Abstract
Introduction
A previous network meta-analysis established 16-week relative efficacy with bimekizumab, an inhibitor of interleukin (IL)-17F in addition to IL-17A, versus other treatments for patients with radiographic axial spondyloarthritis (r-axSpA; i.e., ankylosing spondylitis), including the IL-17A inhibitors secukinumab and ixekizumab. This matching-adjusted indirect comparison (MAIC) assessed 52-week relative efficacy of bimekizumab versus secukinumab and ixekizumab.
Methods
Individual patient data from BE MOBILE 2 (bimekizumab 160 mg; N = 220) were matched to pooled summary data from MEASURE 1/2/3/4 (secukinumab 150 mg), MEASURE 3 (secukinumab 300 mg; escalated dose for inadequate responders), COAST-V (ixekizumab) and COAST-V/-W (ixekizumab). BE MOBILE 2 patients were reweighted using propensity score weights based on age, sex, ethnicity, tumor necrosis factor inhibitor (TNFi) exposure, weight, baseline ASDAS and BASFI (secukinumab) and baseline BASDAI (ixekizumab), and 52-week efficacy outcomes from the trial recalculated. Odds ratios (OR) or mean difference for unanchored comparisons are reported with 95% confidence intervals (CI).
Results
At week 52, MAIC demonstrated that patients may have higher likelihood of improvement in key efficacy outcomes with bimekizumab versus secukinumab 150 mg (e.g., ASAS40: [OR (95% CI): 1.48 (1.05, 2.10); p = 0.026]; effective sample size [ESS] = 177). Differences in 52-week efficacy outcomes between bimekizumab and secukinumab 300 mg dose escalation were non-significant (ESS = 120). Bimekizumab versus ixekizumab 80 mg comparisons (COAST-V only; ESS = 84) also suggested that differences were non-significant for most key efficacy outcomes. Other ixekizumab comparisons (COAST-V/-W; ESS = 45) suggested bimekizumab may have higher comparative efficacy for many of the same efficacy outcomes, however ixekizumab analyses were limited by poor population overlap, likely due to the greater proportion of patients with previous TNFi exposure.
Conclusions
Patients treated with bimekizumab may have a higher likelihood of achieving improved longer-term efficacy versus secukinumab 150 mg, suggesting bimekizumab may be a favorable therapeutic option for r-axSpA. Differences in efficacy outcomes with bimekizumab versus ixekizumab 80 mg were mostly non-significant, depending on the populations considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Due to lack of evidence on comparative efficacy, treatment guidelines for axial spondyloarthritis (axSpA) do not currently distinguish between interleukin (IL)-17 inhibitors in their recommendations. |
The 16-week relative efficacy of bimekizumab, an inhibitor of IL-17F in addition to IL-17A, versus other treatments, including the IL-17A inhibitors secukinumab and ixekizumab, was reported in a previous network meta-analysis. |
These matching-adjusted indirect comparison (MAIC) analyses assessed the longer-term relative efficacy of bimekizumab versus secukinumab and ixekizumab at 52 weeks of treatment across phase 3 trials of patients with radiographic (r-)axSpA. |
What was learned from the study? |
Patients treated with bimekizumab may have a higher likelihood of achieving improved longer-term efficacy versus secukinumab 150 mg, but differences in efficacy outcomes versus ixekizumab 80 mg were mostly non-significant, depending on the populations considered. |
The results suggest that bimekizumab may be a favorable therapeutic option for r-axSpA compared with secukinumab 150 mg, however the MAIC analyses were limited by potential bias from unreported effect modifiers and prognostic factors, as well as reduced overlap in ixekizumab trial populations. |
Introduction
Axial spondyloarthritis (axSpA) is a chronic immune-mediated inflammatory disease predominantly affecting the axial skeleton (i.e., spine and sacroiliac joints [SIJ]), leading to chronic spinal pain and stiffness [1]. The axSpA disease spectrum comprises non-radiographic (nr-) and radiographic (r-)axSpA (i.e., ankylosing spondylitis [AS])[2]; r-axSpA is classified by the presence of definitive structural SIJ damage that can be visualized on plain radiographs, while these changes are not visible on radiographs of nr-axSpA [1]. AxSpA can also affect the peripheral joints (e.g., arthritis, enthesitis, and dactylitis) and is associated with a range of extra-musculoskeletal manifestations, such as psoriasis, uveitis, and inflammatory bowel disease, contributing to its high disease burden [3].
Conventional therapy with non-steroidal anti-inflammatory drugs (NSAIDs) is the first-line treatment for axSpA, and has demonstrated efficacy in treating the disease-associated pain and functional impairment [4]. For patients who do not respond to NSAIDs, targeted therapies including interleukin (IL)-17 inhibitors, tumor necrosis factor inhibitors (TNFis) and Janus kinase (JAK) inhibitors are recommended [5, 6]. Bimekizumab (BKZ), a humanized monoclonal IgG1 antibody that selectively inhibits IL-17F in addition to IL-17A, has recently received European Medicines Agency approval as a novel treatment of axSpA, providing a new therapeutic option to the approved IL-17A inhibitors secukinumab (SEC) and ixekizumab (IXE) [7,8,9]. BKZ treatment has demonstrated sustained long-term efficacy, safety, and tolerability up to 5 years in the phase 2b BE AGILE trial and its open-label extension in patients with active r-axSpA [10,11,12], and up to 52 weeks in patients across the axSpA spectrum in the parallel phase 3 studies BE MOBILE 1 (nr-axSpA) and BE MOBILE 2 (r-axSpA) [13, 14].
In the absence of head-to-head data, efficacy and safety of interventions can be compared indirectly using analytical frameworks such as network meta-analysis (NMA) and matching-adjusted indirect comparison (MAIC) [15, 16]. In the comparison of BKZ with SEC and IXE, a recent NMA demonstrated significantly higher relative efficacy with BKZ versus SEC and similar relative efficacy versus IXE in selected Assessment of SpondyloArthritis international Society (ASAS) responses up to weeks 12–16 in patients with axSpA [17, 18]. However, comparison of these treatments using NMA is not feasible beyond this timepoint due to a disconnected network (i.e., lack of placebo arm data beyond week 16 in the BKZ phase 3 randomized controlled trials [RCTs]) [19].
Unanchored MAIC analyses at week 52 is a feasible alternative that uses individual patient data (IPD) to analyze differences in treatment outcomes across trials after matching for baseline characteristics that may influence treatment responses. Unanchored MAIC analyses have been shown to adjust for cross-trial differences despite the lack of anchoring control, providing the underlying model assumptions are met (e.g., matching for all influential baseline characteristics) [20]. There are currently two key MAIC publications of TNF and IL-17A inhibitors in axSpA; an anchored MAIC comparing adalimumab (ADA) and SEC at weeks 12–16, and an unanchored MAIC comparing ADA and SEC at week 52, both in patients with r-axSpA [21, 22]. However, there is limited information available on comparative longer-term efficacy between IL-17 inhibitors, which are considered for similar patient populations according to guidelines [5, 6]. MAIC analyses of longer-term efficacy may help clinicians decide between IL-17 inhibitor treatment options.
Building upon this literature and the previous NMA performed at weeks 12–16 [18], this manuscript presents results from MAIC analyses assessing longer-term therapeutic efficacy of BKZ compared with SEC 150 mg and IXE 80 mg across key outcomes at 52 weeks in phase 3 trials of patients with r-axSpA.
In addition to the licensed SEC 150 mg dose for r-axSpA, recent real-world evidence from Germany indicated that 63% of patients with axSpA who initiated SEC were receiving the 300 mg dose escalation as a maintenance dose [23]. Similarly, evidence from United States claims databases found that 18/91 (19.8%) patients with r-axSpA initiating SEC began treatment with the escalated 300 mg dose, with 16/76 (21.1%) receiving 300 mg every 4 weeks (Q4W) maintenance dosing and 10/76 (13.2%) found to be dose-escalated (i.e., 150 mg to 300 mg) at follow-up [24]. Therefore, the SEC 300 mg Q4W dose was deemed relevant for clinical practice and pertinent for inclusion in the MAIC as an exploratory analysis.
Methods
The MAIC analyses presented here compared longer-term relative efficacy of therapeutic interventions in r-axSpA at 52 weeks. The methodology followed processes previously described by Signorovitch et al. [15], in accordance with the National Institute for Health and Care Excellence Decision Support Unit Technical Document 18 (NICE DSR TSD-18) [25], which can be applied in the following three key steps: clinical trial data selection through systematic literature review (SLR), identification of outcome measures for comparison, and matching of trial populations [15]. For the latter step, IPD from a trial of a given treatment are matched with the baseline summary statistics reported in trial(s) for another treatment, before reweighting to adjust for cross-trial differences [15]. The impact of reweighting is captured by estimating the effective sample size (ESS) of adjusted data compared to the original sample size (OSS). The ESS is calculated as described in NICE DSR TSD-18, and is defined as the number of independent non-weighted individuals that would be required to give an estimate with the same precision as the weighted sample estimate [25]. The MAIC process always reduces the ESS versus the OSS used in the analysis [25].
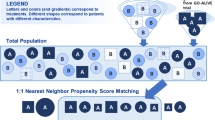
After matching, efficacy outcomes are recalculated for the reweighted trial, enabling comparison of continuous and binary outcomes across balanced trial populations [15]. Specifically, in accordance with NICE DSR TSD-18, the efficacy outcomes of BKZ in the comparator target population are estimated by taking a weighted average of the BKZ outcome in the BKZ trial using the weights estimated by the propensity score logistic regression model, whereby the weight assigned to the ith individual receiving BKZ is equal to the odds of this individual being enrolled in the comparator trial versus the BKZ trial [25]. Figure 1 presents a visual summary of this methodology.
MAIC methodology graphical summary. These MAIC analyses followed processes previously described by Signorovitch et al. [15], in accordance with the NICE DSR TSD-18 [25]. BKZ bimekizumab, IXE ixekizumab, MAIC matching-adjusted indirect comparison, NICE DSR TSD-18 National Institute for Health and Care Excellence Decision Support Unit Technical Document 18, SEC secukinumab
Results are presented as odds ratio (OR) for binary outcomes and as mean difference (MD) for continuous outcomes. All results are presented with 95% confidence intervals (CI) based on robust sandwich estimates of the standard error. All p values were calculated assuming a t-distribution for the treatment effect, with p < 0.05 considered the threshold of statistical significance.
Identification of Trials
Studies reporting 52-week efficacy outcomes for BKZ, SEC, and IXE in phase 3 RCTs in r-axSpA were identified through a published SLR conducted in January 2023, which followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 framework [17, 26]. The identified trials were subsequently assessed for suitability for inclusion in the MAIC analyses (Supplementary Table 1). Trials in patients with AS identified in the SLR were assessed under r-axSpA, due to the interchangeability of AS/r-axSpA terminology [2].
The phase 3 BE MOBILE 2 trial (NCT03928743) was selected for MAIC analyses of BKZ in r-axSpA [13]. Five trials were identified for inclusion in the MAIC analyses with SEC (MEASURE 1, 2, 3, 4, and 5: NCT01358175, NCT01649375, NCT02008916, NCT02159053, and NCT02896127, respectively). The ASTRUM trial with SEC (NCT02763046) was excluded as no week 52 timepoint was reported.
Two trials were identified for inclusion in the MAIC analyses with IXE (COAST-V [NCT02696785] and COAST-W [NCT02696798]). Trial NCT04285229 was excluded as the population consisted of Chinese patients only, and other identified trials had populations of predominantly European descent. Significant variations in disease manifestations of axSpA have been observed between patients in different geographic regions [27], therefore incorporating this trial into the MAIC analyses would introduce heterogeneity, as well as potential for substantial bias, which would be difficult to adjust for.
Selection of Matching Variables
To understand sources of inter-trial heterogeneity, baseline characteristics of patients with r-axSpA (Table 1) were compared between trials, as unanchored MAIC needs to weight for all potential prognostic factors and effect modifiers. Complying with NICE DSU TSD-18 [25], these variables were pre-specified prior to the MAIC analyses and were identified through review of the published unanchored MAIC comparing SEC and ADA at week 52 (Maksymowych et al.) [21], and consensus between clinicians (n = 3; all included as authors). The pre-specified matching variables were: age, baseline Bath Ankylosing Spondylitis Functional Index (BASFI), baseline Ankylosing Spondylitis Disease Activity Score (ASDAS), male (%), previous TNFi exposure (%), body mass index (BMI)/weight, time from diagnosis, time from symptom onset, baseline Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), baseline Patient Global Assessment of Disease Activity (PtGADA), white (%), and sulfasalazine use (%).
Not all included trials had baseline data available for all pre-specified matching variables. Therefore, the matching variables used in the MAIC analyses were the subset of pre-specified variables with available data across the included trials (i.e., BKZ and SEC; BKZ and IXE) and differences at baseline that were expected to have a notable impact on MAIC results, as determined by consultation with the aforementioned clinicians. The final matching variables were: age, male, white, previous TNFi exposure, weight, and baseline ASDAS and BASFI. To adjust for cross-trial differences, patients from BE MOBILE 2 were reweighted to match baseline characteristics in the SEC and IXE trials, respectively, using weights determined by propensity scores. However, although intended, use of the same matching variables for SEC 150 mg, SEC 300 mg, and IXE 80 mg comparisons was not always possible, as baseline ASDAS and BASFI were not reported in the SEC trials. Instead, given ASDAS includes three BASDAI components (BASDAI Q2, Q3, Q6) [28], baseline BASDAI was used as a matching variable in the SEC comparisons, as it is likely to be highly correlated. Baseline characteristics prior to and after adjustment are presented in Supplementary Table 2. Histograms of patient propensity score weights for ASAS40, the primary endpoint in BE MOBILE 2 [13], are provided in Supplementary Fig. 1.
Populations of Interest
Patients in the BE MOBILE 2 trial were adults diagnosed with active axSpA, defined as BASDAI ≥ 4 and spinal pain (BASDAI Q2) ≥ 4, who had r-axSpA fulfilling modified New York (mNY) criteria [14]. All patients in BE MOBILE 2 also fulfilled ASAS criteria [13]. Patients were randomized to receive subcutaneous (SC) BKZ 160 mg Q4W or placebo to week 16; all patients received BKZ 160 mg Q4W from week 16 onwards [13].
For the MAIC analyses with BKZ (MOBILE 2), SEC (MEASURE 1, 2, 3, 4, and 5) and IXE (COAST-V and COAST-W), patients randomized to placebo at baseline were excluded. The majority of BKZ-treated patients in BE MOBILE 2 were TNFi-naïve; 16.7% were TNFi-experienced [13]. Patients in the MEASURE trials were a mixture of TNFi-naïve and TNFi-experienced [29,30,31,32]. COAST-V included TNFi-naïve patients only and COAST-W included TNFi-experienced patients only [33].
Outcomes of Interest
Week 52 outcomes from the BE MOBILE 2 trial of BKZ were compared to available week 52 outcomes from comparator trials. The following pre-specified outcomes were included in the MAIC analyses: ASAS 20% response (ASAS20), ASAS 40% response (ASAS40), ASAS partial remission, BASDAI change from baseline, BASDAI 50% response (BASDAI50), ASDAS < 2.1, BASFI change from baseline, and 36-Item Short Form Survey (SF-36) Physical Component Summary (PCS) score change from baseline.
For binary outcomes (e.g., ASAS20, ASAS40, ASAS partial remission, BASDAI50, ASDAS < 2.1), the MAIC was conducted using non-responder imputation (NRI) in case of missing data. NRI data were derived based on the number of observed responses, or the number of randomized patients for outcomes where the publication reported observed case (OC) data only.
For continuous outcomes (e.g., change from baseline in BASDAI, BASFI, and SF-36 PCS), multiple imputation (MI) was used in the BKZ trial as the primary imputation method and mixed model repeated measures (MMRM) imputation was used in the comparator trials. As it was not possible to use MI data in the MAIC analyses, missing data for continuous outcomes from the BE MOBILE 2 study were imputed using last observation carried forward (LOCF).
Feasible Analyses
A summary of the conducted comparisons and outcomes assessed can be found in Supplementary Table 3. Only feasible analyses of the pre-specified outcomes were conducted; not all MEASURE trials reported all outcomes (details provided in the supplement).
ASAS20, ASAS40, BASDAI change from baseline, and SF-36 PCS change from baseline were the most readily available outcomes across the included comparator trials, therefore these were selected as key outcomes of interest and the primary focus of this study.
Statistical Analyses
Bimekizumab Versus Secukinumab in r-axSpA
In the analysis for BKZ versus SEC 150 mg, IPD from BE MOBILE 2 (BKZ 160 mg Q4W; N = 220) were matched to pooled SEC 150 mg Q4W with loading dose (LD; intravenous [IV]: 10 mg/kg body weight at weeks 0, 2, and 4 or SC: 75 mg or 150 mg at weeks 0, 1, 2, and 3) and no loading dose (NL) summary data from MEASURE 1, 2, 3, and 4 for pairwise comparisons (N = 504). Details on the secondary analysis, with the addition of MEASURE 5 for pairwise comparisons, are provided in the supplement.
Exploratory comparative analyses were also performed for the SEC 300 mg dose escalation, which is licensed for maintenance dosing without IV loading for patients with an inadequate response to the SEC 150 mg dose [7]. IPD from BE MOBILE 2 were matched with SEC 300 mg summary data from MEASURE 3, which had a different dosing regimen to the licensed dose escalation, involving IV loading of SEC before switching to SC administration at week 4.
Bimekizumab Versus Ixekizumab in r-axSpA
For the analyses of BKZ versus IXE, IPD from BE MOBILE 2 were matched for pairwise comparisons to summary data from patients randomized to IXE 80 mg Q4W (LD: 80 mg or 160 mg at week 0) in COAST-V only (i.e., TNFi-naïve patients). The MAIC analyses with COAST-V only were prioritized over analyses with COAST-W only (i.e., TNFi-experienced patients) as the patient population was more similar to BE MOBILE 2 in terms of previous TNFi exposure (TNFi-naïve: 83.3% and 100% in BE MOBILE 2 and COAST-V, respectively). In a separate analysis, IPD from patients randomized to BKZ in BE MOBILE 2 were matched with summary data from patients randomized to IXE 80 mg Q4W (LD: 80 mg or 160 mg at week 0) in COAST-V and COAST-W for pairwise comparisons (N = 195). Although of interest, comparisons between BKZ and IXE 80 mg in TNFi-experienced populations were not possible due to the small number of TNFi-experienced patients in the BE MOBILE 2 trial.
Ethical Approval
This study was non-interventional and based on published secondary data from randomized controlled trials, therefore ethical/institutional review and approval were not required. All included trials obtained informed consent from participants and were conducted in accordance with the Declaration of Helsinki.
Results
Bimekizumab Versus Secukinumab
Bimekizumab 160 mg Versus Secukinumab 150 mg
In the analysis of BKZ 160 mg versus SEC 150 mg for the MEASURE 1–4 trials, the post-matching effective sample size (ESS) for BKZ was 177 (80.5% of original sample size [OSS; i.e., 220]) for ASAS20 and ASAS40, 181 (82.3% of OSS) for BASDAI change from baseline, and 183 (83.2% of OSS) for SF-36 PCS change from baseline. For other outcomes analyzed, the ESS for BKZ was 147 (66.8% of OSS) for ASAS partial remission and 128 (58.2% of OSS) for BASFI change from baseline. As not all MEASURE trials reported BASDAI change from baseline, SF-36 PCS change from baseline, ASAS partial remission, and BASFI change from baseline (Supplementary Table 3), the number of SEC-treated patients with available data for a specific outcome varied, leading to differences in total pre-matching sample size, and subsequently the post-matching ESS.
A summary of the MAIC results for BKZ compared with SEC 150 mg (with or without LD) is provided in Table 2. BKZ-treated patients were significantly more likely to achieve ASAS40 than those treated with SEC 150 mg at week 52 (OR [95% CI]: 1.48 [1.05, 2.10]; p = 0.026; Fig. 2). Patients receiving BKZ also had significantly higher likelihood of achieving greater reductions from baseline in BASDAI and greater increases from baseline in SF-36 PCS than with SEC 150 mg (BASDAI change from baseline MD [95% CI]: − 0.49 [− 0.89, − 0.10]; p = 0.014; SF-36 PCS change from baseline MD: 3.06 [1.53, 4.59]; p < 0.001). Differences observed between BKZ and SEC 150 mg were non-significant for all other outcomes analyzed (Table 2; Fig. 2).
Key outcomes of interest for BKZ versus SEC and BKZ versus IXE. *Statistically significant difference. ASAS20/40 Assessment of SpondyloArthritis international Society 20%/40% response, BASDAI Bath Ankylosing Spondylitis Functional Index, BKZ bimekizumab, CfB change from baseline, CI confidence interval, IXE ixekizumab, MD mean difference, OR odds ratio, PCS Physical Component Summary, SEC secukinumab, SF-36 36-Item Short Form Survey
Results from the secondary analysis with BKZ versus SEC 150 mg, including MEASURE 5, are provided in the supplement (Supplementary Table 4; Supplementary Fig. 2).
Bimekizumab 160 mg Versus Secukinumab 300 mg
For the exploratory MAIC analyses of BKZ compared with SEC 300 mg, the post-matching ESS was 120 (54.5% of OSS). A summary of the MAIC results is provided in Table 2. Differences observed for all outcomes analyzed were non-significant (Table 2; Fig. 2).
Bimekizumab Versus Ixekizumab
All pre-specified outcomes were reported by the COAST trials. In the analysis of BKZ 160 mg versus IXE 80 mg with COAST-V only (i.e., TNFi-naïve patients), the post-matching ESS for BKZ was 84 (38.2% of OSS). MAIC results are summarized in Table 3. There was a significantly higher likelihood of achieving greater increases from baseline in SF-36 PCS with BKZ compared with IXE (MD: 3.31 [0.50, 6.12]; p = 0.021; Fig. 2), however, differences between BKZ and IXE for all remaining analyzed outcomes were non-significant.
In the separate analyses of BKZ 160 mg versus IXE 80 mg with COAST-V and COAST-W, the post-matching ESS for BKZ was 45 (20.5% of OSS). A summary of the MAIC results is provided in Table 3. These analyses suggested that BKZ-treated patients may have a significantly higher likelihood of achieving ASAS20 and ASAS40 at week 52 compared with IXE (ASAS20: OR [95% CI]: 2.06 [1.07, 3.96]; p = 0.030; ASAS40: 2.02 [1.05, 3.89]; p = 0.036; Fig. 2). BKZ-treated patients may also have a significantly higher likelihood of achieving greater reductions from baseline in BASDAI and greater increases from baseline in SF-36 PCS compared with IXE-treated patients (BASDAI change from baseline: MD [95% CI]: − 0.75 [− 1.48, − 0.01]; p = 0.046; SF-36 PCS: 3.88 [1.02, 6.73]; p = 0.008; Fig. 2). Of other outcomes analyzed, evidence suggested that BKZ-treated patients may be significantly more likely to achieve BASDAI50 and have greater reductions from baseline in BASFI compared with IXE, however differences between treatments for ASAS partial remission and ASDAS < 2.1 were non-significant.
Discussion
The MAIC results at week 52 demonstrated that patients with r-axSpA treated with BKZ 160 mg may have a higher likelihood of achieving improved longer-term efficacy compared with SEC 150 mg (MEASURE 1/2/3/4), while differences in longer-term efficacy compared with SEC 300 mg were non-significant (MEASURE 3; exploratory analyses). Relative efficacy of BKZ compared with IXE 80 mg was dependent on the populations considered. Comparisons between BKZ and the COAST-V trial of TNFi-naïve patients suggested that differences between BKZ and IXE 80 mg were non-significant for most key efficacy outcomes. However, comparisons with the mixed TNFi-naïve/experienced population from COAST-V/-W suggested that patients treated with BKZ may have a higher likelihood of achieving improved longer-term efficacy versus IXE 80 mg for many of the same efficacy outcomes.
The analyses of BKZ versus SEC 150 mg (MEASURE 1/2/3/4) provide evidence that patients with r-axSpA treated with BKZ may have a significantly greater likelihood of achieving longer-term ASAS40 response, greater reductions from baseline in BASDAI, and greater increases from baseline in SF-36 PCS. These MAIC results at week 52 are consistent with the NMA analyses at weeks 12–16, in which BKZ achieved higher response rates compared with SEC 150 mg across a range of outcomes in patients with r-axSpA, and provide further evidence supporting the use of BKZ as an effective treatment option in patients with axSpA [17, 18]. However, in the separate analyses with BKZ versus SEC 150 mg including MEASURE 5, significant differences were only observed in SF-36 PCS, with non-significant differences in longer-term efficacy for all other analyzed outcomes. This shows that the MAIC analyses were sensitive to included patient populations, and the heterogeneity introduced by including MEASURE 5, which mainly comprised Asian patients, impacted the results. Although there were differences in LD administration route (i.e., IV versus SC) between MEASURE trials, the impact on week 52 outcomes was expected to be negligible and therefore pooling of SEC 150 mg data was considered appropriate.
The exploratory MAIC analyses comparing BKZ with the escalated SEC 300 mg dose suggested that differences in the likelihood of achieving ASAS20, ASAS40, and reductions from baseline in BASDAI at week 52 with BKZ were non-significant. However, the relative efficacy of SEC 300 mg may be overestimated due to use of additional unlicensed IV loading in MEASURE 3, before switching to SC administration at week 8 [31]. This differs from the SC-only SEC 300 mg escalated dosing, approved without LD for use in patients with an inadequate response to the 150 mg dose [7].
The analyses with BKZ versus IXE 80 mg from COAST-V only (i.e., TNFi-naïve patients) suggested that patients treated with BKZ may have a significantly higher likelihood of achieving greater increases from baseline in SF-36 PCS, however differences observed in 52-week efficacy for all other analyzed outcomes were non-significant. These findings are consistent with those from the NMA at weeks 12–16, in which BKZ demonstrated similar relative efficacy versus IXE 80 mg across ASAS outcomes [17, 18]. In contrast, analyses comparing BKZ with IXE 80 mg (COAST-V/-W) suggested that patients treated with BKZ may have a significantly higher likelihood of 52-week efficacy across ASAS20, ASAS40, BASDAI change from baseline and SF-36 PCS change from baseline. These differences between analyses suggest that the MAIC results were also sensitive to included patient populations. Patients in COAST-W were TNFi-experienced and had lower response rates compared with TNFi-naïve patients enrolled in COAST-V [34]. Therefore, the fewer (16.7%) BKZ-treated TNFi-experienced patients had disproportionate weight in the COAST-V/-W analyses, which may have strengthened the outcomes for BKZ, given that these patients responded well to BKZ treatment in BE MOBILE 2 and did so with similar efficacy to TNFi-naïve patients [13, 14]. This is also evident from the low ESS for BKZ following reweighting in these IXE MAIC analyses (ESS = 45; 20.5% of OSS [COAST-V/-W]), which indicates limited overlap between trial populations.
Treatment guidelines for axSpA do not currently distinguish between IL-17 inhibitors in their recommendations, due to lack of evidence on comparative efficacy [5]. Therefore, by providing one type of comparative efficacy data in the absence of head-to-head trials, these MAIC analyses could contribute initial evidence to help address this data gap. However, these data would need to be interpreted in the context of other comparative efficacy data, as indirect comparisons are subject to uncertainty. A similar approach was applied in psoriatic arthritis, where different types of indirect comparison methodology, including MAIC, have been used to assess comparative effectiveness of TNFis where no head-to-head data were available [35].
There were some key limitations to the MAIC analyses reported here, both intrinsic to the methodology and specific to these comparisons. Matching was limited to characteristics reported by the MEASURE/COAST trials and collected in BE MOBILE 2 (a subset of pre-specified matching variables), resulting in the use of fewer matching variables compared with the unanchored MAIC comparing SEC and ADA at week 52 [21]. For example, baseline symptom duration was intended to be a matching variable, however, these data were not consistently available across comparator trials to enable matching. Therefore, it is possible that patients in one trial may have had a longer average symptom duration, potentially indicative of more established baseline structural damage, than patients in another trial, which could have affected observed efficacy outcomes. Furthermore, imputation methods for continuous outcome data varied in the MAIC analyses due to differences between the BKZ trial and comparator trial methodology, which may have affected outcome data. However, given the low discontinuation rates of patients in long-term r-axSpA trials [14, 30, 31, 33, 36, 37], the difference in imputation methods (LOCF versus MRMM) was expected to have a minor impact on results.
Additionally, ESSs were non-negligibly reduced for some comparisons, linked to heterogeneity between populations across trials, which led to wider CIs and potentially reduced accuracy of estimates. This is particularly evident in the MAIC analyses with IXE, where the ESS for BKZ following reweighting was notably reduced (ESS = 84 [COAST-V only]; ESS = 45 [COAST-V/-W]), indicating limited overlap between trial populations. As the MAICs were unanchored (i.e., not placebo-adjusted) due to lack of placebo arm through week 52 in the included trials, the level of bias in the indirect comparison could be substantial and may exceed the magnitude of treatment effects being estimated [25]. Similarly, the impact of unlicensed IV loading of SEC 300 mg in MEASURE 3 on comparisons could not be determined, however, it is likely that the BKZ versus SEC 300 mg analyses overestimate the relative efficacy of the escalated SEC 300 mg dose.
Finally, although MAIC analyses comparing BKZ and IL-17A inhibitors in patients with nr-axSpA at week 52 were of interest and feasibility was explored, these comparisons were not possible. Fundamental differences in nr-axSpA trial designs (e.g., length of placebo-controlled periods, length of blinding and availability of treatment-switcher data) meant that any attempt to compare BKZ (BE MOBILE 1) with SEC (PREVENT: NCT02696031) and IXE (COAST-X: NCT02757352), respectively, would introduce significant bias and prevent scientifically robust MAIC analyses [13, 38, 39].
Conclusions
In conclusion, MAIC demonstrated that patients with r-axSpA may have a higher likelihood of improved longer-term efficacy with BKZ versus SEC 150 mg, suggesting that BKZ may provide a favorable therapeutic option for r-axSpA. Comparisons with BKZ and IXE 80 mg (COAST-V only) suggested that differences in longer-term efficacy were non-significant for most key clinical efficacy outcomes, however, relative efficacy of BKZ versus IXE 80 mg was dependent on the populations considered, with other IXE comparisons (COAST-V/-W) suggesting that BKZ may have higher comparative efficacy for many of the same outcomes. Although significantly lower responses with BKZ compared with SEC and IXE were not detected, analyses were sensitive to heterogeneity in patient populations and limited by potential bias from unreported effect modifiers and prognostic factors, as well as reduced overlap in IXE trial populations.
Data Availability
Data from the bimekizumab clinical trial used in this analysis may be requested by qualified researchers 6 months after product approval in the US and/or Europe, or if global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymized individual patient data and redacted study documents which may include: raw datasets, analysis-ready datasets, study protocol, blank case report form, annotated case report form, statistical analysis plan, dataset specifications, and clinical study report. Prior to the use of the data, proposals need to be approved by an independent review panel at www.Vivli.org and a signed data-sharing agreement will need to be executed. All documents are available in English only, for a pre-specified time, typically 12 months, on a password-protected portal.
References
Navarro-Compan V, Sepriano A, El-Zorkany B, et al. Axial spondyloarthritis. Ann Rheum Dis. 2021;80(12):1511–21. https://doi.org/10.1136/annrheumdis-2021-221035.
Boel A, Molto A, van der Heijde D, et al. Do patients with axial spondyloarthritis with radiographic sacroiliitis fulfil both the modified New York criteria and the ASAS axial spondyloarthritis criteria? Results from eight cohorts. Ann Rheum Dis. 2019;78(11):1545–9. https://doi.org/10.1136/annrheumdis-2019-215707.
Strand V, Singh JA. Patient burden of axial spondyloarthritis. J Clin Rheumatol. 2017;23(7):383–91. https://doi.org/10.1097/rhu.0000000000000589.
Kroon FPB, van der Burg LRA, Ramiro S, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev. 2015;2015(7):CD010952. https://doi.org/10.1002/14651858.CD010952.pub2.
Ramiro S, Elena N, Alexandre S, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023;82(1):19–34. https://doi.org/10.1136/ard-2022-223296.
Ward MM, Deodhar A, Gensler LS, et al. 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019;71(10):1599–613. https://doi.org/10.1002/art.41042.
European Medicines Agency (EMA). Secukinumab Summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product-information/cosentyx-epar-product-information_en.pdf. Accessed Oct 2023.
European Medicines Agency (EMA). Bimekizumab Summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product-information/bimzelx-epar-product-information_en.pdf. Accessed Feb 2024.
European Medicines Agency (EMA). Ixekizumab Summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product-information/taltz-epar-product-information_en.pdf. Accessed Feb 2024.
van der Heijde D, Gensler LS, Deodhar A, et al. Dual neutralisation of interleukin-17A and interleukin-17F with bimekizumab in patients with active ankylosing spondylitis: results from a 48-week phase IIb, randomised, double-blind, placebo-controlled, dose-ranging study. Ann Rheum Dis. 2020;79(5):595–604. https://doi.org/10.1136/annrheumdis-2020-216980.
Baraliakos X, Deodhar A, Dougados M, et al. Safety and efficacy of bimekizumab in patients with active ankylosing spondylitis: three-year results from a phase IIb randomized controlled trial and its open-label extension study. Arthritis Rheumatol. 2022;74(12):1943–58. https://doi.org/10.1002/art.42282.
Deodhar A, Navarro-Compán V, Poddubnyy D, et al. Long-term safety and efficacy of bimekizumab in patients with active ankylosing spondylitis: 5-year results from a phase 2b study and its open-label extension. Arthritis Rheumatol. 2023;75(suppl 9).
van der Heijde D, Deodhar A, Baraliakos X, et al. Efficacy and safety of bimekizumab in axial spondyloarthritis: results of two parallel phase 3 randomised controlled trials. Ann Rheum Dis. 2023;82(4):515–26. https://doi.org/10.1136/ard-2022-223595.
Baraliakos X, Deodhar A, van der Heijde D, et al. Bimekizumab treatment in patients with active axial spondyloarthritis: 52-week efficacy and safety from the randomised parallel phase 3 BE MOBILE 1 and BE MOBILE 2 studies. Ann Rheum Dis. 2024;83(2):199–213. https://doi.org/10.1136/ard-2023-224803.
Signorovitch JE, Sikirica V, Erder MH, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15(6):940–7. https://doi.org/10.1016/j.jval.2012.05.004.
Dias S, Ades AE, Welton NJ, Jansen JP, Sutton AJ. Network meta-analysis for decision-making. Wiley. 2018.
Deodhar A, Machado PM, Mørup M, et al. Comparative efficacy and safety of bimekizumab in axial spondyloarthritis: a systematic literature review and network meta-analysis. Rheumatology. 2023;63(5):1195–205. https://doi.org/10.1093/rheumatology/kead598.
Deodhar A, Machado PM, Mørup M, et al. Comparative efficacy of bimekizumab in biologic/targeted synthetic DMARD-Naive patients with axial spondyloarthritis: results from a systematic literature review and network meta-analysis. Value Health. 2023;26(6):S406. https://doi.org/10.1016/j.jval.2023.03.2266. (poster number SA50).
Thom H, Leahy J, Jansen JP. Network meta-analysis on disconnected evidence networks when only aggregate data are available: modified methods to include disconnected trials and single-arm studies while minimizing bias. Med Decis Mak. 2022;42(7):906–22. https://doi.org/10.1177/0272989x221097081.
Hatswell AJ, Freemantle N, Baio G. The effects of model misspecification in unanchored matching-adjusted indirect comparison: results of a simulation study. Value Health. 2020;23(6):751–9. https://doi.org/10.1016/j.jval.2020.02.008.
Maksymowych WP, Strand V, Nash P, et al. Comparative effectiveness of secukinumab and adalimumab in ankylosing spondylitis as assessed by matching-adjusted indirect comparison. Eur J Rheumatol. 2018;5(4):216–23. https://doi.org/10.5152/eurjrheum.2018.18162.
Betts KA, Mittal M, Song J, et al. OP0115 relative efficacy of adalimumab versus secukinumab in active ankylosing spondylitis: a matching-adjusted indirect comparison. Ann Rheum Dis. 2016;75(Suppl 2):98–9. https://doi.org/10.1136/annrheumdis-2016-eular.2754.
Anjohrin S, Song J, Abé C, et al. HSD34 real-world usage of biologic disease-modifying anti-rheumatic drugs in patients with axial spondyloarthritis in Germany. Value in Health. 2023;26(12):S300. https://doi.org/10.1016/j.jval.2023.09.1585.
Dubreuil M, Walsh JA, Deodhar A, et al. RWD136 real-world use of biologic disease-modifying anti-rheumatic drugs in US patients with ankylosing spondylitis: persistence, factors associated with discontinuation, and dosing patterns. Value in Health. 2023;26(6):S386–7. https://doi.org/10.1016/j.jval.2023.03.2165.
Phillippo D, Ades AE, Dias S, Palmer S, Abrams KR, Welton N. NICE DSU Technical Support Document 18: methods for population-adjusted indirect comparisons in submissions to NICE (Technical Support Documents). NICE Decision Support Unit. 2016.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71.
Poddubnyy D, Sieper J, Akar S, et al. Characteristics of patients with axial spondyloarthritis by geographic regions: PROOF multicountry observational study baseline results. Rheumatology. 2022;61(8):3299–308. https://doi.org/10.1093/rheumatology/keab901.
van der Heijde D, Lie E, Kvien TK, et al. ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Ann Rheum Dis. 2009;68(12):1811–8. https://doi.org/10.1136/ard.2008.100826.
Baeten D, Sieper J, Braun J, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med. 2015;373(26):2534–48. https://doi.org/10.1056/NEJMoa1505066.
Kivitz AJ, Wagner U, Dokoupilova E, et al. Efficacy and safety of secukinumab 150 mg with and without loading regimen in ankylosing spondylitis: 104-week results from MEASURE 4 study. Rheumatol Ther. 2018;5(2):447–62. https://doi.org/10.1007/s40744-018-0123-5.
Pavelka K, Kivitz A, Dokoupilova E, et al. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res Ther. 2017;19(1):285. https://doi.org/10.1186/s13075-017-1490-y.
Huang F, Sun F, Wan WG, et al. Secukinumab provided significant and sustained improvement in the signs and symptoms of ankylosing spondylitis: results from the 52-week, phase III China-centric study, MEASURE 5. Chin Med J (Engl). 2020;133(21):2521–31. https://doi.org/10.1097/cm9.0000000000001099.
Dougados M, Wei JC, Landewé R, et al. Efficacy and safety of ixekizumab through 52 weeks in two phase 3, randomised, controlled clinical trials in patients with active radiographic axial spondyloarthritis (COAST-V and COAST-W). Ann Rheum Dis. 2020;79(2):176–85. https://doi.org/10.1136/annrheumdis-2019-216118.
Deodhar AA, Mease PJ, Rahman P, et al. Ixekizumab improves spinal pain, function, fatigue, stiffness, and sleep in radiographic axial spondyloarthritis: COAST-V/W 52-week results. BMC Rheumatol. 2021;5(1):35. https://doi.org/10.1186/s41927-021-00205-3.
Kirson NY, Rao S, Birnbaum HG, et al. Matching-adjusted indirect comparison of adalimumab vs etanercept and infliximab for the treatment of psoriatic arthritis. J Med Econ. 2013;16(4):479–89. https://doi.org/10.3111/13696998.2013.768530.
Braun J, Baraliakos X, Deodhar A, et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis. 2017;76(6):1070–7. https://doi.org/10.1136/annrheumdis-2016-209730.
Marzo-Ortega H, Sieper J, Kivitz A, et al. Secukinumab and sustained improvement in signs and symptoms of patients with active ankylosing spondylitis through two years: results from a phase III study. Arthritis Care Res (Hoboken). 2017;69(7):1020–9. https://doi.org/10.1002/acr.23233.
Deodhar A, van der Heijde D, Gensler LS, et al. Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial. Lancet. 2020;395(10217):53–64. https://doi.org/10.1016/s0140-6736(19)32971-x.
Deodhar A, Blanco R, Dokoupilová E, et al. Improvement of signs and symptoms of nonradiographic axial spondyloarthritis in patients treated with secukinumab: primary results of a randomized, placebo-controlled phase III study. Arthritis Rheumatol. 2021;73(1):110–20. https://doi.org/10.1002/art.41477.
Acknowledgements
The authors thank the patients, the investigators, and their teams who took part in these studies. The authors also acknowledge Celia Menckeberg, PhD, UCB Pharma, Breda, The Netherlands, for publication coordination. This study was funded by UCB Pharma.
Medical Writing/Editorial Assistance
The authors acknowledge Evelyn Turner, BSc and James Evry, MSc, Costello Medical, Cambridge, UK, for medical writing and editorial assistance based on the authors’ input and direction. Medical writing and editorial assistance were funded by UCB Pharma.
Funding
This study and the journal’s Rapid Service Fee were funded by UCB Pharma. This article was based on published trials, including one that was sponsored by UCB Pharma. Support for third-party writing assistance for this article, provided by Evelyn Turner, BSc and James Evry, MSc, Costello Medical, Cambridge, UK, was funded by UCB Pharma in accordance with Good Publication Practice (GPP 2022) guidelines (https://www.ismpp.org/gpp-2022).
Author information
Authors and Affiliations
Contributions
Substantial contributions to study conception and design: Walter P Maksymowych, Howard Thom, Michael F Mørup, Vanessa Taieb, Damon Willems, Nikos Lyris, Karl Gaffney; substantial contributions to analysis and interpretation of the data: Walter P Maksymowych, Howard Thom, Michael F Mørup, Vanessa Taieb, Damon Willems, Nikos Lyris, Karl Gaffney; drafting the article or reviewing it critically for important intellectual content: Walter P Maksymowych, Howard Thom, Michael F Mørup, Vanessa Taieb, Damon Willems, Nikos Lyris, Karl Gaffney; final approval of the version of the article to be published: Walter P Maksymowych, Howard Thom, Michael F Mørup, Vanessa Taieb, Damon Willems, Nikos Lyris, Karl Gaffney.
Corresponding author
Ethics declarations
Conflict of Interest
Walter P Maksymowych: Honoraria/consulting fees from AbbVie, BMS, Boehringer-Ingelheim, Celgene, Eli Lilly, Galapagos, Janssen, Novartis, Pfizer, and UCB Pharma; research grants from AbbVie, Galapagos, Pfizer, and UCB Pharma; educational grants from AbbVie, Janssen, Novartis, and Pfizer; Chief Medical Officer for CARE ARTHRITIS; Howard Thom: Owns shares in Clifton Insight which has received consulting fees from Argenx, Bayer, BMS, Daiichi-Sankyo, Eisai, Lundbeck, Novartis, Pfizer, Roche, and UCB Pharma; Michael F Mørup: Employee of UCB Pharma; Vanessa Taieb, Damon Willems, Nikos Lyris: Employee of UCB Pharma; shareholder of UCB Pharma; Karl Gaffney: Speakers bureau for AbbVie, Eli Lilly, Novartis, and UCB Pharma; consultant of AbbVie, Eli Lilly, Novartis, and UCB Pharma; Grant/research support from AbbVie, Gilead, Eli Lilly, Novartis, and UCB Pharma.
Ethical Approval
This study was non-interventional and based on published secondary data from randomized controlled trials, therefore ethical/institutional review and approval were not required. All included trials obtained informed consent from participants and were conducted in accordance with the Declaration of Helsinki.
Consent for Publication
All the results presented in this article are in aggregate form, and no personally identifiable information was used for this study.
Additional information
Prior presentation: This manuscript expands on work previously presented in poster format at ISPOR-EU 2023 (12–15 November, Copenhagen, Denmark).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Maksymowych, W.P., Thom, H., Mørup, M.F. et al. Matching-Adjusted Indirect Comparison of the 52-Week Efficacy of Bimekizumab Versus Secukinumab and Ixekizumab for the Treatment of Radiographic Axial Spondyloarthritis. Rheumatol Ther (2024). https://doi.org/10.1007/s40744-024-00684-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40744-024-00684-z