Abstract
New inorganic and organic geochemical data from thucholite in the Upper Permian (Wuchiapingian) Kupferschiefer (T1) shale collected at the Polkowice-Sieroszowice Cu-Ag mine in Poland are presented. Thucholite, which forms spherical or granular clusters, appears scattered in the T1 dolomitic shale at the oxic-anoxic boundary occurring within the same shale member. The composition of thucholite concretions and the T1 shale differs by a higher content of U- and REE-enriched mineral phases within the thucholite concretions compared to the T1 shale, suggesting a different mineralising history. The differences also comprise higher Ntot, Ctot, Htot, Stot contents and higher C/N, C/S ratios in thucholite than in the T1 shale. The hydrocarbon composition of the thucholite and the surrounding T1 shale also varies. Both are dominated by polycyclic aromatic compounds and their phenyl derivatives. However, higher abundances of unsubstituted polycyclic aromatic hydrocarbons in the thucholite are indicative of its pyrogenic origin. Pyrolytic compounds such as benz[a]anthracene or benzo[a]pyrene are more typical of the thucholite than the T1 shale. Microscopic observations of the thucholite and its molecular composition suggest that it represents well-rounded small charcoal fragments. These charcoals were formed during low-temperature combustion, as confirmed by semifusinite reflectance values, indicating surface fire temperatures of about 400 °C, and the absence of the high-temperature pyrogenic polycyclic aromatic hydrocarbons. Charred detrital particles, likely the main source of insoluble organic matter in the thucholite, migrated to the sedimentary basin in the form of spherical carbonaceous particulates, which adsorbed uranium and REE in particular, which would further explain their different contents and sorption properties in the depositional environment. Finally, the difference in mineral content between thucholite and the T1 shale could also have been caused by microbes, which might have formed biofilms on mineral particles, and caused a change in the original mineral composition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Uranium-bearing thucholite concretions most commonly occur in sedimentary rocks (sandstone or shale) of various geological ages (Hallbauer and van Warmelo 1974; Banaś et al. 1978; Luo et al. 2014). Thucholite is a mineraloid composed of C, O, Th, U, H, N, and P. It is mostly considered a product of polymineralisation of organic matter caused by ionising radiation (Khan et al. 2020). Its presence in a sedimentary basin probably results from the occurrence of radioactive detrital minerals, i.e., monazite, zircon, xenotime, etc., or even a radioactive fluid (Crick et al. 1988; Rasmussen et al. 1989, 1993; Crick 1992; Luo et al. 2014; Khan et al. 2020). The provenance of such radioactive grains in thucholite is still a subject of debate. For example, the origin of thucholite can be explained by an interaction between water and oil rich in radioactive elements (Parnell 1988a, 1996; Parnell and Eakin 1989) or by an allochthonous source (Rasmussen et al. 1993; England et al. 2001). Schidlowski (1981), Luo et al. (2014) and Khan et al. (2020) have suggested that thucholite formation can result from radiation-induced polymerisation of algal mats by radioactive grains.
Thucholite occurs in various environments. It has been reported from pegmatites and hydrothermal veins (Spence 1930; Welin 1966; Chukanov et al. 2009), but it is chiefly associated with sandstone and shale (Ellsworth 1928; Welin 1966; Curiale et al. 1983; Crick et al. 1988; Rasmussen et al. 1989, 1993; Crick 1992; Alexandre and Kyser 2006; Alexandre et al. 2009; Luo et al. 2014; Khan et al. 2020) or very rarely with carbonate strata (Kucha and Przybyłowicz 1999; Banaś et al. 2005). In addition, thucholite can be found in rock formations of different ages, from Precambrian (Witwatersrand sandstone, Finnish Viitaniemi pegmatite) to Permian and younger rocks (Kucha and Przybyłowicz 1999; England et al. 2001; Fuchs et al. 2015; Alderton et al. 2016). Thucholite from the Polkowice-Sieroszowice area in Poland, which is the focus of this study, is built of a polymerised carbonaceous substance containing oxygen and inclusions of graphite, uranium minerals, platinum, palladium, native gold, and organic compounds (Kucha 2007, 2021; Sanz-Robinson 2020). Moreover, U–Pb dating performed on thucholite from the Lubin Cu-Ag deposit gave Jurassic ages (175–180 Ma) (Kucha and Przybyłowicz 1999; Alderton et al. 2016). Later Kucha (2021) used U–Pb dating on uraninite present in thucholite, which gave two groups of ages, i.e., 169–196 Ma (thucholite free of visible mineral inclusions and age similar to that obtained by Kucha and Przybyłowicz 1999) and 58.8–161 Ma (altered uraninite, occurring on the edge of thucholite or cut by microfractures). These discrepancies in different radiometric ages obtained on uraninite, which shows signs of decomposition as reported by Kucha (2021), and release of Pb from uraninite during weathering suggest that the U–Pb dates reported by Kucha and Przybyłowicz (1999) and Kucha (2021) are not reliable. In addition, Kucha and Przybyłowicz (1999) suggested that thucholite from the Lubin mine was associated with noble metals and oxidation processes of the Upper Permian Kupferschiefer (T1) mineralisation, but it was paragenetically separate from main copper minerals. Finally, even though a plethora of methods have been used to decipher the origin of thucholite, none of them reliably explained the genesis of thucholite spheroids/concretions (the most common form), and some even considered thucholite to form fine layers and veins (Banaś et al. 2005). Therefore, to decipher this conundrum, we present here the main hypothesis that thucholite may be derived from charcoal, to which uranyl cations were adsorbed during sedimentation and diagenetic processes in the T1 shale.
Geological setting and sampling material
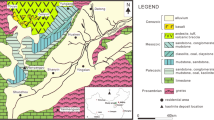
The Polkowice-Sieroszowice mine is located in the central part of the Fore-Sudetic Monocline that borders with the Fore-Sudetic Block (Fig. 1). The polymetallic Cu-Ag deposit in the Fore-Sudetic Monocline is closely related to three lithological levels (members) of the Upper Permian (Wuchiapingian) belonging to the upper part of the Rotliegend Group (Upper Rotliegend) and lower part of the Zechstein Group (PZ1 [Werra] cycle) (Fig. 2): Upper Rotliegend/Lower Zechstein Ws sandstone, Lower Zechstein T1 shale (dolomitic shale, bitumen-calcareous shale) and a series of the Ca1 carbonate strata (dolomite, limestone or mudstone) (Wagner 1994; Słowakiewicz et al. 2009; Oszczepalski et al. 2019). In the western part of the Sieroszowice I mining area, the Cu deposit includes only carbonate series and T1 shale (Fig. 1). There, barren shale in the T1 shale is often observed (Fig. 3). This is related to the proximity of the secondary oxidised zone, which passes in this region through shale and reaches carbonate strata. Shale samples with thucholite are from the area located on the boundary of the oxidised zone, the so-called “Rote Fäule” (Fig. 1). In the area of the Polkowice-Sieroszowice Cu-Ag deposit, oxidised zones occur irregularly, forming, in front of the main zone (Fig. 1), numerous small areas defined in the mining nomenclature as “stone areas”, which are associated with a decrease in the Cu sulphide content < 0.7% (Fig. 3A). In this part of the Cu deposit, the mineralisation with Cu sulphides includes only the T1 shale and the lower part of the Ca1 carbonate complex. The oxidised zone, changing from the Ws sandstone to a bed of the T1 shale, causes partial leaching of Cu sulphides originally found in this deposit, decreasing their content to < 0.5%. A typical geological profile for this zone is shown on the example of the Ra18-168 sample collected from the T1 shale with thucholite (Fig. 3B).
Thucholite sampling site in the mining areas exploited by KGHM Miedź S.A. (1) Głogów Głęboki-Przemysłowy deposit; (2) Polkowice-Sieroszowice mine; (3) Lubin mine; (4) Rudna mine; (5) Boundaries of perspective ore deposits; (6) Boundaries of developed ore deposits; (7) Approximate boundary and extent of the oxidised zone (Rote Fäule) in the T1 shale; (8) Towns: Gł – Głogów, Lu – Lubin, Po – Polkowice; (9) Mining shafts; (10) Thucholite sampling site; (11) Boundary of the Ws sandstone top; (12) Limit of the range of oxidised deposits (Rote Fäule) in the T1 shale; (13) Location and the number of samples collected; (14) Samples with barren shale at the Rote Fäule boundary. A) A close-up view of mine workings with the sampling site marked
(A) Comparison of the stratigraphic position of the Zechstein Cu-bearing series (modified after Oszczepalski 1999; Piestrzyński et al. 2002) with the stratigraphic position of the gold deposit in the Polkowice-Sieroszowice area. Red arrow indicates location of the thucholite-bearing shale. S.O.S. – secondary oxidised zone. a – typical distribution of haematite ‘red spots’ in the oxidised zone within the Ca1 carbonates; (B) Lithological profile with the Ra18-168 sample and vertical distribution of the Cu content (%) at the sampling point (presented in Fig. 3) in the T1 shale with thucholite. The blue dashed line indicates minimal Cu content at 0.7%, determining an economically viable Cu deposit. The red dashed lines indicate the thucholite layer
(A) Lithological profile at the sampling site of the T1 shale with thucholite. Ca1 – Zechstein Limestone, T1 – Kupferschiefer, Ws – Weissliegend sandstone, T1-3 – shale samples, TH1-3 – thucholite samples; (B) slope of the elevation of the Ws sandstone top with a silt-sand heterolith (ht) replacing the T1 shale, RF — red spots of the Rote Fäule oxidising facies in the Ws sandstone top, (C) slope of the elevation of the Ws sandstone top near its culmination with the T1 shale-free zone. Ca1 dolomite lies directly on the silt-laminated (dark laminae) Ws sandstone
The T1 shale with thucholite (Fig. 3A) is located near the elevated structure of the Ws sandstone top, which is thought to be the remnant of a coastal aeolian dune system (Jerzykiewicz et al. 1976; Błaszczyk 1981). This is evidenced by the disturbed sediments replacing the T1 shale on the slopes of the elevation and represents a mud-sandy heterolite with visible sedimentary structures in the form of ripple marks and sediment flow deformation (Fig. 3B). The T1 shale and heterolithic deposits gradually disappear upwards, which is shown by the direct contact of the Ca1 strata with the Ws laminated mudstone and sandstone (Fig. 3C).
Thucholite (TH-1 to -9) and T1 shale (T1-1 to -9) samples were collected between the SG-1 and SW-3 shafts (Fig. 1). The T1-1 sample was collected from the upper zone of the T1 section, a few centimetres above the intermediate layer where thucholite concretions occur, T1-2 from the intermediate layer with thucholite and T1-3 from the lower zone of the T1 section, a few centimetres below the layer with thucholite concretions (Fig. 3A). Samples T1-4 to -9 were collected from the zone directly at the layer (about 1 cm thick) in which thucholite concretions occur. Three thucholite samples (TH-1, -2, and -3) are scattered on the contact between the upper and lower T1 shale and are spaced about 7 m apart whereas the TH-4 to -9 samples are spaced about 0.5 m apart. The T1 lower zone was formed under more oxidising conditions than the upper zone. Thucholite samples were separated from the shale and homogenised by mortar grinding (except for petrographic analyses).
Methods
Mapping and energy-dispersive analysis of thucholite samples was carried out using an FE-SIGMA VP (Carl Zeiss Microscopy GmbH) with an energy-dispersive X-ray spectroscopy (EDS) detector (Quantax XFlash 6|10, Bruker Nano GmbH). For the EDS analysis, small fragments of polished slabs were placed on an aluminium mount with carbon conductive tape. Then, samples were coated with a 20-nm layer of carbon using a vacuum coater (Quorum 150 T ES). Next, carbon tape bridges were prepared for each sample to avoid the excessive accumulation of charge. The procedure of coating and mounting in scanning electron microscope (SEM) was the same as for the EDS analysis. Measurements were carried out with a 120 μm aperture and a 20 kV acceleration voltage with a working distance of 7.8 mm.
The chemical composition of thucholite samples was obtained by means of electron microprobe analysis (EMPA) at the Faculty of Geology, University of Warsaw. Chemical analyses were carried out by a Cameca SXFiveFE equipped with five wavelength dispersive spectrometers (WDS). The operating conditions of the electron microprobe were: 15 kV accelerating voltage, 10 nA probe current, and 2–5 µm beam diameter. For point analyses, the following crystals and X-ray lines (in brackets) were used: thallium acid phthalate crystal (Na Kα, Si Kα, Sr Lα, P Kα, Y Lα, As Lα, Hf Mα), large thallium acid phthalate crystal (Yb Mα, Lu Mα), large pentanerythritol crystal (LPET) (Cl Kα, K Kα, Ca Kα, La Lα, Ce Lα, Pr Lα, Pb Mα, Th Mα, U Mα, S Kα), large lithium fluoride crystal (Fe Kα, Nd Lβ, Sm Lα, Gd Lα, V Kα, Ti Kα, Ba Lα, Dy Lβ, Er Lα, Cu Kα) and large pseudo-crystal (F Kα). The natural and synthetic standards used in the analysis were as follows: albite (Na), SiO2 (Si), tugtupite (Cl), orthoclase (K), apatite (Ca, F), hematite (Fe), SrSO4 (S, Sr), LaPO4 (La), CePO4 (Ce, P), PrPO4 (Pr), NdPO4 (Nd), SmPO4 (Sm), GdPO4 (Gd), DyPO4 (Dy), ErPO4 (Er), YbPO4 (Yb), LuPO4 (Lu), metallic Hf (Hf), cuprite (Cu), crocoite (Pb), ThO2 (Th), UO2 (U), YPO4 (Y), GeAs (As), metallic V (V), TiO2 (Ti) and BaSO4 (Ba). The Φ(ρZ) correction model (X-PHI in the electron microprobe software) developed by Merlet (1994) was used to quantify the chemical composition of the investigated objects. For qualitative mapping of the uranium (U Mα) distribution in thucholite, LPET analysing crystal was used. The operating conditions of the microprobe were: 15 kV accelerating voltage, 50 nA probe current, 3 µm step size, and 0.12 s dwell time. The water content was calculated using a given mineral’s theoretical formula.
The diffraction analyses were conducted with a Malvern Panalytical X’Pert PRO MPD powder diffractometer using the Bragg–Brentano method. The analysis was carried out with a Co Kα lamp at 40 kV and 40 mA in the range of 5.0131–77.9691 2Θ with a step of 0.0260 2Θ. Phase identification was obtained with the use of X’Pert Plus HighScore software with access to the Crystallography Open Database.
Inductively coupled plasma-optical emission spectrometry (ICP-OES) and mass spectrometry (ICP-MS) were used on three shale (T1-1 to -3) and two thucholite (TH-1 and -2) samples. All samples were weighted into PTFE beakers and decomposed in a high-pressure microwave (MW) digestion system, Ethos (Milestone). Decomposition was performed in a stepwise manner. First step: 8.5 mL of 65% HNO3, conditions: MW power 200 W for 20 min, second step: 1 mL of 30% H2O2 and 1 mL of 40% HF were added to the residuum, conditions: MW power 300 W for 20 min. The acid mixture was then evaporated to the state of wet solid, 2.5 mL of 65% HClO4 was added, and evaporation was repeated. The final temperature of the heating block reached 280 °C. The wet solid residue was dissolved in 10 mL of 10% aqueous HCl and diluted to a final volume of 50 mL. Transition metals (Co, Cu, Fe, Ni, Ti, V), metalloids (As, Se), sulphur, and lead were quantified by an ICP-OES (Agilent 5100) due to high concentration levels of these elements in the samples. Solutions were introduced via a concentric pneumatic nebuliser, and an axial setting of intensity collection was applied. The instrument was calibrated by multi-elemental standard solution in the range of 0–20 ppm. Mixed standards were freshly prepared from single element 1000 ppm solutions supplied by Analytika Inc. (Prague). At least two wavelengths free of interferences were used to collect signals of each element, and the concentration was presented as an average value. Non-transition elements were determined separately from the transition metals. In the case of Pb, only the 220.353 nm wavelength was employed. The quality control procedure consisted of a multi-element standard solution (Agilent) applied after every five unknown runs. The standard deviation for ICP-OES measurements is given in online supplementary material (Table S1). Trace and ultra-trace elements were determined by a single-collector sector-field ICP-MS (Thermo Scientific Element 2) starting from the same solutions as for the ICP-OES analyses. The conditions were set up according to the manufacturer’s recommendation (double-pass spray chamber, perfluoroalkoxy [PFA] concentric nebuliser with a flow rate of 50 µL/s, Ni cones, a 1200 W radio frequency power, sampling time 1 min), and the instrument was tuned to achieve the highest sensitivity concurrent with acceptable low oxide formation. Before being introduced into the plasma, the samples were first in-line and admixed with an indium internal standard solution for the correction of the instrumental drift. The system was calibrated using a multi-element calibration solution from commercially available single-element standards (CPAchem) and multi-element standards for REE (CertiPUR) using a blank and 4 calibration points up to 10 ppb of each element. REE, U, Th, Cd, Te, Nb, Mo, Ta, W, and Bi were quantified in a low-resolution setting (LR), while Zn and P were in middle resolution (MR). The standard deviation for ICP-MS measurements is given in online supplementary material (Table S2). The REE + Y contents were normalised to the post-Archaean Australian Shale (PAAS) (Taylor and McLennan 1985). Cerium anomaly (Ce/Ce* = 3CeSN/(2LaSN + NdSN), Pr anomaly (Pr/Pr* = 2PrSN/(CeSN + NdSN) (where ‘SN’ indicates shale normalised) were calculated according to Shields and Stille (2001).
Total organic carbon (Ctot), total nitrogen (Ntot), total hydrogen (Htot) and total sulphur (Stot) contents (%) were measured in three shale (T1-1 to -3) and two thucholite (TH-1 and -2) samples (4 – 6 mg weight) in two duplicates using a Vario CUBE elemental analyser. Sulphanilic acid (NH2C6H4SO3H) was used as a standard. The data are given on a dry mass basis. The analytical errors for Ctot, Ntot, Htot and Stot was 0.6%, 0.18%, 0.21% and 0.43%, respectively.
Six samples (3 from the T1 shale and 3 with thucholite) were powdered to a grain size < 0.2 mm. The mass of 50–100 mg of the sample was placed in a crucible for analysis. The screening pyrolysis was completed with a Vinci Technologies Rock–Eval 6 Turbo apparatus in the Bulk Rock method using the Basic cycle. The quantity of residual TOC fraction (RC, wt%) was assessed from measured by IR amounts of combustion products, e.g., CO and CO2, recorded as S4CO and S4CO2. Standard Rock–Eval parameters such as the production index (PI = S1/(S1 + S2)), hydrogen index (HI = 100 × S2/TOC [mg HC/g TOC], oxygen index (OI = 100 × S3/TOC [mg CO2/g TOC]) and mineral carbon content (MinC, wt%) were calculated from thermogram peaks area using the equations defined by Lafargue et al. (1998).
The Raman measurements were performed using a Renishaw inVia Reflex Raman microscope equipped with an EMCCD detector (Andor Technology Ltd, Oxford Instruments). The Raman signal was acquired using laser radiation with a wavelength of 532 nm. The laser beam was directed to the sample through a 5 × (N.A. = 0.12) objective lens (Leica). The measurement parameters were as follows: acquisition time 3 s and 10 accumulations at each point. The wavelength of the instrument was calibrated using an internal silicon wafer, and the spectrum was centred at 520.5 cm−1. The Raman spectra were acquired from three different locations at sample and a minimum of 100 points in each location. Acquired spectra were processed in WiRE 5.3 software. The spectra were averaged using CasaXPS software.
Geochemical analysis was performed on three shale (T1-1, T1-2, T1-3) and three thucholite (TH-1, TH-2, TH-3) samples. The powdered samples (ca 0.5 to 1 g of thucholite or 10 g of T1 shale) were extracted using an accelerated Dionex ASE 350 solvent extractor with dichloromethane:methanol (DCM:MeOH 1:1, vol./vol.). Prior to the fractionation procedure, the solvent was evaporated at room temperature. The extracts were separated into aliphatic, aromatic, and polar fractions on silica gel (activated at 120 °C for 12 h) using a sequence of organic solvents of different polarity (n-pentane, n-pentane:DCM, 4:1, vol./vol. and DCM:MeOH 1:1, vol./vol.), respectively.
The distribution of compounds containing aliphatic and aromatic fractions was determined with gas chromatography-mass spectrometry (GC–MS) using an Agilent Technologies 7890A gas chromatograph and an Agilent 5975C Network mass spectrometer with Triple-Axis Detector (MSD) and an autosampler at the Faculty of Earth Sciences, Sosnowiec, Poland. Helium (purity > 99.999%) was used as the carrier gas, with a flow rate of 0.5 mL/min. The GC and MS parameters were as follows: separation was obtained with a J&W HP5-MS (60 m × 0.32 mm i.d., 0.25 mm film thickness) fused silica capillary column coated with a chemically bonded phase (5% phenyl, 95% methylsiloxane), for which the GC oven temperature was programmed from 45 °C (1 min) to 100 °C at 20 °C/min, then to 300 °C (hold 60 min) at 3 °C/min, with a solvent delay of 10 min. Mass spectra were recorded from m/z 45–550 (0–40 min) and m/z 50–700 (> 40 min). The ionisation energy was 70 eV. Peak ratio calculations for all compounds were done from the integrated area:area.
Petrological investigations were performed on six representative polished sections, using a Carl Zeiss Axioimager A1m microscope equipped with a PMT photometer, HBO lamp, and 50 × oil immersion objective. Samples were prepared according to the procedure proposed by ASTM D2797 (ASTM 2015). The identification of organic components was carried out under reflected white light (vitrinite and inertinite macerals) and blue light (liptinite macerals). The dispersed organic matter found in the analysed samples was classified using the terminology recommended by the International Committee for Coal and Organic Petrology (ICCP 1998, 2001) and Pickel et al. (2017). The measurements of mean organic matter reflectance were carried out on vitrinite (Ro), solid bitumen (BRo%), fusinite (Rf %) and semifusinite (Rsf %) particles in accordance with the ASTM D 7708–11 (ASTM 2011) guidelines. The photometer was calibrated by the following standards: spinel (R = 0.429%), yttrium–aluminium garnet (R = 0.905%) and gadoliunium-gallium garnet (R = 1.728%). The BRo% values were recalculated to the equivalent vitrinite reflectance values (EqVRo) using formula proposed by Jacob (1989) (EqVRo = 0.618*BRo + 0.4). The maximum palaeotemperatures based on vitrinite reflectance were estimated using Barker and Pawlewicz's (1994) formula (PT = [lnRo + 1.68]/0.0124). Temperatures of palaeo-wildfires using fusinite and semifusinite were calculated after Jones and Lim's (2000) formula (T = 184 + 118*Rf). The quantitative analysis of the organic matter components was based on the point-counting of 300 grains per polished section as recommended by Taylor et al. (1998).
Results
Thucholite distribution
Thucholite was observed in the layer of the T1 dolomitic shale, ~ 35 cm thick (Fig. 3A). The T1 shale shows a distinct duality associated with the change of geochemical zones from oxidised to reduced. The layer with thucholite is located at a depth of 696 m and forms one layer 2–3 m wide and 10 m long. This level is ~ 15 cm above the Ws sandstone top in the lower part of the reducing zone (Fig. 3A). It is mineralised with Cu sulphides up to 10 vol% (Fig. 2), and the TOC content is < 11 wt%. The visible accumulation of thucholite in this layer is in the form of scattered spherical or granular clusters (online supplementary material Fig. S1). Thucholite concretions are < 4 mm in size (online supplementary material Fig. S1A), and in their vicinity, numerous fine thucholite aggregates with a diameter of < 1 mm are visible (online supplementary material Fig. S1B). In the bottom part of the T1 shale, in the layer below the thucholite level (~ 15 cm) (Fig. 3A), the presence of red laminae indicates that Fe-rich oxidising solutions passed through. This is confirmed by the decrease in the content of Cu sulphides to 0.3 vol% (Fig. 2).
T1 shale distribution
Remains of Late Permian terrestrial flora and microflora are observed in the T1 shale. The macroflora is represented by the remains of biostructures in the form of shrub root casts or fragments of branches, cones and needles of conifers, in many cases with traces of charred material (Błaszczyk 1984; Diedrich 2009). The presence of charred remains of conifers found in Zechstein deposits in the German Basin (NW-Hesse) is a proof of frequent fires having occurred during this period (Uhl and Kerp 2003; Diedrich 2009). This type of biogenic structures is observed in the middle part of the T1 shale and their dimensions do not exceed 70 cm. The microflora described from occurrences in the T1 shale is mainly represented by myospores (Dybova-Jachowicz 2001; Gibson and Wellman 2021). The most common pollen grains in the T1 shale belong to conifers of the genus Lueckisporites virkkiae (Potonié et Klaus) Jansonius (Dybova-Jachowicz 2001) or Majonicaceae, e.g. Majonica alpina/Pseudovoltzia liebeana (Gibson and Wellman 2021). Pollen of L. virkkiae in the T1 shale was found in the Rudna Północna and Lubin Główny mines located in the Fore-Sudetic Monocline (Fijałkowska-Mader 2012).
Microscopic observations
Mineral matter
The SEM results indicate the presence of several mineral phases in thucholite. The EDS confirmed the occurrence of a uraninite-like phase (Fig. 4A) and an unknown U-Si-P-Ca-Y phase (Fig. 4B). The uraninite crystals occur as small lamellar grains in a carbon matrix, which are nanometre in size (Fig. 4A). The unknown U-Si-P-Ca-Y phase has needle-like crystals, a few μm in diameter (Fig. 4B). In addition, secondary U minerals were observed in cracks and voids in thucholite concretions, such as uranyl carbonates, arsenates, silicates, and sulphates. The uranyl arsenates are represented by the phase whose chemical composition was similar to nováčekite (Fig. 4C). This phase forms lamellar spherical clusters of a few μm in diameter (Fig. 4C). A copper uranyl silicate was also found and probably represents cuprosklodowskite (Fig. 4D). It occurs as needle-shaped aggregates (Fig. 4D). The EDS analyses of uranyl sulphates also helped to identify natrozippeite (Fig. 4E) of lamellar habit (Fig. 4E). The uranyl carbonates are represented by the phase whose chemical composition is similar to liebigite (Fig. 4F), which forms large lamellar crystals (Fig. 4F).
SEM images of a thucholite sample (TH-1). (A) uraninite grains in thucholite; (B) an unknown U-Si-P-Ca-Y phase in thucholite; (C) cuprosklodowskite-like phase; (D) natrozippeite-like phase; (E) liebigite-like phase; (F) nováčekite-like phase. Yellow dots indicate EDS analysis and EDS spectra are presented on the right panel
The WDS and EDS uranium mapping (online supplementary material Fig. S2) reveals high spatial concentrations of thucholite concretions but no U enrichment in the thucholite-bearing shale.
Organic matter
The maceral composition of the analysed samples was counted separately for the T1 shale and TH concretions and is presented in Table 1.
Solid bitumen (Fig. 5A, B, D) dominates in organic matter dispersed in the T1 shale and occupies 29 to 36.7 vol%. The vitrinite macerals occur in small amount (0.5–2.8 vol%). Liptinite maceral group is represented by alginate (Fig. 5C), liptodetrinite, sporinite and trace amounts of exsudatinite with ∑L (sum of liptinite macerals) of trace to 13.0 vol%. Inertinite macerals are represented mainly by semifusinite, liptodetrinite and fusinite. In three samples (T1-4 – T1-6), hydrocarbon evidence was observed. The analysed T1 shale is rich in organic matter, varying from 34.2 to 47.6 vol%.
Photomicrographs of organic matter dispersed in the T1 shale and thucholite concretions. Explanation: reflected white light, sample T1-4 (B); sample TH-3 (D); thucholite concretion, sample TH-7 (G); sample TH-8 (A, H) which allows for detailed investigations of the organic particles observed in them; sample TH-9 (I); fluorescent light; sample T1-5 (C); sample TH-9 (E, F). All photomicrographs were taken with an oil immersion objective 50 ×
The TH concretions (Fig. 5D, H) were observed on polished surfaces in five out of six analysed samples (TH-5 – TH-9). The concretions were most abundant in TH-7, TH-8 and TH-9 and their diameters varied between 1 and 3 mm. The maceral composition of concretions was very homogenous in each sample. Thucholite is composed mainly of an organic mineral matrix (50 vol%) and a smaller amount of highly reflective semifusinite (21 vol%), (Fig. 5G, I), macrinite (22 vol%), (Fig. 5G, I) and inertodetrinite (5 vol%). The organic component in the mix of organic-mineral matrix is probably collodetrinite (Fig. 5). The mineral matter occurs as a mixed mineral-organic groundmass and in 2 vol% as individual particles, but also as uraninite (Fig. 5E, F).
Semifusinite is formed in the peat-forming stages by weak humification, dehydration, and redox processes as well as during wildfires, whereas macrinite probably originates from a flocculated humic matrix that underwent dehydration and redox processes (ICCP 2001).
The reflectance measurements of organic matter were carried out for the T1 shale (Fig. 5A, Table 2) and separately for thucholite concretions (Fig. 5B, Table 2). For the T1 shale, reflectance measurements were carried out on solid bitumen and fusinite particles (Table 2). The BRo% values in the analysed T1 samples varied from 0.83 to 1.02% with low standard deviations for these measurements (Fig. 6A, Table 2). The EqVRo values obtained from Jacob’s (1989) formula varied from 0.91 to 1.03% (Table 2). Based on the EqVRo results, the maximum palaeotemperature affecting the analysed T1 shale was calculated using the formula proposed by Barker and Pawlewicz (1994) (Table 2). This geothermometer can be successfully applied if the thermal history of the studied samples is poorly known and organic matter had sufficient time to reach its stabilisation. Therefore, vitrinite or EqVRo measurements can be transformed into maximum palaeotemperature. Additionally, fusinite reflectance (Rf %) was measured in the analysed T1 shale (Table 2; Fig. 6A). Based on the reflectance values, three populations of fusinite were found with Rf (%) varying from 2.23 to 2.27%; 2.90 to 3.38%, and 4.31 to 4.92% for each population, respectively. The palaeo-wildfire temperatures based on the fusinite reflectance values were estimated using Jones and Lim's (2000) formula of TRf (Table 2) and range from 447 to 696 °C.
Histograms of reflectance values. A – T1 shale samples; B – thucholite concretions in the T1 shale samples. SB – solid bitumen reflectance measurements; BRo% – mean solid bitumen reflectance values; V – vitrinite reflectance measurements; Ro% – mean vitrinite reflectance values F1, F2, F3 – reflectance measurements of fusinite populations; Sf—reflectance measurements of semifusinite; Rf% – mean fusinite reflectance values; RSf% – mean semifusinite reflectance values; n – number of measured particles; std. – standard deviation of measured reflectance values; min., max. – minimum and maximum reflectance values
Reflectance of thucholite concretions was measured on collodetrinite (vitrinite) and is presented as mean Ro values and on high-reflecting semifusinite (Rsf) (Fig. 6B, Table 3). The reflectance values of organic matter components in the TH samples range from 1.50 to 1.62% and 1.86 to 2.06% for vitrinite and semifusinite, respectively. The Ro values are highest in the centre of the TH concretions (Ro ~ 1.70%) and decrease towards the T1 shale boundary (Ro ~ 1.30%). The results of the reflectance measurements show higher values in the TH concretions than in the T1 shale. Maximum palaeotemperatures (PT °C) calculated from Barker and Pawlewicz's (1994) equation range from 168 to 174 °C. The palaeo-wildfire temperatures were estimated by Jones and Lim’s (2000) formula. As was concluded by Zakrzewski et al. (2022), the origin of high-reflecting semifusinite may be related to wildfires, therefore this component was used as an input parameter for the temperature estimation (Table 3). The temperature values of the calculated palaeo-wildfires vary from 403 to 427 °C.
Trace element contents
REE-Y abundances in the T1 shale and thucholite samples are presented in online supplementary material (Tables S3 and S4). Total REE contents vary from 139 (arithmetic mean; n = 3) to 1367 mg/kg (arithmetic mean; n = 2) in the T1 shale and thucholite samples, respectively, with the highest value in an unknown U-Si-P-Ca-U phase (15,889 mg/kg). The Ce/Ce* and Pr/Pr* values are 0.85 and 0.51, and 1.07 and 0.97 for the T1 shale and thucholite samples, respectively. The T1 shale and thucholite samples show a different REE + Y pattern (Fig. 7). The former has positive Y anomalies, whereas the latter has positive Eu and negative Gd anomalies.
Representative PAAS-normalised (Taylor and McLennan 1985) REE + Y patterns of average thucholite (TH) and T1 shale (T1)
The thucholite is depleted in MREE and LREE and enriched in HREE, whereas the T1 shale is depleted in HREE and MREE and enriched in LREE (online supplementary material Table S4). An unknown U-Si-P-Ca-Y phase is characterised by a high content of HREE and depletion in LREE. The Th/La and Nb/La ratios are between 0.22–0.25 and 0.29–0.32 for the T1 shale and 0.20–0.21 and 0.23–0.25 for thucholite, respectively.
Trace and major element contents are listed in online supplementary material (Tables S5 and S6). Both thucholite and T1 shale contain significant amounts of Mo, Zn, Cu, S, P, Ti, Fe, Ni, Pb, Se, and U. Thucholite has a higher content of REE, Y, U, Pb, Bi, Co, As, W, and P than the T1 shale, which in turn contains greater amounts of Nb, Se, La, Te, Ti, S, Cu, and V.
XRD
The XRD and semiquantitative XRD results showed that the thucholite is highly amorphous. The diffractogram's high background results from increased natural radiation from thucholite concretions due to the high U content therein. However, the crystallite phase of thucholite mainly consists of graphite (~ 65%), illite (~ 20%), uraninite (~ 10%), quartz (~ 3%), and fergusonite (~ 2%) (online supplementary material Fig. S3).
Carbon, nitrogen, hydrogen and sulphur contents
The Ctot, Ntot, Htot, and Stot contents in the T1 shale (T1 – 3) and thucholite (TH-1 and -2) samples vary from 11.2–11.3%, 0.25–0.27%, 0.33–0.44%, 0.9–1.1% for the T1 samples respectively and 70.1–72.3%, 0.39–0.43%, 2.9–3%, 1.57–1.6% for thucholite samples respectively (Table 4). The C/N ratios differ from 41.1–45.3 for the T1 shale and 162.2–184.3 for thucholite samples, respectively (online supplementary material Table S7). The H/C ratios in the T1 shale and thucholite samples are similar whereas the C/S ratios vary from 26.3 to 32.5 and 119.3 to 120.7 in the T1 shale and thucholite samples, respectively (online supplementary material Table S7).
Rock–Eval pyrolysis
The pyrolysis results for three T1 shale and three thucholite samples are presented in Table 4. The obtained values of parameters and indicators for each of the analysed samples show some differences. Thucholite is very rich in organic matter with mean TOC (sum of pyrolysable carbon-PC and residual carbon-RC) values of 57.8 wt%, whereas the mean TOC content in the T1 shale is as low as 4.0 wt%. The TOC richness in the TH samples is related to high RC (mean 54.0 wt%) with a mean value of 3.8 wt% PC (amount of TOC represented by S1 and S2). For the T1 shale samples, the PC and RC content is 0.17 and 3.8 wt%, respectively. The mean total content of hydrocarbons generated from the analysed samples (S1 and S2 peaks) is 0.22 and 0.58 mg HC/g rock for the T1 shale and 3.4 and 27.8 mg HC/g rock for the thucholite samples. The hydrogen index (HI) values for the T1 shale are lower (mean 15 mg HC/g TOC) than in the thucholite samples (mean 48 mg HC/g TOC) possibly due to a higher concentration of organic matter in thucholite concretions whereas the oxygen index is higher in the T1 shale (mean 65 mg CO2/g TOC) compared to the thucholite samples (mean 20 mg CO2/g TOC). Plotting the hydrogen index vs. Tmax temperature indicates that organic matter is represented by kerogen type III in the thucholite and T1 shale samples (Fig. 8). However, the late oil generation phase could preclude the unequivocal determination of the genetic type of kerogen (see e.g., Hunt 1995).
Hydrogen index vs. Tmax temperature. Maturation paths for kerogens after Espitalié et al. (1985). TH – thucholite samples; T1 – shale samples; %Ro – vitrinite reflectance
Organic compounds extracted from shale and thucholite samples
Aliphatic hydrocarbons
The molecular composition of thucholite varies slightly from that of the surrounding T1 shale. In the T1 shale extracts (samples T1 to T3), the aliphatic fraction is dominated by short-chain n-alkanes in the range between n-C16 and n-C18. In all shale samples and one thucholite sample, even-carbon short-chain n-alkanes predominate. This is reflected in the lower than 1 CPI(TOTAL) values (online supplementary material Table S8). Pristane/phytane ratios (Pr/Ph) are similar for the T1-T3 samples and range from 0.4 to 0.6 (online supplementary material Table S8). In the thucholite samples, long-chain n-alkanes predominate (online supplementary material Fig. S4), and in the TH-2 sample, n-alkanes show a bimodal distribution, with long-chain homologues predominating. In contrast, the Pr/Ph ratios are similar to those measured in the T1 shale samples, although, in the TH-1 sample, these isoprenoids are below the detection limit. Between the cyclic biomarkers, hopanes and small concentrations of steranes are present in samples of thucholite and the surrounding T1 shale (see hopane/sterane ratios in Table S8). The distribution of hopanes is characterised by the dominance of C29αβ and/or C30αβ hopanes (online supplementary material Fig. S5). Homohopanes are present in small amounts, with C31αβ epimers predominating. Equilibrium was reached for the C31 22S/(S + R) ratio in all samples (online supplementary material Table S8). No significant differences were noted between hopane distribution in thucholite and shale (online supplementary material Fig. S5), which is reflected by similar values for typical parameters calculated based on the hopane distribution (online supplementary material Table S8). Steranes are not abundant. They were identified in samples T-1, T-2 and TH-3. In other samples they are below the detection limit. The distribution of steranes is C27 > C29 > C28, with similar abundance of C27 and C29 clearly dominating over C28. The calculated values of sterane parameters are also similar between thucholite and surrounding shales (online supplementary material Table S8).
Polycyclic aromatic compounds
Polycyclic aromatic compounds (PAC) are present in large quantities in both thucholite and the surrounding T1 shale. Their composition is complex and diverse. Phenanthrene and dibenzothiophene predominate in all the T1 shale and TH-1 and TH-3 samples, whereas in TH-2, the predominant compounds are benzo[b]naphtho[2,1-d]thiophene, chrysene and triphenylene (online supplementary material Fig. S6). Other organic compounds abundant in all samples include biphenyl, dibenzofuran, and four- and five-ring polycyclic aromatic hydrocarbons (PAHs) such as fluoranthene, pyrene, chrysene, triphenylene, benz[a]anthracene, benzofluoranthenes, benzo[e]pyrene and benzo[a]pyrene (online supplementary material Fig. S6). Methyl derivatives are also present in the analysed samples, although in lower amounts than unsubstituted compounds. Methylnaphthalenes, which are common in the T1 shale, are found in small amounts here. In contrast, aromatic sulphur compounds such as benzothiophenes, dibenzothiophenes, benzonaphthothiophenes, dinaphthothiophenes, and benzobisbenzothiophenes are common (Rospondek et al. 1994). The thucholite samples (TH-1 and -2) are depleted in two and three-ring PAHs (online supplementary material Fig. S6). Only TH-3 contains phenanthrene and dibenzothiophene as important but not dominant compounds. In addition, phenyl derivatives of aromatic compounds are present in all samples, generally with intensities matching those of the main molecules. Phenylnaphthalenes (one of the main groups of compounds in the fraction), terphenyls, phenyldibenzofurans, phenyldibenzothiophenes (Marynowski et al. 2002; Rospondek et al. 2007, 2008), phenylphenanthrenes (Rospondek et al. 2009), and diphenylnaphthalenes have been identified. In addition, phenyl derivatives of fluoranthene and pyrene and M+228 compounds (mainly chrysene and triphenylene) were tentatively identified. Methyl derivatives of phenylated aromatic compounds are also present but in much lower amount.
Raman spectroscopy
The results of the Raman measurements are presented in online supplementary material (Figure S7 and Table S9). The position of the disordered (D) band and graphite (G) band peaks represented the first-order region of organic matter and their Raman spectra are 1347 and 1598 cm−1, respectively. Based on D (1347 cm−1) and G (1598 cm−1) bands, Raman parameters such as Raman band separation RBS = 251 cm−1 (G-D); full width at half maximum (FWHM) of the D and G bands such as D-FWHM = 225 cm−1 and G-FWHM = 91 cm−1; ratio of Raman band height (R1-0.67) and the ratio of Raman band areas (R2 = 0.56) were calculated.
Discussion
Chemical composition of thucholite concretions
Our microscopic and chemical analyses reveal noticeable differences in the mineral and chemical composition of thucholite and T1 shale. Thucholite is significantly enriched in U, which can reach 88 wt.% in uraninite (Piestrzyński 1990). According to Kucha and Pawlikowski (1986) and Piestrzyński (1990), the major source of U could be Upper Permian Rotliegend sandstone or Zechstein rock salt, from which U and other metals may have been remobilised. Alternatively, it is suggested here that charred organic particles adsorbed significant amounts of U from the seawater in T1 time. A similar process takes place during the well-known adsorption of metals on activated carbon and carbonised porous materials, which is widely used in water treatment technologies. However, the occurrence of U minerals in thucholite such as uraninite, brannerite, masuyite (Kucha and Przybyłowicz 1999), liebigite, natrozippeite, cuprosklodovskite, and nováčekite, can be interpreted as secondary minerals formed during the oxidation of thucholite and the T1 shale in the Polkowice-Sieroszowice mine. The oxidation of uraninite grains has also an implication on the U–Pb dating of uraninite due to possible migration of Pb during this process (Janeczek and Ewing 1995; Alexandre and Kyser 2005; Martz et al. 2019). Moreover, metamictisation of the uraninite structure caused by U decay may also influence the Pb content in uraninite grains (Bowles 2015).
A non-identified U-Si-P-Ca-Y phase is a naturally occurring phosphosilicate that contains Ca, lanthanoides, and Y, OH− and F− (e.g., Kaisbeek et al. 1990; Oberti et al. 2001; Kamel et al. 2007), and can be derived from apatite (Ardhaoui et al. 2006; Lan et al. 2021). The crystallisation of britholite group minerals is commonly associated with late-magmatic crystallisation or the alteration of primary minerals in alkali-rich igneous rocks. Given that thucholite contains significant contents of Au, Pd and Pt adsorbed by organic matter aggregated in thucholite due to catalytic autooxidation at the oxic-anoxic boundary or catalytic autooxidation by radiation (Kucha 1982), it is very plausible that the presence of this not identified U-Si-P-Ca-Y phase, which is an important REE-bearing mineral in thucholite concretions, can be explained by similar processes.
The PAAS-normalised REE patterns for the T1 shale (Fig. 7) are typical of mudstone in the oxidised zone in the Polkowice-Sieroszowice mine (Sawlowicz 2013; Oszczepalski et al. 2016). The REE-Y pattern and REE contents of thucholite significantly differ from those of the T1 samples. This would suggest different mineralisation histories in both cases. Such a possibility is also indicated by a different chemical composition of the T1 shale and thucholite samples.
The XRD analysis revealed the presence of graphite among other minerals inside the thucholite concretions which is similar to graphite reported by Kucha and Przybyłowicz (1999). Of note is that our XRD measurements were semi-quantitative, therefore the exact percentages of the obtained mineral phases may be disturbed by the poor signal to noise ratios caused by the presence of amorphous organic and inorganic phases. In addition, such a poor ratio may be caused by high radiation levels.
The high C/N ratio in thucholite is characteristic of charred particles of wood because charcoals have C/N ratios ranging from 56 to 571 (Gaskin et al. 2008; Lehmann et al. 2011). A much lower C/N ratio measured in the T1 shale could indicate an input of terrigenous organic matter (Bechtel and Püttmann 1997) dominated, e.g., by conifers (Meyers and Ishiwatari 1995). Nitrogen contents of the T1 shale (2500–2700 ppm) are typical of Phanerozoic shales (100 to 2800 ppm; Bottrell and Miller 1990; Compton et al. 1992), whereas in the thucholite samples N contents are much higher (~ 4000 ppm; online supplementary material Table S7), likely suggesting coal-derived material (Nelson et al. 1992). The similar H/C ratios in all studied samples imply organic matter with comparable H/C whereas the C/S ratios which differ significantly between the T1 shale and thucholite samples suggest that in both cases they can be characteristic of non-marine coals (Berner 1984) but deposited under varied anoxic conditions.
Organic matter in the T1 shale and thucholite
The organic matter in the T1 shale has been described as being of marine type, with some terrestrial components (Sawlowicz et al. 2000). This notion is also supported by our study as organic matter in the T1 shale is present mostly as solid bitumen and alginite (Table 1). However, the bottom part of the T1 shale, at the boundary with the underlying Ws sandstone (Fig. 3), was in many places influenced by migrating fluids (Püttmann et al. 1989; Sun et al. 1995; Sun 1998). This resulted in the partial oxidation of the kerogen and a significant reduction of the HI values (Fig. 8) and, consequently, a transition from type II kerogen to type III (Więcław et al. 2007). Low-temperature (100–130 °C) oxidising solutions migrating from Rotliegend sandstones were carriers of precious metals (Au, Pt, Pd) precipitating on the boundary of the reduced and oxidised zones (Bechtel et al. 1995, 2002).
The molecular composition of the thucholite samples and the surrounding T1 shale is, in general, different. In both cases, the distribution of hopanes with a predominance of C29αβ and C30αβ hopanes and PAC with a high abundance of phenyl-PAC derivatives is quite close. This interaction resulted in numerous phenylated PACs derivatives forming via a free-radical phenylation process (Marynowski et al. 2002; Rospondek et al. 2009, 2008, 2007; Grafka et al. 2015).
The first difference observed in the analysed samples is the predominance of long-chain n-alkanes in thucholite and a bimodal distribution in the T1 shale, with a majority of even-carbon short-chain n-alkanes (online supplementary material Fig. S4). A similar distribution of n-alkanes for ore-bearing shales with a majority of short-chain n-alkanes was also described by Sawlowicz et al. (2000) and Bechtel et al. (2001), indicating a predominance of marine algal-type organic matter. Long-chain n-alkanes are more typical of higher plants (Eglinton and Hamilton 1967) and could potentially occur as relics of burned wood. In addition, the predominance of even-carbon n-alkanes in all analysed T1 samples and one thucholite sample may indicate reducing depositional conditions (Welte and Ebhardt 1968; Kostova et al. 2022). It is manifested by low (< 1) CPI values (online supplementary material Table S7). Although T1 deposits were formed under reducing conditions (e.g., Grice et al. 1996), excessive thermal maturity precludes the determination of more reliable euxinic indicators (such as isorenieratane and its derivatives). The Pr/Ph ratios for T1-T3 shales (0.4 to 0.6) are lower than those reported by Püttmann et al. (1989), Sawlowicz et al. (2000) and Bechtel et al. (2001), indicating some heterogeneity in depositional conditions within the T1 shale and more reducing environments associated with thucholite. An even more intriguing difference in composition concerns PAHs. Although phenyl (Ph) derivatives are present in both shale and thucholite, their abundances in relation to PAHs are different. Thucholite is characterised by slightly higher abundances of PAHs vs. Ph-PAHs relative to shale. However, the differences between the T1 shale and thucholite are not large but are noticeable (Fig. 9, Table 5). Furthermore, PAHs typical of pyrolytic processes, such as benz[a]anthracene and benzo[a]pyrene (Killops and Massoud 1992; Finkelstein et al. 2005), are present in thucholite (Fig. 9). In contrast, they are absent, or present only in trace amounts, in the surrounding T1 shale. These compounds were likely absorbed in the charcoal during incomplete combustion (e.g., Marynowski et al. 2014). However, high-mass pyrolytic PAHs (such as coronene) were not found in the thucholite and T1 shale samples, confirming low-temperature charcoal-forming fires (Kaiho et al. 2021).
Irradiation in thucholite can influence the extractable organic matter composition in our samples. This could be manifested by the removal of short-chain n-alkanes, or a change in the Pr/Ph ratio. It is known that n-alkanes and isoprenoids are the most susceptible to irradiation influence (Dahl et al. 1988; Schulz et al. 2019), and undergo aromatisation to simple aromatic compounds (Court et al. 2006). Also, two- and three-ring PAHs were degraded in thucholite (Fig. S6). The much less susceptible high-molecular-weight PAHs (Dahl et al. 1988) do not show large changes in their distribution.
In summary, thucholite differs from the T1 shale by specific details such as the different distribution of n-alkanes and the presence of typically pyrolytic organic compounds. Some of these differences are due to radiation effects in U-enriched thucholite, and some (such as the presence of specific PAHs) are due to the derivation of the thucholite from charcoal fragments.
Thucholite formation temperature
Thermal maturity of thucholite
The vitrinite reflectance of thucholite concretions ranges from 1.50 to 1.60% (Table 2, Fig. 6) and indicates thermally mature organic matter corresponding to the “gas window”. Lower values were obtained from the T1 shale samples (from 0.93 to 1.03 EqRo%, Table 1, Fig. 6). Thermal maturity obtained from Tmax (Table 4) of the analysed samples and calculated from various formulae (Jarvie et al. 2001; Evenick 2021; Waliczek et al. 2021) gives %Ro (Tmax) in the range of 0.90—1.00%, which is lower than Ro for the thucholite samples (Table 2). %Ro (Tmax) for the T1 shale recalculated by Jarvie et al. (2001), Evenick (2021), and Waliczek et al. (2021), is in agreement with the measured Ro values (Table 1). Therefore, the lithology of the analysed T1 shale samples could be a key factor here. The equivalent vitrinite reflectance values (Rc and Rcs) based on the aromatic fraction (online supplementary material Table S7) indicate a similar thermal maturity of organic matter for the T1 shale and thucholite. The calculated vitrinite reflectance from methylphenanthrenes (Rc) using the formula proposed by Radke et al. (1986) ranges from 0.55 to 0.59% for the T1 shale and 0.52 to 0.61% for thucholite concretions, whereas the recalculated vitrinite reflectance values from methyldibenzothiophenes (Rcs) using the equation suggested by Radke and Willsch (1994) range from 0.75 to 0.77% for the T1 shale and 0.62 to 0.76% for the thucholite. However, the aromatic fraction maturity parameters seem to give underestimated equivalent vitrinite reflectance values and unreliable thermal maturity for organic matter dispersed both in the T1 shale and thucholite. Higher thermal maturity is also confirmed by Raman maturity parameters. G-FWHM (91 cm−1) and D1A/GA (1.28) in this study (online supplementary material Table S8) indicate vitrinite reflectance for TH-3 of ≤ 1.5% Ro (Henry et al. 2019). RBS of 251 cm−1 corresponds to the vitrinite reflectance value of about 1.7–1.8% for coal (Jin et al. 2021).
Raman spectroscopy- and organic matter-based temperature
Two different temperatures were calculated using the organic matter reflectance. Based on vitrinite reflectance measurements, the maximum palaeotemperature (PT, Table 3) was obtained. By applying semifusinite reflectance values, palaeo-wildfire temperature was estimated (TRsf, Table 3) indicating surface fire temperatures of ~ 400 °C (Table 3). The PT temperature correlates well with the geothermometer from Raman parameters, however, the most coherent results indicate higher maturity grade (Henry et al. 2019 and references therein).
The calculated PT is ~ 170 °C and the temperature calculated from the R1 and R2 Raman parameters using Rahl et al.’s (2005) equation (T = 737.3 + 320.9 *R1 − 1067*R2- 80*638 R12) is 319 °C. The differences between temperatures from Raman spectroscopy and Ro may result from different reasons. The thermometer proposed by Rahl et al. (2005) is applicable for temperatures from 100 to 700 °C. However, the value R1 = 0.67 herein, compared to apatite and zircon temperatures presented by Rahl et al. (2005), is characteristic of temperatures equal to, or lower than, 180 °C or > 440 °C. Therefore, 319 °C calculated from the Rahl et al.'s (2005) formula may be incorrect. The differences in the estimated temperatures calculated herein can result from the Ro measurement error as well. The reflectance measurements were carried out on detrovitrinite mixed with mineral matter and the standard deviation of single measurements is generally higher than 0.10 (Table 3). As a source of the higher radiation irregular radiolitic alteration could be considered. Moreover, the Ro values are higher in the centre of thucholite (Ro≈1.7%) and decrease towards the edge (Ro≈1.3%) which could be caused by radiation (Zumberge et al. 1978; Sassen 1984; Leventhal et al. 1986; Dahl et al. 1988). A similar phenomenon has been described by Havelcová et al. (2022), who noted an increase in reflectance from rim to core of solid bitumen that had been radiolytically altered by adjacent uraninite. It is possible that U-organic complexes which have been placed in the centre of the thucholite with unstable U-bearing clastic grains degraded and transformed into uraninite.
Possible role of microorganisms
Due to the different REE and organic matter contents of thucholite in comparison with the T1 shale, the possible role of microorganisms should be considered. Many bacteria can create a biofilm on mineral particles at different stages of sedimentation. Interactions between microorganisms and mineral particles may affect the composition of organic matter and the trace metal biosignatures (Playter et al. 2017). It is also commonly known that microorganisms can effectively grow on the surface of carbonised/charred particles, which is widely used in technology. Moreover, the formation of biofilms may entail an increase in the rate of biotransformation of organic compounds (Xia et al. 2011; Folwell et al. 2016). The presence of biofilm may also influence the degradation processes of organic matter via UV radiation. This process of simultaneous action of microorganisms and UV radiation, known as photobiodegradation, is also used in practice (Xia et al. 2011). Therefore, it can be assumed that the charred debris at the "pre-thucholite" stage could have been subject to intensive colonisation by microorganisms, which could affect the biotransformation of organic matter. Indeed, the organic matter analysis in thucholite did reveal the presence of typical markers of bacterial mediation such as hopanes. These compounds have a similar distribution to that of the T1 shale, suggesting that the charred (terrigenous) particles were subsequently colonised by the same groups of bacteria in the marine realm, which also contributed to the total biomass of the T1 shale. Another problem, which can be explained by the presence of microorganisms, concerns the differences in the REE and U contents between thucholite and the T1 shale. Firstly, the charred particles may have different sorption properties than the mineral particles. Secondly, the presence of biofilms can further enhance such effects. Thirdly, exopolymeric substances (EPS) secreted by bacteria can strongly absorb metal ions (Toner et al. 2006). Fourthly, it is also known that U can be efficiently adsorbed by organic matter (Bone et al. 2017). Hence, could microorganisms growing on the charred particles lead to an increase in the concentration of some metals at the pre-sedimentation stage? The above considerations are somewhat speculative, however, in light of the presence of microbial activity markers (hopanes), they are worth being considered. Moreover, following the principle of actualism, it should be assumed that the charred material entering the depositional basin must have been subject to microbial activity. It is commonly known that microorganisms can readily colonise carbonised particles, thus it is conceivable that such processes occurred also in the past.
Charred detrital particles can also adsorb metals before they migrate into the sedimentary basin or through interaction with petroleum-derived metal-rich organic matter (Parnell 1988b; Parnell and Eakin 1989). If one assumes the processes of adsorption of charred particles during migration also in the hinterland, this does not contradict the hypotheses presented herein. The key assumption here is the combustion origin of the charred matter, which can both interact with microorganisms and strongly adsorb metals, which may explain such a local occurrence of thucholite within the T1 shale. If one accepts another hypothesis about the interaction of radioactive detrital grains (e.g., uraninite) with petroleum-derived organic matter (England et al. 2001), it is difficult to compare it with the hypothesis presented herein. It has been assumed that the interaction of detrital uraninite with petroleum-derived organic matter occurred during sedimentation, rather than during transport of sediment to the basin. On the other hand, some similarities between thucholite from the T1 shale and bitumen grains from the Witwatersrand (England et al. 2001), which could hypothetically have resulted from such interactions, may simply be an example of convergence, which means a similar effect as a result of different processes.
Conceptual model of the thucholite formation
Based on our results, it is possible to speculate on a hypothetical sequence of events that led to the formation of thucholite in the environment of T1 shale sedimentation (Fig. 10). Some of the most important features of thucholite should be considered. Firstly, these structures are characterised by a different REE abundance pattern. Secondly, some differences in organic matter composition were found (e.g., more intense typical pyrolytic compounds in thucholite, presence of hopanes). Thirdly, Raman spectroscopy revealed a higher maturity level of thucholite’s organic matter macromolecular structure. All these properties may indicate a low-temperature combustion process, such as the slow combustion of peats (Fig. 10 (1)). It is likely that low-temperature combustion debris (charred detrital particles) was the source of organic matter aggregated in the thucholite (Fig. 10 (2)). Moreover, it is possible that these charred particles were subjected to mechanical abrasion during their migration to the sedimentary basin, taking the form of spherical porous carbon structures (Fig. 10 (3)). The physicochemical properties of these structures also determined different sorption properties compared to the other non-charred material deposited in the environment (Fig. 10 (4)). As a result, thucholite differ in their REE content, which may also explain their increased radioactivity. The latter, as well as the different surface chemical reactivity of charred particles that were the precursors of thucholite, could also have resulted in a different organic matter composition in relation to the non-carbonised matter deposited in the environment (Fig. 10 (5)). Similarly, the activity of microorganisms creating biofilms on the surface of charred debris can also change the organic matter composition (Fig. 10 (6)). However, the processes leading to the formation of the source material for thucholite do not have to be common, which explains the occurrence of thucholite only in the form of incidental layers within carbonate strata, dolomitic shale, and Ws sandstone (Fig. 10 (7)). The role of coal particles in formation of U-bearing mineral phases in fluvio-lacustrine environments was previously discussed by Rallakis et al. (2021). Similarly, the charred particles of “pre-thucholite” probably were able to adsorb U and REE leading to formation of thucholite concretions. In hydrous solutions, the most stable U species are the uranyl ions (UO22+). However, at pH > 6 other species such as UO2(OH)2 and complexed (UO2)3(OH)5+ are also stable (Allard et al. 1980). The standard redox potential for the reduction reaction: UO22+ + 4H+ + 2e− ⇌ U4+ + 2H2O is + 0.32 V (Allard et al. 1980). Such a potential implies that uranyl ions can be relatively easily reduced to + IV by many different reduction agents. However, considering that uraninite (detected by XRD) is easily weathered, it is reasonable to assume that the reduction of adsorbed + VI uranyl ions took place after sedimentation and during diagenesis. Alternatively, the accumulation of U in the thucholite concretions may be associated with the redox boundary near Rote Fäule. Uranium in the T1 shale migrated as uranyl-rich fluids from the Rotliegend deposits (Sun et al. 2005). It was subsequently adsorbed by charred debris (thucholite) and reduced by organic matter present in the T1 shale. However, the presence of Cu compounds can induce the oxidation of uraninite to secondary phases such as cuprosklodovskite (detected by SEM and EDS). The presented hypothetical sequence of events, based on the geochemical data presented in this paper, most probably led to the formation of thucholite observed in the Cu-bearing shale layers. In the area where thucholite occurs, no changes indicating the impact of hydrothermal solutions related to tectonics were found. The nearest faults are located approximately 400 m from the studied area and are represented by reverse faults extending along the E-W line, which is associated with the beginning of the Laramian phase (Salski 1977).
Conclusions
Mineralogical and organic matter studies of the thucholite and T1 shale samples suggest a different origin for the thucholite concretions. The chemical and organic matter composition of thucholite and T1 shale show that the T1 shale and thucholite are characterised by a varied organic composition, suggesting the dominance of organic matter of marine-terrigenous origin in the former, and terrigenous-marine origin in the latter. This is particularly reflected by different Ntot, Ctot, Htot and Stot contents but also the C/N and C/S ratios irrespective of later catagenetic changes of organic matter. However, the presence of typically pyrolytic PAHs in thucholite, such as benz[a]anthracene and benzo[a]pyrene, indicates a pyrolytic origin of some of the organic matter trapped therein. Based on semifusinite reflectance, it is concluded that the organic matter of the thucholite samples was subjected to a temperature of 400 °C, which may correspond to low-temperature fires. Thus, the data obtained suggest that the low-temperature fires may have released charred particles that were subsequently transported and deposited as thucholite concretions within the T1 shale.
References
Alderton DHM, Selby D, Kucha H, Blundell DJ (2016) A multistage origin for Kupferschiefer mineralization. Ore Geol Rev 79:535–543. https://doi.org/10.1016/j.oregeorev.2016.05.007
Alexandre P, Kyser TK (2005) Effects of cationic substitutions and alteration in uraninite, and implications for the dating of uranium deposits. Can Mineral 43:1005–1017. https://doi.org/10.2113/gscanmin.43.3.1005
Alexandre P, Kyser TK (2006) Geochemistry of uraniferous bitumen in the Southwest Athabasca Basin, Saskatchewan, Canada. Econ Geol 101:1605–1612. https://doi.org/10.2113/gsecongeo.101.8.1605
Alexandre P, Kyser K, Thomas D et al (2009) Geochronology of unconformity-related uranium deposits in the Athabasca Basin, Saskatchewan, Canada and their integration in the evolution of the basin. Miner Deposita 44:41–59. https://doi.org/10.1007/s00126-007-0153-3
Allard B, Kipatsi H, Liljenzin JO (1980) Expected species of uranium, neptunium and plutonium in neutral aqueous solutions. J Inorg Nucl Chem 42:1015–1027. https://doi.org/10.1016/0022-1902(80)80394-0
Ardhaoui K, Coulet MV, Ben Chérifa A et al (2006) Standard enthalpy of formation of neodymium fluorbritholites. Thermochim Acta 444:190–194. https://doi.org/10.1016/j.tca.2006.03.013
ASTM (2011) ASTM D7708–14. Standard test method for microscopical determination of the reflectance of vitrinite dispersed in sedimentary rocks. West Conshohocken, Pennsylvania, p 2
ASTM (2015) ASTM D2797/D2797M-11a: standard practice for preparing coal samples for microscopical analysis by reflected light. West Conshohocken, Pennsylvania, p 10
Banaś M, Jarosz J, Salamon W (1978) Thucholite from the Permian copper-bearing rocks in Poland. Mineral Pol 9:3–24
Banaś M, Kwiecińska B, Starnawska E (2005) The association of uranium, vanadium and organic matter in the copper deposits in Weissligend sandstones (Fore-Sudetic Monocline, Poland). Mineral Pol 36:145–159
Barker CE, Pawlewicz MJ (1994) Calculation of vitrinite reflectance from thermal histories and peak temperatures. In: Mukhopadhyay PK, Dow WG (eds) Vitrinite Reflectance as a Maturity Parameter. American Chemical Society, pp 216–229
Bechtel A, Püttmann W (1997) Palaeoceanography of the early Zechstein Sea during Kupferschiefer deposition in the Lower Rhine Basin (Germany): A reappraisal from stable isotope and organic geochemical investigations. Palaeogeogr Palaeoclimatol Palaeoecol 136:331–358. https://doi.org/10.1016/S0031-0182(97)00104-1
Bechtel A, Püttmann W, Hoernes S (1995) Reconstruction of the thermal history of the Kupferschiefer within the Zechstein basin of Central Europe: A stable isotope and organic geochemical approach. Ore Geol Rev 9:371–389. https://doi.org/10.1016/0169-1368(94)00020-O
Bechtel A, Gratzer R, Püttmann W, Oszczepalski S (2001) Variable alteration of organic matter in relation to metal zoning at the Rote Fäule front (Lubin-Sieroszowice mining district, SW Poland). Org Geochem 32:377–395. https://doi.org/10.1016/S0146-6380(01)00002-X
Bechtel A, Gratzer R, Püttmann W, Oszczepalski S (2002) Geochemical characteristics across the oxic/anoxic interface (Rote Fäule front) within the Kupferschiefer of the Lubin-Sieroszowice mining district (SW Poland). Chem Geol 185:9–31. https://doi.org/10.1016/S0009-2541(01)00395-3
Berner RA (1984) Sedimentary pyrite formation: An update. Geochim Cosmochim Acta 48:605–615. https://doi.org/10.1016/0016-7037(84)90089-9
Błaszczyk JK (1981) Wpływ paleomorfologii stropu białego spągowca na zmienność facjalną serii złożowej w Zagłębiu Lubińskim. Geol Sudet 16:195–217
Błaszczyk JK (1984) Biostruktury w łupkach miedzionośnych na monoklinie przedsudeckiej. Geol Quart 28:655–674
Bone SE, Dynes JJ, Cliff J, Bargar JR (2017) Uranium(IV) adsorption by natural organic matter in anoxic sediments. Proc Natl Acad Sci 114:711–716. https://doi.org/10.1073/pnas.1611918114
Bottrell SH, Miller MF (1990) The geochemical behaviour of nitrogen compounds during the formation of black shale hosted quartz-vein gold deposits, north Wales. Appl Geochem 5:289–296. https://doi.org/10.1016/0883-2927(90)90004-O
Bowles JF (2015) Age dating from electron microprobe analyses of U, Th, and Pb: geological advantages and analytical difficulties. Microsc Microanal 21:1114–1122. https://doi.org/10.1017/S1431927615000446
Chukanov NV, Ermolaeva VN, Pekov IV, Lahti S (2009) Carbonaceous matters in permatites of different genetic types and their role in formation of mineral associations. New Data Miner 44:11–23
Compton JS, Williams LB, Ferrell RE (1992) Mineralization of organogenic ammonium in the Monterey Formation, Santa Maria and San Joaquin basins, California, USA. Geochim Cosmochim Acta 56:1979–1991. https://doi.org/10.1016/0016-7037(92)90324-C
Court RW, Sephton MA, Parnell J, Gilmour I (2006) The alteration of organic matter in response to ionising irradiation: Chemical trends and implications for extraterrestrial sample analysis. Geochim Cosmochim Acta 70:1020–1039. https://doi.org/10.1016/j.gca.2005.10.017
Crick IH (1992) Petrological and maturation characteristics of organic matter from the Middle Proterozoic McArthur Basin, Australia. Aust J Earth Sci 39:501–519. https://doi.org/10.1080/08120099208728042
Crick IH, Boreham CJ, Cook AC, Powell TG (1988) Petroleum geology and geochemistry of Middle Proterozoic McArthur Basin, northern Australia II: assessment of source rock potential. AAPG Bull 72:1495–1514
Curiale JA, Bloch S, Rafalska-Bloch J, Harrison WE (1983) Petroleum-related origin for uraniferous organic-rich nodules of southwestern Oklahoma. AAPG Bull 67:588–608
Dahl J, Hallberg R, Kaplan IR (1988) Effects of irradiation from uranium decay on extractable organic matter in the Alum Shales of Sweden. Org Geochem 12:559–571. https://doi.org/10.1016/0146-6380(88)90147-7
Diedrich CG (2009) A coelacanthid-rich site at Hasbergen (NW Germany): taphonomy and palaeoenvironment of a first systematic excavation in the Kupferschiefer (Upper Permian, Lopingian). Palaeobio Palaeoenv 89:67–94. https://doi.org/10.1007/s12549-009-0004-6
Dybova-Jachowicz S (2001) Miospory. In: Pajchlowa M, Wagner R (eds) Atlas skamieniałości przewodnich i charakterystycznych. Cz. 1c. Z. 3. Państwowy Instytut Geologiczny, Warszawa, pp 106–162
Eglinton G, Hamilton RJ (1967) Leaf epicuticular waxes. Science 156:1322–1335. https://doi.org/10.1126/science.156.3780.1322
Ellsworth H (1928) Thucholite, a remarkable primary carbon mineral from the vicinity of Parry Sound, Ontario. Am Miner 13:419–441
England GL, Rasmussen B, Krapež B, Groves DI (2001) The origin of uraninite, bitumen nodules, and carbon seams in Witwatersrand gold-uranium-pyrite ore deposits, based on a Permo-Triassic analogue. Econ Geol 96:1907–1920. https://doi.org/10.2113/gsecongeo.96.8.1907
Espitalié J, Deroo G, Marquis F (1985) La pyrolyse Rock-Eval et ses applications. Revue De L’institut Français Du Pétrole 40–41(563–579):755–784. https://doi.org/10.2516/ogst:1985045
Evenick JC (2021) Examining the relationship between Tmax and vitrinite reflectance: An empirical comparison between thermal maturity indicators. J Nat Gas Sci Eng 91:103946. https://doi.org/10.1016/j.jngse.2021.103946
Fijałkowska-Mader A (2012) Odzwierciedlenie stresu środowiskowego w palinoflorze późnego permu w Polsce. Biuletyn Państwowego Instytutu Geologicznego Nr 452:23–31
Finkelstein DB, Pratt LM, Curtin TM, Brassell SC (2005) Wildfires and seasonal aridity recorded in Late Cretaceous strata from south-eastern Arizona, USA. Sedimentology 52:587–599. https://doi.org/10.1111/j.1365-3091.2005.00712.x
Folwell BD, McGenity TJ, Whitby C (2016) Biofilm and planktonic bacterial and fungal communities transforming high-molecular-weight polycyclic aromatic hydrocarbons. Appl Environ Microbiol 82:2288–2299. https://doi.org/10.1128/AEM.03713-15
Fuchs S, Schumann D, Williams-Jones AE, Vali H (2015) The growth and concentration of uranium and titanium minerals in hydrocarbons of the Carbon Leader Reef, Witwatersrand Supergroup, South Africa. Chem Geol 393–394:55–66. https://doi.org/10.1016/j.chemgeo.2014.11.018
Gaskin JW, Steiner C, Harris K et al (2008) Effect of low-temperature pyrolysis conditions on biochar for agricultural use. Trans ASABE 51:2061–2069
Gibson ME, Wellman CH (2021) The use of spore–pollen assemblages to reconstruct vegetation changes in the Permian (Lopingian) Zechstein deposits of northeast England. Rev Palaeobot Palynol 288:104399. https://doi.org/10.1016/j.revpalbo.2021.104399
Grafka O, Marynowski L, Simoneit BRT (2015) Phenyl derivatives of polycyclic aromatic compounds as indicators of hydrothermal activity in the Silurian black siliceous shales of the Bardzkie Mountains, Poland. Int J Coal Geol 139:142–151. https://doi.org/10.1016/j.coal.2014.09.006
Grice K, Schaeffer P, Schwark L, Maxwell JR (1996) Molecular indicators of palaeoenvironmental conditions in an immature Permian shale (Kupferschiefer, Lower Rhine Basin, north-west Germany) from free and S-bound lipids. Org Geochem 25:131–147. https://doi.org/10.1016/S0146-6380(96)00130-1
Hallbauer DK, van Warmelo KT (1974) Fossilized plants in thucholite from Precambrian rocks of the Witwatersrand, South Africa. Precambr Res 1:199–212. https://doi.org/10.1016/0301-9268(74)90010-2
Havelcová M, Sýkorová I, René M et al (2022) Geology and petrography of uraniferous bitumens in Permo-Carboniferous sediments (Vrchlabí, Czech Republic). Minerals 12:544. https://doi.org/10.3390/min12050544
Henry DG, Jarvis I, Gillmore G, Stephenson M (2019) Raman spectroscopy as a tool to determine the thermal maturity of organic matter: Application to sedimentary, metamorphic and structural geology. Earth Sci Rev 198:102936. https://doi.org/10.1016/j.earscirev.2019.102936
Hunt J (1995) Petroleum geochemistry and geology, 2nd ed. W. H. Freeman, New York, p 743
ICCP (1998) The new vitrinite classification (ICCP System 1994). Fuel 77:349–358. https://doi.org/10.1016/S0016-2361(98)80024-0
ICCP (2001) The new inertinite classification (ICCP System 1994). Fuel 80:459–471. https://doi.org/10.1016/S0016-2361(00)00102-2
Jacob H (1989) Classification, structure, genesis and practical importance of natural solid oil bitumen (“migrabitumen”). Int J Coal Geol 11:65–79. https://doi.org/10.1016/0166-5162(89)90113-4
Janeczek J, Ewing RC (1995) Mechanisms of lead release from uraninite in the natural fission reactors in Gabon. Geochim Cosmochim Acta 59:1917–1931. https://doi.org/10.1016/0016-7037(95)00117-4
Jarvie DM, Claxton BL, Henk F, Breyer JT (2001) Oil and shale gas from Barnett Shale, Ft. Worth Basin, Texas. AAPG Search and Discovery Article #90906
Jerzykiewicz T, Kijewski P, Mroczkowski J, Teisseyre AK (1976) Geneza osadów białego spągowca monokliny przedsudeckiej. Geol Sudet 11:57–100
Jin Y, Wu S, Gao L et al (2021) Raman thermal maturity of coal and Type II kerogen based on surface-enhanced Raman spectroscopy (SERS). ACS Omega 6:18504–18508. https://doi.org/10.1021/acsomega.1c02730
Jones TP, Lim B (2000) Extraterrestrial impacts and wildfires. Palaeogeogr Palaeoclimatol Palaeoecol 164:57–66. https://doi.org/10.1016/S0031-0182(00)00175-9
Kaiho K, Miura M, Tezuka M et al (2021) Coronene, mercury, and biomarker data support a link between extinction magnitude and volcanic intensity in the Late Devonian. Global Planet Change 199:103452. https://doi.org/10.1016/j.gloplacha.2021.103452
Kaisbeek N, Larsen S, Rønsbo JG (1990) Crystal structures of rare earth elements rich apatite analogues. Z fur Krist - Cryst Mater 191:249–264. https://doi.org/10.1524/zkri.1990.191.14.249
Kamel N, Aït-Amar H, Zahri A (2007) The use of a soaking procedure combined with the sintering process to reach a high cerium content in a cerium-bearing Y-britholite. Prog Nucl Energy 49:351–364. https://doi.org/10.1016/j.pnucene.2007.03.002
Khan I, Zhong N, Luo Q et al (2020) Maceral composition and origin of organic matter input in Neoproterozoic-Lower Cambrian organic-rich shales of Salt Range Formation, upper Indus Basin, Pakistan. Int J Coal Geol 217:103319. https://doi.org/10.1016/j.coal.2019.103319
Killops SD, Massoud MS (1992) Polycyclic aromatic hydrocarbons of pyrolytic origin in ancient sediments: evidence for Jurassic vegetation fires. Org Geochem 18:1–7. https://doi.org/10.1016/0146-6380(92)90137-M
Kostova I, Zdravkov A, Bechtel A et al (2022) Characterization of organic matter from the Cretaceous sedimentary and volcano-sedimentary strata from Livingston Island, Antarctic Peninsula: Insights from organic petrology, molecular proxies and carbon and hydrogen isotopes. Int J Coal Geol 252:103940. https://doi.org/10.1016/j.coal.2022.103940
Kucha H (1982) Platinum-group metals in the Zechstein copper deposits, Poland. Econ Geol 77:1578–1591. https://doi.org/10.2113/gsecongeo.77.6.1578
Kucha H (2007) Mineralogia kruszcowa i geochemia ciała rudnego złoża Lubin-Sieroszowice. Biuletyn Państwowego Instytutu Geologicznego 423:77–94
Kucha HH (2021) New minerals and the first mineral occurrences in the Kupferschiefer (U, REE, HgS, chloride minerals PtAs, Pt(Sb, Bi), PtBi), Poland, and their genetic meaning. Mineralogia 52:31–42. https://doi.org/10.2478/mipo-2021-0004
Kucha H, Pawlikowski M (1986) Two-brine model of the genesis of strata-bound Zechstein deposits (Kupferschiefer type), Poland. Miner Deposita 21:70–80. https://doi.org/10.1007/BF00204365
Kucha H, Przybyłowicz W (1999) Noble metals in organic matter and clay-organic matrices, Kupferschiefer, Poland. Econ Geol 94:1137–1162. https://doi.org/10.2113/gsecongeo.94.7.1137
Lafargue E, Marquis F, Pillot D (1998) Rock-Eval 6 Applications in Hydrocarbon Exploration, Production, and Soil Contamination Studies. Revue De L’institut Français Du Pétrole 53:421–437. https://doi.org/10.2516/ogst:1998036
Lan X, Gao J, Li Y, Guo Z (2021) Phase equilibria of CaO–SiO2–CaF2–P2O5–Ce2O3 system and formation mechanism of britholite. Ceram Int 47:11966–11972. https://doi.org/10.1016/j.ceramint.2021.01.038
Lehmann J, Rillig MC, Thies J et al (2011) Biochar effects on soil biota – A review. Soil Biol Biochem 43:1812–1836. https://doi.org/10.1016/j.soilbio.2011.04.022
Leventhal JS, Daws TA, Frye JS (1986) Organic geochemical analysis of sedimentary organic matter associated with uranium. Appl Geochem 1:241–247. https://doi.org/10.1016/0883-2927(86)90008-9
Luo Q, Zhong N, Qin J et al (2014) Thucholite in Mesoproterozoic shales from northern north China: Occurrence and indication for thermal maturity. Int J Coal Geol 125:1–9. https://doi.org/10.1016/j.coal.2014.01.009
Martz P, Mercadier J, Perret J et al (2019) Post-crystallization alteration of natural uraninites: Implications for dating, tracing, and nuclear forensics. Geochim Cosmochim Acta 249:138–159. https://doi.org/10.1016/j.gca.2019.01.025
Marynowski L, Rospondek MJ, Meyer zu Reckendorf R, Simoneit BRT (2002) Phenyldibenzofurans and phenyldibenzothiophenes in marine sedimentary rocks and hydrothermal petroleum. Org Geochem 33:701–714. https://doi.org/10.1016/S0146-6380(02)00040-2
Marynowski L, Kubik R, Uhl D, Simoneit BRT (2014) Molecular composition of fossil charcoal and relationship with incomplete combustion of wood. Org Geochem 77:22–31. https://doi.org/10.1016/j.orggeochem.2014.09.003
Merlet C (1994) An accurate computer correction program for quantitative electron probe microanalysis. Microchim Acta 114:363–376. https://doi.org/10.1007/BF01244563
Meyers PA, Ishiwatari R (1995) Organic matter accumulation records in lake sediments. In: Lerman A, Imboden DM, Gat JR (eds) Physics and Chemistry of Lakes. Springer, Berlin, Heidelberg, pp 279–328
Nelson PF, Buckley AN, Kelly MD (1992) Functional forms of nitrogen in coals and the release of coal nitrogen as NOx precursors (HCN and NH3). Symp (Int) Combust 24:1259–1267. https://doi.org/10.1016/S0082-0784(06)80148-7
Oberti R, Ottolini L, Ventura GD, Parodi GC (2001) On the symmetry and crystal chemistry of britholite: New structural and microanalytical data. Am Miner 86:1066–1075. https://doi.org/10.2138/am-2001-8-913
Oszczepalski S (1999) Origin of the Kupferschiefer polymetallic mineralization in Poland. Miner Deposita 34:599–613. https://doi.org/10.1007/s001260050222
Oszczepalski S, Chmielewski A, Mikulski SZ (2016) Controls on the distribution of rare earth elements in the Kupferschiefer series of SW Poland. Geol Quart 60:811–826. https://doi.org/10.7306/gq.1325
Oszczepalski S, Speczik S, Zieliński K, Chmielewski A (2019) The Kupferschiefer deposits and prospects in SW Poland: past, present and future. Minerals 9:592. https://doi.org/10.3390/min9100592
Parnell J (1988a) Lacustrine petroleum source rocks in the Dinantian Oil Shale Group, Scotland: a review. Geol Soc London Spec Publ 40:235–246. https://doi.org/10.1144/GSL.SP.1988.040.01.20
Parnell J (1988b) Metal enrichments in solid bitumens: A review. Miner Deposita 23:191–199. https://doi.org/10.1007/BF00204301
Parnell J (1996) Phanerozoic analogues for carbonaceous matter in Witwatersrand ore deposits. Econ Geol 91:55–62. https://doi.org/10.2113/gsecongeo.91.1.55
Parnell J, Eakin P (1989) Thorium–bitumen mineralization in Silurian sandstones, Welsh Borderland. Mineral Mag 53:111–116. https://doi.org/10.1180/minmag.1989.053.369.13
Pickel W, Kus J, Flores D et al (2017) Classification of liptinite – ICCP System 1994. Int J Coal Geol 169:40–61. https://doi.org/10.1016/j.coal.2016.11.004
Piestrzyński A (1990) Uranium and thorium in the Kupferschiefer formation, Lower Zechstein, Poland. Miner Deposita 25:146–151. https://doi.org/10.1007/BF00208858
Piestrzyński A, Pieczonka J, Głuszek A (2002) Redbed-type gold mineralisation, Kupferschiefer, south-west Poland. Miner Deposita 37:512–528. https://doi.org/10.1007/s00126-002-0256-9
Playter T, Konhauser K, Owttrim G et al (2017) Microbe-clay interactions as a mechanism for the preservation of organic matter and trace metal biosignatures in black shales. Chem Geol 459:75–90. https://doi.org/10.1016/j.chemgeo.2017.04.007
Püttmann W, Merz C, Speczik S (1989) The secondary oxidation of organic material and its influence on Kupferschiefer mineralization of southwest Poland. Appl Geochem 4:151–161. https://doi.org/10.1016/0883-2927(89)90046-2
Radke M, Willsch H (1994) Extractable alkyldibenzothiophenes in Posidonia Shale (Toarcian) source rocks: Relationship of yields to petroleum formation and expulsion. Geochim Cosmochim Acta 58:5223–5244. https://doi.org/10.1016/0016-7037(94)90307-7
Radke M, Welte DH, Willsch H (1986) Maturity parameters based on aromatic hydrocarbons: Influence of the organic matter type. Org Geochem 10:51–63. https://doi.org/10.1016/0146-6380(86)90008-2
Rahl JM, Anderson KM, Brandon MT, Fassoulas C (2005) Raman spectroscopic carbonaceous material thermometry of low-grade metamorphic rocks: Calibration and application to tectonic exhumation in Crete, Greece. Earth Planet Sci Lett 240:339–354. https://doi.org/10.1016/j.epsl.2005.09.055
Rallakis D, Michels R, Cathelineau M et al (2021) Conditions for uranium biomineralization during the formation of the Zoovch Ovoo roll-front-type uranium deposit in East Gobi Basin, Mongolia. Ore Geol Rev 138:104351. https://doi.org/10.1016/j.oregeorev.2021.104351
Rasmussen B, Glover JE, Alexander R (1989) Hydrocarbon rims on monazite in Permian-Triassic arenites, northern Perth Basin, Western Australia: Pointers to the former presence of oil. Geology 17:115–118. https://doi.org/10.1130/0091-7613(1989)017%3c0115:HROMIP%3e2.3.CO;2
Rasmussen B, Glover JE, Foster CB (1993) Polymerisation of hydrocarbons by radioactive minerals in sedimentary rocks: Diagenetic and economic significance. In: Parnell J, Kucha H, Landais P (eds) Bitumens in Ore Deposits. Springer, Berlin, Heidelberg, pp 490–509
Rospondek MJ, de Leeuw JW, Baas M et al (1994) The role of organically bound sulphur in stratiform ore sulphide deposits. Org Geochem 21:1181–1191. https://doi.org/10.1016/0146-6380(94)90162-7
Rospondek MJ, Marynowski L, Góra M (2007) Novel arylated polyaromatic thiophenes: Phenylnaphtho[b]thiophenes and naphthylbenzo[b]thiophenes as markers of organic matter diagenesis buffered by oxidising solutions. Org Geochem 38:1729–1756. https://doi.org/10.1016/j.orggeochem.2007.05.008
Rospondek MJ, Szczerba M, Malek K et al (2008) Comparison of phenyldibenzothiophene distributions predicted from molecular modelling with relevant experimental and geological data. Org Geochem 39:1800–1815. https://doi.org/10.1016/j.orggeochem.2008.06.012
Rospondek MJ, Marynowski L, Chachaj A, Góra M (2009) Novel aryl polycyclic aromatic hydrocarbons: Phenylphenanthrene and phenylanthracene identification, occurrence and distribution in sedimentary rocks. Org Geochem 40:986–1004. https://doi.org/10.1016/j.orggeochem.2009.06.001
Salski W (1977) Rozwój tektoniczny obszaru miedzionośnego monokliny przedsudeckiej. Rocz Pol Towarz Geol 47:27–48
Sanz-Robinson J (2020) An experimental investigation of the potential role of liquid hydrocarbons as ore fluids for sediment-hosted ore deposits. McGill University, PhD thesis, p 190
Sassen R (1984) Effects of radiation exposure on indicators of thermal maturity. Org Geochem 5:183–186. https://doi.org/10.1016/0146-6380(84)90004-4
Sawlowicz Z (2013) REE and their relevance to the development of the Kupferschiefer copper deposit in Poland. Ore Geol Rev 55:176–186. https://doi.org/10.1016/j.oregeorev.2013.06.006
Sawlowicz Z, Gize AP, Rospondek M (2000) Organic matter from Zechstein copper deposits (Kupferschiefer) in Poland. In: Glikson M, Mastalerz M (eds) Organic Matter and Mineralisation: Thermal Alteration, Hydrocarbon Generation and Role in Metallogenesis. Springer, Netherlands, Dordrecht, pp 220–242
Schidlowski M (1981) Uraniferous constituents of the Witwatersrand conglomerates: Ore-microscopic observations and implications for the Witwatersrand metallogeny. U.S. Geological Survey Professional Paper 1161:N1-N29
Schulz H-M, Yang S, Panova E, Bechtel A (2019) The role of Pleistocene meltwater-controlled uranium leaching in assessing irradiation-induced alteration of organic matter and petroleum potential in the Tremadocian Koporie Formation (Western Russia). Geochim Cosmochim Acta 245:133–153. https://doi.org/10.1016/j.gca.2018.10.029
Shields G, Stille P (2001) Diagenetic constraints on the use of cerium anomalies as palaeoseawater redox proxies: an isotopic and REE study of Cambrian phosphorites. Chem Geol 175:29–48. https://doi.org/10.1016/S0009-2541(00)00362-4
Słowakiewicz M, Kiersnowski H, Wagner R (2009) Correlation of the Middle and Upper Permian marine and terrestrial sedimentary sequences in Polish, German, and USA Western Interior Basins with reference to global time markers. Palaeoworld 18:193–211. https://doi.org/10.1016/j.palwor.2009.04.009
Spence HS (1930) Pegmatite minerals of Ontario and Quebec. Am Miner 15:474–496
Sun Y-Z (1998) Influences of secondary oxidation and sulfide formation on several maturity parameters in Kupferschiefer. Org Geochem 29:1419–1429. https://doi.org/10.1016/S0146-6380(98)00128-4
Sun Y, Püttmann W, Speczik S (1995) Differences in the depositional environment of basal Zechstein in southwest Poland: implication for base metal mineralization. Org Geochem 23:819–835. https://doi.org/10.1016/0146-6380(95)80003-A
Sun Y-Z, Jinxi W, Shifeng L et al (2005) Mechanism of uranium accumulation in the Kupferschiefer from Poland and Germany. Energy Explor Exploit 23:463–473. https://doi.org/10.1260/014459805776986902
Taylor SR, McLennan SM (1985) The continental crust: Its composition and evolution. Blackwell Scientific Publications, p 312
Taylor GH, Teichmüller M, Davis A et al (1998) Organic petrology. Gerbrüder Borntraeger, Berlin, Stuttgart, p 704
Toner B, Manceau A, Webb SM, Sposito G (2006) Zinc sorption to biogenic hexagonal-birnessite particles within a hydrated bacterial biofilm. Geochim Cosmochim Acta 70:27–43. https://doi.org/10.1016/j.gca.2005.08.029
Uhl D, Kerp H (2003) Wildfires in the Late Palaeozoic of Central Europe – The Zechstein (Upper Permian) of NW-Hesse (Germany). Palaeogeogr Palaeoclimatol Palaeoecol 199:1–15. https://doi.org/10.1016/S0031-0182(03)00482-6
Wagner R (1994) Stratygrafia osadów i rozwój basenu cechsztyńskiego na Niżu Polskim. Prace Państwowego Instytutu Geologicznego 146:1–71
Waliczek M, Machowski G, Poprawa P et al (2021) A novel VRo, Tmax, and S indices conversion formulae on data from the fold-and-thrust belt of the Western Outer Carpathians (Poland). Int J Coal Geol 234:103672. https://doi.org/10.1016/j.coal.2020.103672
Welin E (1966) The Occurrence of Asphaltite and thucholite in the Precambrian bedrock of Sweden. Geologiska Föreningen i Stockholm Förhandlingar 87:509–526. https://doi.org/10.1080/11035896609448319
Welte DH, Ebhardt G (1968) Distribution of long chain n-parafflns and n-fatty acids in sediments from the Persian Gulf. Geochim Cosmochim Acta 32:465–466. https://doi.org/10.1016/0016-7037(68)90080-X
Więcław D, Kotarba MJ, Pieczonka J et al (2007) Rozmieszczenie strefy redukcyjnej, przejściowej i utlenionej w łupku miedzionośnym na monoklinie przedsudeckiej na podstawie wskaźników materii organicznej. Biuletyn Państwowego Instytutu Geologicznego 423:125–138
Xia S, Yan N, Zhu J, Zhang Y (2011) Biofilm coupled with UV irradiation for phenol degradation and change of its community structure. Bioprocess Biosyst Eng 34:607–614. https://doi.org/10.1007/s00449-010-0509-4
Zakrzewski A, Waliczek M, Kosakowski P (2022) Geochemical and petrological characteristics of the Middle Jurassic organic-rich siliciclastic sediments from the central part of the Polish Basin. Int J Coal Geol 255:103986. https://doi.org/10.1016/j.coal.2022.103986
Zumberge JE, Sigleo AC, Nagy B (1978) Molecular and elemental analyses of the carbonaceous matter in the gold and uranium bearing Vaal Reef carbon seams, Witwatersrand sequence. Miner Sci Eng 10:223–246
Acknowledgements
We would like to thank the management of the division of Zakłady Górnicze "Polkowice-Sieroszowice" KGHM Polska Miedź S.A. for organising a visit to the mine in 2018 and for permitting geological sampling and photographic documentation. Grzegorz Kaproń is thanked for conducting XRD and Dariusz Więcław for Rock-Eval analyses. We are grateful for extensive and helpful comments of Hartwig Frimmel, Christoph Gauert, Maurice E. Tucker and Bernd Lehmann which improved our manuscript. The partial analyses were also performed under a subsidy of the Faculty of Geology, Geophysics and Environmental Protection at the AGH University of Krakow (16.16.140.315).
Author information
Authors and Affiliations
Contributions
M.D.S. designed the experiments, performed mineralogical, scanning electron microscopy and electron microprobe analyses; P.P. collected the samples and provided geological background; L.M. performed organic geochemistry; M.W. performed organic matter petrology; A.B. partially conducted chromatographic analysis; J.R., Š.M., I.S. conducted chemical analyses; M.L., B.J. conducted Raman spectroscopy analysis; M.S. conceptualisation, supervised the experiments; L.M., M.W., A.B., A.N.M., P.P. and M.S. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Editorial handling: H. E. Frimmel
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Syczewski, M.D., Panajew, P., Marynowski, L. et al. Geochemical implications of uranium-bearing thucholite aggregates in the Upper Permian Kupferschiefer shale, Lubin district, Poland. Miner Deposita (2024). https://doi.org/10.1007/s00126-024-01279-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00126-024-01279-y