Abstract
Introduction
To evaluate the efficacy and safety of secukinumab 150 mg, with or without a loading regimen, using a self-administered prefilled syringe in patients with ankylosing spondylitis (AS) over 104 weeks from the MEASURE 4 study.
Methods
Patients (N = 350) with active AS were randomized (1:1:1) to receive subcutaneous secukinumab 150 mg with loading dose (150 mg), without loading dose (150 mg no load), or placebo. All patients received secukinumab or placebo at baseline, weeks 1, 2, and 3 and every 4 weeks starting at week 4. The primary endpoint was the Assessment of SpondyloArthritis international Society criteria for 20% improvement (ASAS20) at week 16.
Results
A total of 96.9% of patients (339/350) completed 16 weeks and 82.6% (289/350) completed 104 weeks of treatment. The ASAS20 response rate at week 16 was 59.5% and 61.5% with 150 and 150 mg no load groups, respectively, versus placebo (47%; P = 0.057 and 0.054, respectively); the primary endpoint was not met. Increases in response rates achieved with secukinumab for ASAS20 at week 16 were sustained through week 104. The safety profile of secukinumab 150 mg, with or without a loading regimen, showed no new or unexpected safety signals.
Conclusions
Secukinumab 150 mg, with or without loading regimen, provided rapid and sustained decreases in the signs and symptoms of patients with AS, but the differences were not statistically significant at week 16 due to higher than expected placebo responses. The responses and safety profile were consistent with previous phase 3 studies and sustained through 2 years.
Trial registration
ClinicalTrials.gov identifier, NCT02159053.
Funding
Novartis Pharma AG, Basel, Switzerland.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ankylosing spondylitis (AS), a chronic inflammatory disease belonging to the spondyloarthritis family, is characterized by involvement of the axial skeleton and sacroiliac joints, but also affects peripheral joints, entheses, and extra-articular organ systems [1,2,3]. AS-associated inflammatory back pain and stiffness lead to functional impairments and reduced quality of life (QoL) [1]. Conventional therapies according to the Assessment of SpondyloArthritis International Society (ASAS) and the European League Against Rheumatism (EULAR), include nonsteroidal anti-inflammatory drugs (NSAIDs) and disease-modifying anti-rheumatic drugs (DMARDs); however, these are often reported to be inefficacious in treating AS-associated symptoms [4]. Biologics, such as tumor necrosis factor-alpha inhibitors (TNFi) and interleukin-17A (IL-17A) inhibitors, have been shown to be effective in controlling AS-associated symptoms and are recommended by ASAS and EULAR for the management of AS [5].
Secukinumab, a fully human monoclonal IgG1κ antibody to IL-17A, has shown significant reductions in the signs and symptoms of AS in the two pivotal phase 3 studies, MEASURE 1 and MEASURE 2 [6]. In these studies, subcutaneous (s.c.) secukinumab 150 mg (approved) and 75 mg doses, following either intravenous (i.v.) or s.c. loading regimens, demonstrated sustained efficacy and safety over 3 years [6,7,8,9,10]. MEASURE 4 is the first phase 3 study evaluating self-administered s.c. secukinumab 150 mg, with or without a loading regimen, followed by maintenance dosing, using pre-filled syringe in patients with active AS. Herein, we present the efficacy and safety results of s.c. secukinumab 150 mg over 104 weeks (2-year) of treatment from the MEASURE 4 study.
Methods
Patients
Patients ≥ 18 years of age, with active AS with prior documented radiological evidence (X-ray) fulfilling the modified New York criteria for AS were enrolled in the study [11]. Other inclusion criteria included a score of 4 or higher on the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) [12] and a score for spinal pain of 4 cm or more on a 10-cm visual analog scale (VAS), despite treatment with the maximum tolerated doses of NSAIDs. Patients on scheduled NSAIDs were required to be on a stable dose for at least 2 weeks before randomization and had to refrain from any NSAID intake for at least 24 h before a visit with disease activity assessment. After the week 20 assessment, changes in NSAID dose were permitted. Previous use of DMARDs was allowed; a washout period for DMARDs, other than sulfasalazine and methotrexate, was required before initiation of the study treatment. Patients previously treated with not more than one TNFi could participate if they had an inadequate response to an approved dosage for ≥ 3 months or were intolerant to at least one dose (hereafter collectively referred to as patients with an inadequate response to TNFi [TNFi-IR]). Patients could continue to receive the following medications at a stable dose: sulfasalazine (≤ 3 g per day), methotrexate (7.5–25 mg per week), prednisone or equivalent (≤ 10 mg per day), and NSAIDs. Key exclusion criteria were total spinal ankylosis, evidence of infection or cancer on chest radiography, active systemic infection within 2 weeks before baseline, history of ongoing, chronic, or recurrent infectious disease or evidence of tuberculosis infection, and previous treatment with cell-depleting therapies or biologic agents other than TNFi.
MEASURE 4 (NCT02159053) was conducted in accordance with the Declaration of Helsinki [13] and was approved by institutional review boards or independent ethics committees at each participating center. Written informed consent was obtained from all enrolled patients.
Study Design
MEASURE 4 is a multicenter, randomized, double-blind, placebo-controlled, parallel-group, 2-year study (104 weeks), conducted at 85 centers in 19 countries (Australia, Austria, Bulgaria, Canada, the Czech Republic, Denmark, Finland, Germany, Greece, Italy, Netherlands, Norway, Poland, Russian Federation, Slovakia, Spain, Switzerland, the United Kingdom, and the United States).
After a 10-week initial screening period, eligible patients were randomly assigned (1:1:1) by means of an Interactive Response Technology to one of three treatment groups: s.c. secukinumab 150 mg with loading dose (secukinumab 150 mg), s.c. secukinumab 150 mg without loading dose (secukinumab 150 mg no load), or placebo (Fig. S1 in Supplementary Appendix). All patients received s.c. secukinumab 150 mg or placebo at baseline and weeks 1, 2, 3, and every 4 weeks (q4w) starting at week 4. At week 16, all placebo patients were switched to s.c. secukinumab 150 mg q4w. Thus, starting at week 16, patients in all three arms received secukinumab 150 mg q4w in an open-label fashion, although study participants and investigators remained blinded to the original group assignment. Randomization of patients was stratified according to previous use of TNFi therapy (i.e., patients who were naïve to TNFi therapy [TNFi-naïve] versus those who were TNFi-IR). The study was planned to enroll no more than 40% TNFi-IR patients.
Data were collected in accordance with Good Clinical Practice guidelines by the study investigators and were analyzed by the sponsor. Data presented here, from the primary analysis at week 16 to end of study analysis at week 104 (2-year), were collected from May 18, 2015 (first patient first visit) to Jan 02, 2018 (last patient last visit).
Efficacy Outcomes
The primary endpoint was to demonstrate that the efficacy of secukinumab 150 mg, with or without a loading regimen, was superior to placebo based on the proportion of patients achieving an ASAS20 response at week 16. ASAS20 is defined as a relative improvement of ≥ 20% and an absolute improvement of ≥ 1 unit (on a 10-unit scale) in at least three of the four main ASAS domains (patient global assessment of disease activity, back pain, physical function, and inflammation), with no worsening of ≥ 20% and ≥ 1 unit (on a 10-unit scale) in the remaining domain [14].
Secondary endpoints assessed as part of the pre-specified hierarchical hypothesis testing strategy at week 16 included the following: (a) ASAS40 response criteria (improvement of ≥ 40% and absolute improvement of ≥ 2 units [on a 10-unit scale] in at least three of the four main ASAS domains, with no worsening in the remaining domain), (b) change from baseline in high-sensitivity C-reactive protein (hsCRP) levels, (c) ASAS5/6 response (≥ 20% improvement in five of the six ASAS response domains: four main ASAS domains, hsCRP, and lateral spinal mobility), (d) change from baseline in total BASDAI (questions on a 0–10 scale captured as a continuous VAS, pertaining to the five major symptoms of AS: fatigue, spinal pain, joint pain/swelling, areas of localized tenderness [enthesitis or inflammation of tendons and ligaments], and morning stiffness duration and severity), (e) change from baseline in Short Form-36 Physical Component Summary (SF-36 PCS; scores range from 0 [maximum disability] to 100 [no disability] for individual domains, with a normative composite summary score of 50), (f) the score on the ASQoL scale (scores range from 0 [best quality] to 18 [poorest quality]), and (g) the proportion of patients achieving ASAS20 and ASAS40 responses at week 4 [14,15,16,17]. All endpoints were assessed through week 104.
Pre-specified subgroup analyses based on previous use of TNFi therapy were performed for key efficacy endpoints. Interactions between treatment and baseline demographics or disease characteristics were also analyzed for ASAS20 response at week 16 to check if treatment effect was influenced by any of the baseline demographics or disease characteristics including age, gender, race, weight, hsCRP, erythrocyte sedimentation rate (ESR), human leukocyte antigen (HLA) B-27, TNFi-IR, time since first diagnosis of AS, methotrexate and sulfasalazine use at randomization, patient’s global assessment of disease activity, and total back pain. Additionally, the treatment effect of secukinumab versus placebo in three separate regions: (1) Western Europe, (2) Eastern Europe, and (3) North America and Australia were analyzed for all primary and secondary endpoints at week 16.
Safety
The overall safety and tolerability of secukinumab 150 mg compared with placebo was assessed by adverse events (AEs), serious AEs (SAEs), vital signs, and clinical laboratory value monitoring. Safety data during the entire treatment period (from baseline through to the week 104 visit of each patient) are presented in the two secukinumab treatment groups, and in the Any secukinumab 150 mg group that included all patients who received a dose of secukinumab (i.e., those originally randomized to secukinumab 150 mg [with and without load] and those who switched from placebo to secukinumab 150 mg at week 16).
Statistical Analysis
The sample size for MEASURE 4 was calculated to have 99% and 97% power for secukinumab 150 mg and 150 mg no load, respectively, versus placebo with a 2.5% type-I error rate two-sided for each comparison between secukinumab and placebo using the Fisher’s exact test. The ASAS20 response rate (primary endpoint) was assumed to be 61% for the secukinumab 150 mg and 56% for the secukinumab 150 mg no load groups, both compared with placebo (27%) at week 16. Based on these assumptions, at least 108 patients were needed in each study group to achieve 99% and 97% power for the secukinumab 150 mg and 150 mg no load groups, respectively.
Analyses of primary and secondary efficacy endpoints at week 16 included all patients according to the treatment assigned at randomization. Closed testing procedures were used to maintain a family-wise error rate of 5% across the secukinumab groups and endpoints. The hypotheses for the primary objective in either secukinumab treatment group versus placebo were tested simultaneously at the 0.025 level. Based on the rejection of one or both of these hypotheses, analyses of the secondary endpoints were completed according to a pre-specified hypothesis testing hierarchy in the sequence described in Fig. S2 of the Supplementary Appendix. Adjusted P values are presented unless otherwise stated.
Comparative efficacy analyses (i.e., inferential efficacy comparisons versus placebo) were performed on the full analysis set, which was comprised of all patients who were randomized. The primary endpoint and other binary endpoints were evaluated using logistic regression, with treatment and TNFi use as factors and weight as a covariate. Missing values, including those due to discontinuation of study treatment, were imputed as non-response. Between-treatment differences in continuous variables were evaluated using a mixed-effect model repeated-measures (MMRM) approach, which is valid under the missing at random assumption. Treatment, analysis visit, and TNFi use were used as factors, with baseline score and weight as covariates. Treatment and baseline score by analysis visit were included as interaction terms in the model. For the change in hsCRP level, the loge ratio of the post-baseline value to the baseline value was used to normalize the distribution of the hsCRP level at each assessment time point. Interactions between treatment and baseline demographics or disease characteristics for ASAS20 response at week 16 were evaluated using a logistic regression model. Safety assessment included all patients who received at least one dose of the study drug; AE rates were summarized descriptively.
Results
Patients
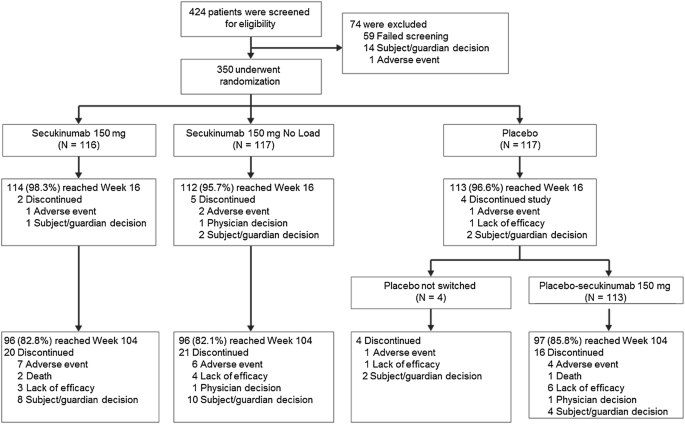
Of the 424 patients screened, 350 patients (82.5%) underwent randomization to receive secukinumab 150 mg (N = 116), secukinumab 150 mg no load (N = 117), or placebo (N = 117). Of these patients, 96.9% (339/350) completed the 16-week evaluation period and 82.6% (289/350) patients completed 104 weeks; 61 patients discontinued the study before week 104. The details of patient disposition up to week 104 and the reasons for discontinuation are outlined in Fig. 1. There were no clinically meaningful differences across the treatment groups in demographics, baseline disease characteristics, or relevant medical history (Table 1). A history of inflammatory bowel disease was either reported infrequently or not at all across the treatment groups whereas a history of uveitis was reported more frequently. Most of the patients (95.4%) were < 65 years of age, with the median age ranging from 41 to 44 years among the groups. About two-thirds (68.6%) of the patients were male, and 98.3% were Caucasian. The majority of patients (72.3%) enrolled were TNFi-naïve. The rate of NSAID intake at baseline was 85.3%, 83.8%, and 74.4% in the secukinumab 150 mg, secukinumab 150 mg no load, and placebo groups, respectively. Cumulative NSAID score was comparable among the treatment groups at baseline (Table 1).
Patient disposition through week 104. The secukinumab groups received either s.c. secukinumab 150 mg loading dose weekly followed by a maintenance dose q4w starting at week 4 or s.c. secukinumab 150 mg without loading dose at baseline (with placebo doses at weeks 1, 2, and 3), followed by q4w dosing starting at week 4. Placebo was given on the same dosing schedule as the loading regimen, and all placebo patients were switched to s.c. secukinumab 150 mg q4w at week 16 in an open-label fashion. q4w, every 4 weeks; s.c., subcutaneous
Efficacy
Short-term (16-week) Efficacy
The primary endpoint was not met with either secukinumab regimen at week 16; the ASAS20 response rate was 59.5% (P = 0.057) with secukinumab 150 mg and 61.5% (P = 0.054) with secukinumab 150 mg no load versus 47% with placebo (Fig. 2a). Subsequently, secukinumab 150 mg, with or without a loading regimen, was not superior to placebo at week 16 for any secondary endpoint as assessed in the pre-specified hierarchy. A summary of results for all other pre-specified secondary endpoints at week 16 is presented in Table 2. Similar to the pattern of ASAS20 responses, ASAS40 response rates (Fig. 2b) at week 16 for secukinumab 150 mg (38.8%; P = 0.188) and secukinumab 150 mg no load (35.9%; P = 0.356) were numerically higher than placebo (28.2%). Early ASAS20/40 response rates at week 4 were comparable between the two secukinumab groups (49.1%/29.3% in 150 mg and 53.8%/26.5% in 150 mg no load; all P = 0.356) and numerically higher than placebo (39.3%/17.9%).
ASAS20 (a) and ASAS40 (b) response rates through week 16 (placebo-controlled phase). Shown are the proportions of patients with an ASAS20 response (a improvement of ≥ 20% and absolute improvement of ≥ 1 unit [on a 10-unit scale] in at least three of the four main ASAS domains, with no worsening by ≥ 20% in the remaining domain) and the proportion with ASAS40 responses (b improvement of ≥ 40% and absolute improvement of ≥ 2 units [on a 10-unit scale] in at least three of the four main ASAS domains, with no worsening in the remaining domain). *P < 0.0001; §P < 0.01; ‡P < 0.05 versus placebo (P values at week 16 were adjusted for multiplicity of testing); missing data were imputed as non-response through week 16. ASAS Assessment of SpondyloArthritis International Society, N number of patients randomized
In the pre-specified subgroup analysis by TNFi use at week 16, ASAS20/40 response rate was numerically higher with both secukinumab regimens versus placebo in TNFi-naïve (150 mg: 60%/40%; 150 mg no load: 62.4%/38.8%; placebo: 49.4%/30.1%) and TNFi-IR patients (150 mg: 58.1%/35.5%; 150 mg no load: 59.4%/28.1%; placebo: 41.2%/23.5%). Numerically greater improvements were also observed with both secukinumab regimens versus placebo for other efficacy endpoints at week 16, regardless of TNFi therapy status (Table 3).
Interactions between treatment and baseline demographics or disease characteristics for ASAS20 response at week 16 are presented in Table S1 of the Supplementary Appendix. There were no significant interactions reported. Comparison of the effect of secukinumab versus placebo at week 16 in the three different geographic regions have been presented for all efficacy endpoints in Table S2 of the Supplementary Appendix. The ASAS20 response rate was numerically higher with both secukinumab regimens versus placebo in Western Europe (150 mg: 59.6%; 150 mg no load: 53.8%; placebo: 46.2%) and Eastern Europe (150 mg: 62.3%; 150 mg no load: 68.5% [unadjusted P = 0.030]; placebo: 47.3%). This trend was not observed with the secukinumab 150 mg regimen in North America and Australia (150 mg: 45.5%; 150 mg no load: 63.6%; placebo: 50%). Similar results were also observed across other efficacy endpoints.
Two-year (104-week) Efficacy
Clinical responses observed at week 16 in the primary and secondary endpoints with both secukinumab regimens were sustained or further improved through 104 weeks of therapy. Summary of 52 and 104 week results for all efficacy endpoints by multiple imputation/MMRM analyses are presented in Table 2. Outcomes using observed data across all efficacy endpoints through weeks 52 and 104 are presented in Table S3 of the Supplementary Appendix. Patients originally randomized to placebo showed numerical increases in ASAS20 and ASAS40 response rates to 77.7% (n = 94) and 60.6% (n = 94), respectively, at week 104 after switching to secukinumab 150 mg q4w at week 16. Similarly, numerical improvements were observed through week 104 across all other efficacy endpoints in placebo patients switched to secukinumab (observed data; Table S4 in Supplementary Appendix). Improvements reported at week 16 in the sub-groups of patients by TNFi therapy status were also sustained or further improved through week 104 (observed data; Table S5 in Supplementary Appendix).
Safety
Placebo-controlled Period (16-week)
Treatment-emergent AEs up to week 16 were comparable across all three-treatment groups, with the rate in placebo patients (54.7%) falling between the two secukinumab groups (150 mg: 62.1%; 150 mg no load: 50.4%). The majority of AEs reported up to week 16 were mild or moderate in severity. The incidence of AEs possibly related to study drug were comparable across all treatment groups up to week 16: secukinumab 150 mg (24.1%), secukinumab 150 mg no load (18.8%), and placebo (23.1%). The most frequent treatment-emergent AEs were nasopharyngitis, upper respiratory tract infection, and diarrhea (Table 4). The rate of discontinuations due to any AE was low across all groups (Table 4). During the 16-week period, one patient (0.9%) from each treatment group discontinued due to an AE; reasons for discontinuation were oral candidiasis, Crohn’s disease, and malignant melanoma in the secukinumab 150 mg, secukinumab 150 mg no load, and placebo groups, respectively. Oral candidiasis and Crohn’s disease, although suspected by the investigator to be related to study treatment, were not considered SAEs; malignant melanoma was an SAE and not suspected to be related to study treatment.
The frequency of SAEs was low and comparable across the treatment groups (150 mg: 1.7%, 150 mg no load: 1.7%, placebo: 3.4%). One treatment-emergent serious infection (erysipelas) was reported in a patient receiving secukinumab 150 mg and was suspected by the investigator to be related to study treatment; however, this did not lead to study discontinuation. There were no deaths or major adverse cardiovascular events (MACE) reported. Grade 3 neutropenia was reported in one patient in the secukinumab 150 mg group, but it did not lead to study treatment discontinuation. Transient treatment-emergent anti-drug antibodies (i.e., negative at baseline and positive at week 16) were detected in two patients, one each in the 150 mg and 150 mg no load treatment arms. Neither of these patients had neutralizing antibodies.
Entire Treatment Period (104-week)
During the entire treatment period, the mean exposure was 636.0 days in the Any secukinumab 150 mg group (Table 4). The absolute and relative frequencies for treatment-emergent AE and SAE were 83.5% and 12.4% in the Any secukinumab 150 mg group, respectively (Table 4). The frequent treatment-emergent AEs were the same as over the 16-week placebo-controlled period (Table 4), with infections and infestations being the most common (58.7%) AE by primary system organ class in the Any secukinumab 150 mg group. In total, 5.8% of patients discontinued treatment due to any AEs in the Any secukinumab 150 mg group. The incidence of non-fatal SAEs (11.3%) and serious infections (2.3%) were low in the Any secukinumab 150 mg group. Crohn’s disease was reported in four patients with relevant medical history (one during the 16-week placebo-controlled period and other three thereafter); two cases resulted in treatment discontinuation.
During the entire treatment period, grade 3 neutropenia was reported in one patient in the Any secukinumab 150 mg group, which resolved and did not lead to discontinuation of study treatment. Eight cases of Candida infection were reported in the Any secukinumab 150 mg group. Oral candidiasis was reported in three patients in the Any secukinumab 150 mg group, including one case during the 16-week placebo-controlled period that led to study discontinuation. Uveitis was reported in six patients (three de novo cases and three with a history of uveitis) in the Any secukinumab 150 mg group, none of which led to treatment discontinuation. Transient treatment-emergent anti-drug antibodies (i.e., negative at baseline and positive at week 104) were detected in four patients, two cases in the 150 mg and one each case in the 150 mg no load and placebo-150 mg treatment arms. None of these patients had neutralizing antibodies.
There were four deaths in the study, three of which were adjudicated as MACE. One case (on day 159) was due to acute myocardial infarction in a 55-year-old man randomized to receive secukinumab 150 mg, who was a smoker with multiple baseline cardiac risk factors (obesity, sleep apnea, high low-density lipoprotein [LDL] cholesterol, and abnormal electrocardiogram) and was on concomitant hypertension medication. The second case (on day 193) was due to myocardial ischemia in a 54-year-old man with multiple baseline cardiac risk factors (obesity, chronic gastritis, hypertension, and intermittent high LDL cholesterol), who was initially randomized to receive placebo and switched to secukinumab at week 16. The third case (on day 398) was due to acute cardiac failure in a 41-year-old man randomized to receive secukinumab 150 mg, who was a smoker and was on concomitant hypertension medication. The forth case (on day 716) was due to basal ganglia hemorrhage in a 74-year-old man with active medical conditions including polyneuropathy, peripheral artery disease, and hypertension, who was initially randomized to receive placebo and switched to secukinumab at week 16. All cases were considered by the investigator to be unrelated to study medication.
Discussion
This randomized, double-blind, placebo-controlled phase 3 multicenter study of s.c. secukinumab 150 mg, with and without a loading regimen, assessed efficacy, safety, and tolerability in patients with active AS over 104 weeks. The treatment regimens were well balanced with respect to demographics, disease history, and baseline characteristics. The majority (83%) of patients enrolled at baseline remained in the study for 104 weeks of secukinumab treatment, reflecting a high retention rate. At week 16, both secukinumab 150 mg and secukinumab 150 mg no load regimens showed numerically higher response rates than placebo with respect to the primary endpoint of ASAS20 response, but the difference was not significant in either secukinumab group (P = 0.057 and 0.054, respectively) due to higher than expected placebo responses, which were seen across all subjective patient-reported outcome (PRO) endpoints.
ASAS20 and ASAS40 response rates at week 16 in both the secukinumab 150 mg and 150 mg no load groups were consistent with those observed in previous phase 3 studies of secukinumab 150 mg with either i.v. or s.c. loading regimens followed by maintenance dosing, including MEASURE 1 and 2 [6, 18]. However, the present study reported the highest placebo response rates (ASAS20/40: 47%/28%) amongst the four phase 3 studies of secukinumab in AS (MEASURE 1, 2, 3, and 4), with the response rate being two times greater than the placebo rates observed in the pivotal phase 3 trials, MEASURE 1 and MEASURE 2 (ASAS20/40: 29%/13% and 28%/11%, respectively) [6].
The higher than expected placebo response rates observed in this study indicates that the power estimations undervalued the predicted placebo response for the primary endpoint. Thus, the sample size would have needed to be higher to demonstrate statistical differentiation between secukinumab and placebo. Higher than expected placebo responses in subjective PRO measures may have occurred as both patients and investigators became increasingly aware of the established efficacy of secukinumab in AS during study conduct, given the dissemination of data from the MEASURE 1 and 2 studies to the medical community.
Similar findings were observed across all secondary endpoints examined in the hierarchical analysis at week 16, in that secukinumab response rates were comparable with those seen in prior secukinumab AS studies [6, 18], while placebo response rates were unexpectedly high. A notable exception was the reduction in hsCRP levels, in which a placebo effect was not observed, thereby reinforcing that placebo response rates occurred only in subjective PROs. Additionally, pharmacokinetic and drug-specific immunoglobulin data at week 16 confirmed that secukinumab was not detected in any placebo-treated patients, ruling out treatment administration errors as a possible cause of the unexpectedly high placebo response rates.
Interaction analysis of treatment and baseline demographics or disease characteristics was performed to evaluate any underlying differences between the treatment groups due to influence from any of the baseline demographics or disease characteristics. The treatment groups were well balanced with respect to (1) demographics (age, gender, race, ethnicity), (2) medical history (AS duration and disease-specific medication use), and (3) baseline disease characteristics (disease activity), with no underlying biases identified. There were no significant interactions observed, therefore, the treatment effect on ASAS20 at week 16 was not influenced by any of the baseline demographics or disease characteristics. To evaluate whether disproportionate efficacy responses in certain geographic regions may have contributed to the overall higher than expected placebo response rates [19], a sub-analysis by geographic region was conducted, which did not reveal any unexpected patterns in efficacy responses at week 16 across different regions of the world.
Despite the lack of differentiation from placebo at week 16, treatment responses in the primary and secondary endpoints were sustained or further improved from week 16 through week 104 for both secukinumab regimens, regardless of previous TNFi therapy status. Placebo patients who switched to secukinumab 150 mg at week 16 without a loading regimen also demonstrated rapidly increased treatment responses across all efficacy endpoints up to week 104. These patterns of response to secukinumab at week 104 were consistent with clinically meaningful efficacy responses to secukinumab 150 mg reported in prior phase 3 trials [7, 9].
The safety profile of secukinumab 150 mg, either with or without a loading regimen, did not reveal any new or unexpected safety signals. AE or SAE rates up to week 16 for both secukinumab regimens were comparable to placebo, and no clinically meaningful differences in the safety profile of either secukinumab regimen was observed over the entire treatment period. This indicates there was no increased safety risk with the secukinumab loading regimen. Moreover, the incidence of SAEs (including infections) was low and reported at a similar frequency between both secukinumab groups over the entire treatment period. Thus, both the short- and long-term safety profiles of secukinumab were consistent with previous reports of phase 3 studies of secukinumab in patients with active AS [6, 18].
As with the previous phase 3 secukinumab studies [6, 18], the majority of the patients in this study were recruited from Europe and United States. Although ethnic differences in disease epidemiology and therapeutic responses are known in AS, no unexpected patterns in efficacy responses were reported in the sub-analysis of these data by geographic region. In addition, baseline disease activity and duration in this study were consistent with previous phase 3 AS studies with secukinumab and were not related to the placebo response rates observed in this study.
Conclusions
While there were no clinically meaningful differences in the primary and secondary endpoint response rates between the two secukinumab 150 mg groups up to week 16, it cannot be inferred from this study whether there is a difference in efficacy between the two secukinumab regimens, given that neither secukinumab regimen differentiated statistically from placebo at week 16. Nonetheless, the findings of this study support the clinical benefit of secukinumab in AS that has already been established in several prior phase 3 trials [6, 18], by virtue of the similar response rates on all efficacy outcomes for secukinumab 150 mg as seen in all prior phase 3 AS studies.
References
Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007;369:1379–90.
Dougados M, Baeten D. Spondyloarthritis. Lancet. 2011;377:2127–37.
Garg N, van den Bosch F, Deodhar A. The concept of spondyloarthritis: where are we now? Best Pract Res Clin Rheumatol. 2014;28:663–72.
Braun J, van den Berg R, Baraliakos X, et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis. 2011;70:896–904.
van der Heijde D, Ramiro S, Landewe R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76:978–91.
Baeten D, Sieper J, Braun J, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med. 2015;373:2534–48.
Braun J, Baraliakos X, Deodhar A, et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis. 2017;76:1070–7.
Baraliakos X, Kivitz AJ, Deodhar A, et al. Long-term effects of interleukin-17A inhibition with secukinumab in active ankylosing spondylitis: 3-year efficacy and safety results from an extension of the phase 3 MEASURE 1 trial. Clin Exp Rheumatol. 2018;36:50–5.
Marzo-Ortega H, Sieper J, Kivitz AJ, et al. Secukinumab and sustained improvement in signs and symptoms of patients with active ankylosing spondylitis through 2 years: results from a phase III study. Arthritis Care Res (Hoboken). 2017;69:1020–9.
Marzo-Ortega H, Sieper J, Kivitz A, et al. Secukinumab provides sustained improvements in the signs and symptoms of active ankylosing spondylitis with high retention rate: 3-year results from the phase III trial, MEASURE 2. RMD Open. 2017. https://doi.org/10.1136/rmdopen-2017-000592.
van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–8.
Garrett S, Jenkinson T, Kennedy LG, et al. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994;21:2286–91.
World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–4.
Sieper J, Rudwaleit M, Baraliakos X, et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis. 2009;68:1–ii44.
Braun J, Davis J, Dougados M, et al. First update of the international ASAS consensus statement for the use of anti-TNF agents in patients with ankylosing spondylitis. Ann Rheum Dis. 2006;65:316–20.
Ware JE Jr. SF-36 health survey update. Spine. 2000;25:3130–9.
Doward LC, Spoorenberg A, Cook SA, et al. Development of the ASQoL: a quality of life instrument specific to ankylosing spondylitis. Ann Rheum Dis. 2003;62:20–6.
Pavelka K, Kivitz A, Dokoupilova E, et al. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res Ther. 2017;19:285.
Kivitz AJ, Gutierrez-Urena SR, Poiley J, et al. Peficitinib, a JAK inhibitor, in the treatment of moderate-to-severe rheumatoid arthritis in patients with an inadequate response to methotrexate. Arthritis Rheumatol. 2017;69:709–19.
Acknowledgements
The authors thank the patients who participated in this study; the study investigators; Suzanne McCreddin, clinical scientific expert, Novartis Pharma AG, Switzerland; and John Gallagher, medical consultant, Novartis Pharma AG, Switzerland.
Funding
The study was sponsored by Novartis Pharma AG, Basel, Switzerland, and designed by the scientific steering committee and Novartis personnel. Article processing charges were funded by Novartis Pharma AG, Basel, Switzerland. Medical writing support was funded by Novartis. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical Writing and/or Editorial Assistance
Medical writing support, under the guidance of the authors, was provided by Niladri Maity, senior scientific writer for Novartis, India; Martin Wallace, expert scientific writer for Novartis Ireland Ltd., Ireland; and Neeta Pillai, scientific editor for Novartis, India. The first draft of this manuscript was written by Niladri Maity based on input from all the authors.
Disclosures
A Kivitz received consulting fees from Celgene, Janssen, Pfizer, Genentech, Novartis, and Sanofi; and in speakers bureau of Celgene, Pfizer, Genentech, and Novartis. U Wagner received consulting fees from AbbVie, MSD, BMS, Novartis, Pfizer (Wyeth), and Roche. R Martin is an employee of Novartis and owns Novartis stock. Z Talloczy is an employee of Novartis and owns Novartis stock. HB Richards is an employee of Novartis and owns Novartis stock. B Porter is an employee of Novartis and owns Novartis stock. E Dokoupilova and J Supronik have nothing to disclose.
Compliance with Ethics Guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available. Novartis is committed to sharing with qualified external researchers access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved the basis of scientific merit. All data provided is anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. The data may be requested from the corresponding author of the manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.6887093.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kivitz, A.J., Wagner, U., Dokoupilova, E. et al. Efficacy and Safety of Secukinumab 150 mg with and Without Loading Regimen in Ankylosing Spondylitis: 104-week Results from MEASURE 4 Study. Rheumatol Ther 5, 447–462 (2018). https://doi.org/10.1007/s40744-018-0123-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-018-0123-5