. values on daf basis) shows that theselignites contain 54.0 % carbon, 5.4 % hydrogen, 0.8 % nitrogen, 35.9 % oxygen and 3.8 % sulfur (Table 2). The vertical variation of the chemical components along the seam profile is shown in Fig. 4. Volatile matter shows an increasing trend towards upper part of seam while fixed carbon shows a reverse trend. Carbon and sulfur show an increasing trend towards upper part while other ultimate components like hydrogen, nitrogen and oxygen do not show any definite trend. Variable concentrations and dimensions of undecomposed, partly decomposed and completely decomposed wood have been noticed in the Kasnau-Matasukh lignites. This appears to have affected the proximate and ultimate composition of this lignite from bottom to top because these three components have variation in the organic geochemical constitution.
4.3 Hydrocarbon potential
These lignites of Nagaur basin have attained a thermal maturity indicated by vitrinite reflectance (VRo) between 0.23 % and 0.30 % (Table 3) which put them as ‘low rank C’ coals as per ISO-11760 (2005). The analytical results of Rock–Eval pyrolysis of Kasnau-Matasukh lignites show that S1 values (free hydrocarbon distilled out of samples at initial heating of 300 °C) vary from 1.16 to 2.83 mg HC/g. Considering 1 mg HC/g as its cut-off value,t his lignite may be considered as a good source rock. Similarly, S2 values (hydrocarbons generated through thermal cracking which actually indicate the quantity of hydrocarbons that the lignite may potentially produce) are many-fold higher than the free hydrocarbons (already generated oil in the lignite and occur as free hydrocarbons in lignite samples) and it varies from 48.65 to 87.84 mg HC/g (av. 68.99 mg HC/g). Taking 5 as its cut-off value, it also indicates a good source rock for hydrocarbon generation. The S3 values vary from 24.34 to 29.89 mg CO2/g and represent the trapped carbon-di-oxide which is released during the pyrolysis up to a temperature of 390 °C. This is also proportional with the oxygen present in the Kasnau-Matasukh lignites of Nagaur basin. Total organic carbon (TOC) content of these lignite samples exhibits a wide range from 3.27 % to 43.92 % with an average of 31.08 % while the total inorganic carbon (TIC) values also have the similar trend and the value ranges from 1.85 % to 2.73 %.
The vertical variation of the Rock-Evaldata from the base of the lignite seam is shown in Fig. 5. It is evident from this figure and the data (Table 4), that S1 valueis low at the bottom while S2 value is more at the middle part of the seamwhich decreases towards the top as well as towards the bottom part (Table 4; Fig. 5). Total organic carbon content has an increasing trend towards the mid of the seam while decreases at the upper part. The total inorganic carbon (TIC) values also have the similar trend and are derived from the carbonates. In this seam the sulfur content (varies from 3.2 % to 4.4 %) maintains a strong negative correlation (r = −0.81; P value = 0.399) with the TOC content and also with TIC (r = −0.78; P value = 0.433). The organic matter (OM, obtained by deducting ash content from hundred) shows a variation from 81.8 % to 97 %.
Coal acts as good source rock for hydrocarbon generation. The H/C ratio, in 0.8–0.9 range, is a good indicator of a source rock having hydrocarbon potential (Powell and Boreham 1994). Certain coals with low liptinite content have hydrogen-rich vitrinite which generates oil (Bertrand 1989; Newman et al. 1997; Petersen et al. 2000; Singh 2012; Singh et al. 2013). The generated hydrocarbon products have a finite storage capacity and until this capacity is exceeded, no oil expulsion takes place (Powell 1978; Mc Auliffe 1979; Durand 1983; Tissot and Welte 1984; Inan et al. 1998). Singh (2012) and Singh et al. (2016) have studied the hydrocarbon potential of lignites of Cambay basin and Bikaner basin (India) respectively while Singh et al. (2013) investigated the sub-bituminous coals of east Kalimantan (Indonesia) for its liquid hydrocarbon potential. The cross plot of hydrogen index (HI) with oxygen index (OI) and T max of Kasnau-Matasukh lignite (Fig. 6) indicates its immaturity. This lignite falls closer to the zone of organic rich type-III kerogen which is formed under topogenous condition as also revealed by a cross plot between total organic carbon (TOC) and sulfur content (Fig. 7). This plot is proposed by Jasper et al. (2010) who have grouped the coals in three domains. The coal, in Group-A, is for high TOC and low sulphur content (<2 %) which evolves under ombrogenous mires having raised bogs. Such mires are fed by rainwater. Group-C is characterized by coals with high sulphur content and is formed under topogenous mires. Group-B, however, shows an intermediate condition. On this plot the Kasnau-Matasukh lignite falls under Group-C which is characterized by coals having high sulphur content and is formed under topogenous mires as per Jasper et al. (2010). The H/C atomic ratio of 1.2 (Table 2),and high concentration (>80 %) of reactive macerals (huminite + liptinite), in Kasnau-Matasukh lignites (Table 2), are indication of their good hydrocarbon potential as per Cudmore (1977) and Davis et al. (1976). These ligniteshave potential of generating mainly gaseous hydrocarbons. The cross plot between vitrinite reflectance and HI (Fig. 8) also indicates that these lignites are mainly gas prone. The details of the maturity and oil generating potential of the lignites of entire Bikaner-Nagaur basin have been discussed in detail by Singh et al. (2016).
Cross plot between Sulfur and total organic carbon (TOC) (after Jasper et al. 2010)
Cross plot between Hydrogen Index (HI) and Vitrinite Reflectance (VRo) (after Petersen 2005)
4.4 X-ray diffraction (XRD) and Fourier transform infrared (FTIR) studies
XRD spectra of whole coal and low temperature ash samples of the Kasnau-Matasukh lignites are shown in Fig. 9a, b. The minerals in these coals were identified by comparing ‘d’ values as per Lindholm (1987). The common minerals identified from XRD spectrum of whole coal sample are biotite, gypsum, chlorite, goethite/laumontite, quartz, barite, dolomite, haematite and marcasite. The minerals identified in the low temperature ash include goethite/laumontite, anorthite, quartz, haematite and mixed clay. Kaolinite, illite and chlorite are the major mixed clay minerals. Haematite, goethite and marcasite are major iron containing minerals while gypsum, dolomite and laumontite are calcium rich. FTIR spectra are useful for the identification of minerals associated with the coal structures (Karr 1978). The peaks in FTIR spectra of coal between 1100 and 400 cm−1 are of clay minerals such as quartz, kaolinite, illite and montmorillonite groups. The different absorption peaks with their bonds and functional groups are furnished in Table 5 and shown in Fig. 9c. The broad absorption bands in coal ranging from 3618 to 3628 and 3696 to 3699 cm−1 belong to clay minerals (kaolinite and illite).The absorption bands at 3694.90 and 2920.08 cm−1 in the coals are due to O–H groups while the absorption bands at 3397.37 cm−1 are due to O–H and N–H groups. Strong aliphatic absorptions are observed at 2920–2850 cm−1. The intensity of peaks at 2920 cm−1 indicates the presence of long aliphatic chains in the Kasnau-Matasukh lignites. Low intensity aromatic bands were observed in 900–700 cm−1 regions in these lignites. The peak 1701.7 cm−1 appears due to the presence of carbonyl (C=O) group while the peak 1621.54 cm−1 appears due to the presence of 1° amines (N–H). The peak 1435.98 cm−1 indicates the presence of aromatics (C–O stretch in ring). The peaks near 1172 and 1113.75 cm−1 indicate the presence of aliphatic amines (C–N). The oxygen containing functional groups are phenols, alcohols, ethers, carboxylic acid and carbonyls. The region of 1000–1300 cm−1 in the spectra is of C–O bonds. The weak band at 756.64 cm−1 could be due to C–Cl bond while the weak band at 699.1 cm−1 may be due to C≡C–H; C–H bonds. The peak at 667.65 cm−1 may be due to C≡C–H; C–H bonds. The weak bands at 603.21 and 538.47 cm−1 is due to C–Br bond. The present study is in agreement with the studies of Georgakopoulos et al. (2003), Saikia et al. (2007) and Zodrow et al. (2010).
4.5 Geochemistry of major/minor and trace elements
The study of trace elements in coal is being given more impetus during last few decades owing to their environmental implications. Mode of occurrence of major, minor and trace elements in coal may be known through direct and indirect methods (Eskenzy and Stefanova 2007). In the lignite samples of Kasnau-Matasukh, the mode of occurrence of elements has been studied through indirect method. Here, correlation coefficients of the elements with ash yield, petrographic content and also among themselves have been calculated.
The concentration of elements in the analysed samples has been compared with the world average in lignite. As we can see from the Table 6 the concentration of Cu is very high in all the bands and over 70 times, in KM-7 band, as compared to world average in brown coals. Similarly, Cd is 2–3 times high in almost all the bands while Zn is high in KM-3 band. Rests of the elements have a normal concentration in Kasnau-Matasukh lignites. The vertical variation of various major/minor and trace elements is shown in Fig. 10 along the lignite seam profile. Though, there is not a prominent trend of distribution of these elements yet the concentration of elements like Mn, Na, Cu, Ni, Co, Cr, Pb and Cd is higher towards the upper part of the seam as revealed in Fig. 10. Sulfur concentration is high in Kasnau-Matasukh lignites. Pyrite is formed, in coal, from H2S and Fe in solution which involves bacterial reduction of SO4 to H2S at pH 7–4.5 (Ryan and Ledda 1997). It occurs in various forms in Kasnau-Matasukh lignites. As analyzed under microscope, disseminated pyrite in these lignites, dominates (av. 41 %) over the other pyrite forms and is followed by discrete pyrite grains (av. 23.8 %) and massive pyrite (av. 11.7 %) (Table 7). Some photomicrographs of pyrite of Kasnau-Matasukh lignites are shown in Fig. 11. The clustered framboidal pyrites are more common in the middle part of the seam while single framboids are more towards the upper part. Based on the values of correlation coefficient, preferential enrichment of Ni, Pb, and Co is seen in pyrite. Finkelman (1994) also has reported the association of Co with sulfides, though, it is also found associated with clays and organic matter. Co may also occur as siegenite and cattierite (Dai et al. 2015a, b).Dale et al. (1999) have reported the occurrence of Co associated with silicates in Australian coals. Framboidal pyrite has shown preferential enrichment of Cu, Pb, Co, Cr, and Ni; disseminated pyrite shows an affinity with Ni and Co while discrete pyrite grains with Pb and Co. Similarly massive pyrite has a close affinity with Fe and Zn while pyrite occurring as fissure and crack fillings has affinity with Cd and Mg. Cadmium is normally associated with sphalerite (ZnS) though it is also found in other sulphides (Finkelman 1994). This has also been documented by Swaine (1990), Goodarzi (2002), and Dale et al. (1999). Ash yield shows a strong affinity with Mn (r = 0.728) and Na (r = 0.744) among the major elements and with Pb (r = 0.786) and Co (r = 0.65) among the trace elements. Eskenzy (2009) also reported a positive correlation of ash content with Mn and Co and observed the association of Pb with organic as well as inorganic fractions in Bulgarian coal. On the other hand inertinite maceral group has shown a strong affinity with Mg and Zn while huminite has a strong affinity with Mn, and liptinite relates well with Cu and Cr. These elements could either be associated with the organic molecules or with the minerals occurring as intergrown with the macerals. On the other hand some elements could be related to those minerals which occur as surface blanketing or as superficial mounting over the surface of the macerals (Singh et al. 2010). While working on Shenbei Tertiary lignites of China, Ren et al. (2004) reported Cr, Co, Ni, Cu, V and Zn to be associated with organic macromolecules and they have suggested their enrichment during coal-forming or early diagenesis process. Eskenzy and Stefanova (2007) believe that organically bound parts of the elements are generally higher in low-rank coals. Due to lack of evidence regarding mode of occurrence of Ni in coal, its relation is yet to be precisely established (Finkelman 1994; Riley et al. 2012). It may be organically bound or it could also be associated with sulfides. Dale et al. (1999) reported Ni from both monosulphides and organic matter. Ni relates with Na, K and Co in Kasnau-Matasukh lignites which is in agreement with the work of Singh et al. (2015a, b) on the nearly located Barsingsar and Gurhalignites of Bikaner-Nagaur basin. KM-3, which is matrix rich stratified band, contains high concentration of Zn, Cu and Cd. Zinc is considered as a notorious contaminant and occurs in all coals in HCl soluble phase (Riley et al. 2012).
Photomicrographs of minerals in the lignite samples of Kasnau-Matasukh; a framboidal pyrite, disseminated pyrite, discrete pyrite and massive pyrite; b framboidal pyrite and discrete pyrite occuring in textinite maceral; c massive pyrite, discrete pyrite, pyrite occurring as fissure and also in funginite maceral; d massive pyrite, disseminated pyrite, pyrite occupying the fissures in the lamellar textinite; e massive pyrite; f massive and framboidal pyrite; abbreviations: Fra. Py. framboidal pyrite, Diss. Py. disseminated pyrite, Disc. Py. discrete pyrite, Mass. Py. massive pyrite, Fiss. Py. fissure pyrite
As revealed in the correlation matrix among the elements in the Kasnau-Matasukh lignites, Cd shows a strong affinity with Cu while Pb has a strong affinity with Co and Ni. Pb also has an affinity with sulfides especially pyrite. Cr relates strongly with Cu and occurs in sulphides while Co maintains a positive affinity with Na and Mn (Table 8).
5 Conclusion
-
1.
These lignites are predominantly composed of huminite group of macerals while liptinite and inertinite macerals occur in less concentration. Huminite shows a high concentration at the upper indicating anaerobic degradation during that period. Mineral matter is more towards the upper part of the seam indicating a wet environment.
-
2.
Volatile matter content is high while ash yield is moderate.Sulfur content of these lignites is moderately high. There is increase in volatile matter, carbon and sulphur contents towards the upper part of seam.
-
3.
S1 values are low at the bottom while S2 values are more at the middle part of the seam and decreases towards the top as well as bottom. Total organic carbon content is more in the middle part of the seam and decreases towards the top. Study reveals that these lignites are type-III kerogen and are mainly gas prone.
-
4.
XRD study reveals the presence of mixed clay minerals including kaolinite, illite and chlorite. The peaks in FTIR spectra between 1100 and 400 cm−1 further support the presence of these clay minerals.
-
5.
The concentration of Cu is very high in all the samples and over 70 times in KM-7 band. Similarly, Cd is 2–3 times high in almost all the samples while Zn is high in KM-3 band. The concentration of elements like Mn, Na, Cu, Ni, Co, Cr, Pb and Cd is higher towards the upper part of the seam. Preferential enrichment of Ni, Pb, and Co is seen in pyrite.
-
6.
Ash content shows a strong affinity with Mn, among the major elements, and with Co among the trace elements. On the other hand, inertinite maceral has an affinity with Mg and Zn while huminite with Mn, and liptinite with Cu and Cr. Cadmium shows a strong affinity with Mg and Cu while Pb has a strong affinity with Mn, Na, Co and Ni. Chromium relates strongly with Cu,Pb with pyrite, Co with Na and Mn; and Ni with Na, K and Co.
Nevertheless, the results warrant further study for formulating any strategy for proper utilization of the Kasnau-Matasukh lignites of Rajasthan.
References
Bertrand PR (1989) Microfacies and petroleum properties of coals as revealed by a study of North Sea Jurassic coals. Int J Coal Geol 13(1–4):575–595
BIS (2003) Methods of test for coal and coke (2nd revision of IS: 1350). Part I, Proximate analysis. Bureau of Indian Standard 1–29
Blanford WT (1876) On the physical geography of the Great Indian Desert with special reference to the former existence of the sea in the Indus Valley; and on the origin and mode of formation of the sand hills. J Asiat Soc Bengal 45(2):86–103
Bouška V, Pešek J, Sýkorová I (2000) Probable modes of occurrence of chemical elements in coal. Acta Mont Ser B 10(117):53–90
Cudmore JF (1977) Evaluation of coals for conversion to liquid hydrocarbons. In: International: coal borehole evaluation, Victoria, Australia. The Australasian Institute of Mining and Metallurgy, pp 146–158
Dai SF, Ren DY, Tang YG, Yue M, Hao LM (2005) Concentration and distribution of elements in Late Permian coals from western Guizhou Province, China. Int J Coal Geol 61(1–2):119–137
Dai SF, Zhang WG, Ward CR, Seredin VV, Hower JC, Li X, Song WJ, Wang XB, Kang H, Zheng LC, Wang PP, Zhou D (2013) Mineralogical and geochemical anomalies of late Permian coals from the Fusui Coalfield, Guangxi Province, southern China: influences of terrigenous materials and hydrothermal fluids. Int J Coal Geol 105:60–84
Dai SF, Yang JY, Ward CR, Hower JC, Liu HD, Garrison TM, French D, O’Keefe JMK (2015a) Geochemical and mineralogical evidence for a coal-hosted uranium deposit in the Yili Basin, Xinjiang, northwestern China. Ore Geol Rev 70:1–30
Dai SF, Hower JC, Ward CR, Guo WM, Song HJ, O’Keefe JMK, Xie PP, Hood MM, Yan XY (2015b) Elements and phosphorus minerals in the middle Jurassic inertinite-rich coals of the Muli Coalfield on the Tibetan Plateau. Int J Coal Geol 144–145:23–47
Dale LS, Chapman JF, Buchanan SJ, Lavrencic SA (1999) Mechanisms for trace element partitioning in Australian coals—Project 4.2 Final report, Co-operative Research Centre for Black Coal Utilisation
Davis A, Spackman W, Given PH (1976) The influence of the properties of coals on their conversion to clean fuels. Energy Sources Part A 3:55–81
Durand B (1983) Present trends in organic geochemistry in research on migration of hydrocarbons. In: Bjorey M et al (eds) Advances in organic geochemistry 1981. Wiley, Chichester, pp 117–128
Eaton AD, Clesceri LS, Greenberg AE (1995) Standard methods for the examination of water and waste water, 19th edn. American Public Health Association, Washington.
Eskenzy GM (2009) Trace elements geochemistry of the Dobrudza coal basin, Bulgaria. Int J Coal Geol 78(3):192–200
Eskenzy GM, Stefanova YS (2007) Trace elements in the Goze Delchev coal deposit, Bulgaria. Int J Coal Geol 72(3–4):257–267
Espitalié J, Laporte JL, Madec M, Marquis F, Leplat P, Poulet J, Boutefeu A (1977) Rapid method of characterizing source rocks and their petroleum potential and degree of maturity. Revue de l’InstitutFrancais du Petrole 32:23–42
Espitalié J, Marquis F, Barsony I (1984) Geochemical logging. In: Voorhees KJ (ed) Analytical pyrolysis: techniques and applications. Butterworth, London, pp 276–304
Espitalié J, Deroo G, Marquis F (1986) La pyrolyse Rock-Evaletses applications, partie III. Rev Inst Fr Pet 41:73–89
Faruqi NH (1978–79) Report on the elucidation of solid geology of sand and alluvium covered areas in part of Bikaner and Ganganagar district, Rajasthan. Geological Survey of India, Government of India. (Unpublished report)
Faruqi NH (1982–83) Report on the Geological mapping of pre-quaternaries in parts of Bikaner and Ganganagar district, Rajasthan. Geological Survey of India, Government of India. (Unpublished report)
Finkelman RB (1994) Modes of occurrence of potentially hazardous elements in coal: levels of confidence. Fuel Process Technol 39:21–34
Finkelman RB (1995) Modes of occurrence of environmentally-sensitive trace elements in coal. In: Swain DJ, Goodarzi F (eds) Environmental aspects of trace elements in coal. Kluwer Academic Publishers, Dordrecht, pp 24–50
Georgakopoulos A, Iordanidis A, Karma V (2003) Study of low rank Greek coals using FTIR spectoscopy. Energy Sources A 25:995–1005
Ghose KP (1983) Generalised structural and metamorphic map around Batia, Udaipur District, Rajasthan; a study. Indian J Earth Sci 10:228–231
Goodarzi F (2002) Mineralogy, elemental composition and modes of occurrence of elements in Candian feed-coals. Fuel 81(9):1191–1213
Gowrisankaran S, Sethi PP, Hariharan R, Agrawal, KP (1987) Lignite deposits of India- their occurrences, depositional features and characteristics. In: Singh. R. M. (ed). Proceedings of national seminar coal resources of India. Tara Printing Works, Kamachha, Varanasi, pp 481–553
Inan S, Yalcin MN, Mann U (1998) Expulsion of oil from petroleum source rocks: inferences from pyrolysis of samples of unconventional grain size. Org Geochem 29(1–3):45–61
International Commitee for Coal and Organic Petrology (2001) The new inertinite classification (I.C.C.P. System 1994). Fuel 80:459–471
ISO 11760 (2005) Classification of coals. International standard, pp 1–9
ISO 7404-5 (2009) Methods for the petrographic analysis of coals—part 5: method of determining microscopically the reflectance of vitrinite 1–14
Jasper K, Hartkopf-Froder C, Flajs G, Littke R (2010) Evolution of Pennsylvanian (Late Carboniferous) peat swamps of the Ruhr Basin, Germany: comparison of palynological, coal petrographical and organic geochemical data. Int J Coal Geol 83(4):346–365
Jodha BS (2008) Report on search for lignite by scout drilling in Nagrasar area, Palana basin, Bikaner and Jaisalmer districts, Rajasthan. Geological Survey of India, Government of India
Jodha BS (2009) Report on search for lignite by scout drilling in Borana East area, Palana basin, Bikaner and Jaisalmer districts, Rajasthan. Geological Survey of India, Government of India
Karr C Jr (1978) Analytical methods for coal and coal products, vol II. Academic Press, New York
Ketris MP, Yudovich YE (2009) Estimations of Clarkes for carbonaceous biolithes: world average for trace element contents in black shales and coals. Int J Coal Geol 78(2):135–148
Koeverdon JHV, Karlsen DA, Backer-Owe K (2011) Carboniferous non-marine source rocks from Spitsbergen and Bajornoya: coparision with western Arctic. J Pet Geol 34(1):53–66
Krevelen V (1961) Coal. Elsevier, Amsterdam, pp 162–210
Kulshrestha SK, Singh RY, Atta, Sobeh V (1989) Stratigraphy of lower tertiary sediments in Bikaner, Western Rajasthan. In: Proceedings of XIII Indian Colloq, Micropal. Strat., pp 193–202
Lal B, Regar RL (1991) Geophysical investigation for lignite in Sujasar-Kesardesar area, Bikaner district, Rajasthan. Geological Survey of India, Government of India. (Unpublished report)
Li Z, Ward CR, Gurba WL (2010) Occurrence of non-mineral inorganic elements in macerals of low-rank coals. Int J Coal Geol 81(4):242–250
Lindholm R (1987) A practical approach to sedimentology. Allen & Unwin (New Zealand) Ltd in association with the Port Nicholson Press Ltd 60 Cambridge Terrace, Wellington, pp 124–132
Mc Auliffe CD (1979) Oil and gas migration-chemical and physical constraints. Am Assoc Pet Geol Bull 63(5):761–781
Mukhopadhyay AK (1974–1975) Report on the elucidation of solid geology of sand and alluvium covered areas in part of Churu and Bikaner district, Rajasthan. Geological Survey of India, Government of India. (Unpublished report)
Munshi RL (1975–77) Report on the elucidation of solid geology of sand and alluvium covered areas in part of Bikaner and Ganganagar district, Rajasthan. Geological Survey of India, Government of India. (Unpublished report)
Newman J, Price LC, Johnston JH (1997) Hydrocarbon source potential and maturation in Eocene New Zealand vitrinite-rich coals. J Pet Geol 20:137–163
Petersen HI (2005) Oil Generation from coal source rocks: the influence of depositional conditions and stratigraphic age. Geol Survey Denmark Greenl Bull 7:9–12
Petersen HI, Andsbjerg J, Bojesen-Koefoed JA, Nytoft HP (2000) Coal-generated oil: source rock evaluation and petroleum geochemistry of the Lulita oilfield, Danish North Sea. J Pet Geol 23(1):55–90
Pickhardt WV (1989) Trace elements in minerals of German bituminous. Int J Coal Geol 14(1–2):137–153
Powell TG (1978) An assessment of the hydrocarbon source rock potential of the Canadian Arctic Islands. Geological Survey of Canada, pp 78–12
Powell TG, Boreham CJ (1994) Terrestrial sourced oils: where do they exist and what are our limits of knowledge?—a geochemical perspective. In: Scott AC, Fleet AJ (eds) Coal and coal bearing strata as oil-prone source rocks, vol 77. Geological Society Special Publication, London, pp 11–29
Prachiti PK, Manikyamba C, Singh PK, Balram V, Lakshminarayana G, Raju K, Singh MP, Kalpana S, Arora M (2011) Geochemical systematics and precious metal content of the sedimentary horizons of Lower Gondwanas from the Sattupalli coal field, Godavari Valley, India. Int J Coal Geol 88(2–3):83–100
Querol X, Alastuey A, Zhuang XG, Hower JC, Lopez-Soler A, Plana F, Zeng RS (2001) Petrology, mineralogy and geochemistry of the Permian and Triassic coals in the Leping area, Jiangxi Province, southeast China. Int J Coal Geol 48(1–2):23–45
Ren DY (1996) Mineral matter in coal. In: Han DX (ed) Coal petrology of China. Publishing House of China University of Mining and Technology, Xuzhou, pp 67–77
Ren DY, Xu DW, Zhao FH (2004) A preliminary study on the enrichment mechanism and occurrence of hazardous trace elements in the Tertiary lignite from the Shenbei coalfield, China. Int J Coal Geol 57(3–4):187–196
Riley KW, French DH, Farrell OP, Wood RA, Huggins FE (2012) Modes of occurrence of trace and minor elements in some Australian coals. Int J Coal Geol 94:214–224
Roy AB, Jakhar SR (2002) Geology of Rajasthan (Northwest India) precambrian to recent. Scientific Publishers, Jodhpur, pp 1–421
Ryan B, Ledda A (1997) A review of sulphur in coal: with specific reference to the Telkwa deposit, north-western British Columbia. Geological Fieldwork, 22 pp
Saikia BK, Boruah RK, Gogoi PK (2007) XRD and FT-IR investigation of sub-bituminous Assam coals. Bull Mater Sci 30(4):421–426
Schopf JM (1960) Field description and sampling of coal beds. U.S. Geological Servey Bulletin B 1111, pp 25–69
Shah SCD, Kar RK (1971) Palynostratigraphic evolution of the Lowest Eocene sediments of India. In: Proceedings of sem. paleopalynology and Indian strat. Calcutta University Publication Calcutta, pp 255–264
Singh PK (2012) Petrological and geochemical considerations to predict oil potential of Rajpardi and Vastan Lignite Deposits of Gujarat, Western India. J Geol Soc India 80:759–770
Singh PK, Singh MP (2013) Coal resource of India in context of recent developments in clean coal technologies. In: Sharma PR, Yadav RS, Sharma VN (eds) Interdisciplinary advances in environmental and earth system studies, I edn. RK Books, New Delhi, pp 198–212
Singh PK, Singh GP, Naik AS (2010) Petrological considerations for benificiation of Indian coals. J Sci Res 54(1–2):51–60
Singh PK, Singh AL, Aniruddha K, Singh MP (2011) A study on removal of selected major elements from Indonesian coal through bacteria: environmental implications. World Acad Sci Eng Technol 75:925–935
Singh PK, Singh AL, Aniruddha K, Singh MP (2012) Mixed bacterial consortium as an emerging tool to remove hazardous trace metals from coal. Fuel 102:227–230
Singh PK, Singh MP, Singh AK, Arora M, Naik AS (2013) The prediction of the liquefaction behavior of the East Kalimantan Coals of Indonesia: an appraisal through petrography of selected coal samples. Energy Sources Part A 35(18):1728–1740
Singh AL, Singh PK, Aniruddha K, Yadav A, Singh MP (2014) Experimental study on demineralization of coal with Pseudomonas mendocina strain B6-1 bacteria to obtain clean fuel. Energy Explor Exploit 32(5):831–846
Singh PK, Rajak PK, Singh MP, Naik AS, Singh VK, Raju SV (2015a) Environmental geochemistry of selected elements in lignite from Barsingsar and Gurha Mines of Rajasthan, Western India. Geol Soc India 86(1):23–32
Singh AL, Singh PK, Singh MP, Kumar A (2015b) Environmentally sensitive major and trace elements in Indonesian coal and their geochemical significance. Energy Sources Part A 37(17):1836–1845
Singh PK, Rajak PK, Singh VK, Singh MP, Naik AS, Raju SV (2016) Studies on thermal maturity and hydrocarbon potential of lignites of Bikaner-Nagaur basin, Rajasthan. Energy Explor Exploit 34(1):140–157. doi:10.1177/0144598715623679
Swaine DJ (1990) Trace elements in coal. Butterworths & Co, London, p 278
Sýkorová I, Pickel W, Christanis M, Wolf K, Taylor GH, Flores D (2005) Classification of huminite. ICCP system 1994. Int J Coal Geol 62:85–106
Tang Y, Chang C, Zhang Y, Li W (2009) Migration and distribution of fifteen toxic trace elements during the coal washing of the Kailuan Coalfield, Hebei Province, China. Energy Explor Exploit 27:143–151
Taylor GH, Teichmüller M, Davis A, Diessel CFK, Littke R, Robert P (1998) Organic petrology. Gebrüder Borntraeger, Berlin, p 704
Tissot BP, Welte DH (1984) Petroleum formation and occurrence, 2nd edn. Springer, Berlin, p 699
Turiel JLF, Carvalho WD, Cabanas M, Querol X, Soler AL (1994) Mobility of heavy metals from coal flyash. Environ Geol 23:264–270
Valkovic VV (1983) Trace elements in coal, vol 1. CRC Press, Inc, Florida, p 207
Wang WF, Qin Y, Sang SX, Zhu YM, Wang CY, Weiss DJ (2008) Geochemistry of rare earth elements in a marine influenced coal and its organic solvent extracts from the Antaibao mining district, Shanxi, China. Int J Coal Geol 76(4):309–317
Ward CR (2002) Analysis and significance of mineral matter in coal seams. Int J Coal Geol 50(1–4):135–138
Ward CR, Spears DA, Booth CA, Staton I, Gurba LW (1999) Mineral matter and trace elements in coals of the Gunnedah Basin, New South Wales, Australia. Int J Coal Geol 40(4):281–308
Ward CR, Matulis CE, Tayler JC, Dale LS (2001) Quantification of mineral matter in the Argonne Premium coals using interactive Rietveld-based X-ray diffraction. Int J Coal Geol 46(2–4):67–82
Zodrow EL, Mastalerz M, Werner-Zwanziger U, D’Angelo JA (2010) Medullosalean fusain trunk from the roof rocks of a coal seam: insight from FTIR and NMR (Pennsylvanian Sydney Coalfield, Canada. Int J Coal Geol 82(1–2):116–124
Acknowledgments
The authors thankfully acknowledge the Department of Geology, Banaras Hindu University for extending the laboratory and other facilities. The help received for Rock–Eval-6 pyrolysis from the R & D department of Oil India Ltd, Duliajan, is thankfully acknowledged. The authors also thank CMPDI, Ranchi for carrying out ultimate analysis. The help rendered by the officials in the Kasnau-Matasukh mine is thankfully acknowledged. We are also thankful to the editor for editorial handling of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Singh, P.K., Rajak, P.K., Singh, M.P. et al. Geochemistry of Kasnau-Matasukh lignites, Nagaur Basin, Rajasthan (India). Int J Coal Sci Technol 3, 104–122 (2016). https://doi.org/10.1007/s40789-016-0135-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40789-016-0135-0