Abstract
Introduction
ABP 501 was an adalimumab (ADA) biosimilar approved for treating immune-mediated inflammatory diseases (IMIDs) including rheumatoid arthritis (RA), psoriatic arthritis (PsA), and ankylosing spondylitis (AS). In this retrospective study, we aimed to examine the treatment patterns of ABP 501 among patients with these IMIDs using German and French pharmacy claims databases.
Methods
Patients with RA, PsA, or AS who initiated ABP 501 between October 2018 and March 2020 and were observed continuously for ≥ 365 days both before and after ABP 501 initiation were included. Descriptive analyses of persistence and switch after ABP 501 discontinuation were conducted and reported for each disease cohort by prior use of ADA products (patients naïve to ADA or patients experienced with ADA).
Results
Median (95% confidence interval) persistence on ABP 501 was 9.4 (8.6–10.3), 10.2 (9.0–11.7), and 12.1 (11.0–13.1) months in German patients, and 11.7 (9.9–13.3), 7.1 (5.8–8.4), and 10.8 (9.6–11.9) months in French patients for RA, PsA, and AS, respectively. For patients who switched from ABP 501 to another targeted therapy during the first 12 months of follow-up, switching patterns varied between patients naïve to ADA and patients experienced with ADA in both Germany and France, with patients naïve to ADA switching most frequently to other targeted therapies including non-ADA tumor necrosis factor inhibitor (TNFi), non-TNFi biologic, or Janus Kinase inhibitor (JAKi) and patients experienced with ADA switching most frequently back to ADA reference product (RP).
Conclusions
Across three rheumatologic diseases, about half of patients persisted on ABP 501 at the end of 12 months after treatment initiation in both Germany and France. Patients experienced with ADA were more likely to switch back to ADA RP, regardless of indication and country, suggesting a possible nocebo effect. Future studies are warranted to understand reasons of discontinuation and switching.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
ABP 501, the first adalimumab (ADA) biosimilar approved by the European Medicines Agency and the United States (US) Food and Drug Administration for the treatment of certain immune-mediated inflammatory diseases including rheumatoid arthritis (RA), psoriatic arthritis (PsA), and ankylosing spondylitis (AS), has been available in Europe since October 2018 and in the US since January 2023. |
Real-world data of ABP 501 from European countries can provide information regarding patient experience and utilization patterns to medical communities in the US upon the market entry of ADA biosimilars, especially for indications such as PsA and AS that were approved based upon extrapolation. |
What was learned from this study? |
Real-world data from German and French pharmacy claims databases demonstrate that there were no substantial differences in ABP 501 treatment patterns between indications approved based on extrapolation (PsA, AS) and an indication for which comparative clinical trial data were available (RA). |
Across three immune-mediated inflammatory disease indications (RA, PsA, AS), about half of the study population persisted on treatment with ABP 501 during the 12 months after ABP 501 initiation. |
Patterns of switching varied by prior exposure to ADA products: patients naïve to ADA switched most frequently to other targeted therapies including non-ADA tumor necrosis factor inhibitor (TNFi), non-TNFi biologic, or Janus Kinase inhibitor; patients experienced with ADA switched most frequently back to ADA reference product, suggesting a possible nocebo effect. |
Although reasons for switching and barriers to biosimilar utilization need to be further studied, providing evidence-based communication to patients and their treating physicians may help improve treatment persistence and outcomes. |
Introduction
Immune-mediated inflammatory diseases (IMIDs), including rheumatoid arthritis (RA), psoriatic arthritis (PsA), and ankylosing spondylitis (AS), are chronic, progressive, and disabling conditions that cause joint pain and, if untreated, can result in structural damage with associated loss of function [1,2,3,4,5]. Management of these diseases has been improved over the past decades with employment of early aggressive treatment strategies and the availability of effective treatment options [6,7,8]. Tumor necrosis factor inhibitor (TNFi) biologics (e.g., adalimumab [ADA], infliximab, etanercept, golimumab, certolizumab) can improve disease activity and other clinical outcomes, particularly for patients who do not tolerate or respond adequately to conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) [6,7,8].
With regulatory approval of TNFi biosimilars in recent years, additional treatment options become available to patients and have the potential to increase access [9,10,11]. ABP 501 (AMGEVITA® in the European Union or AMJEVITA™ in the United States [US]; adalimumab-atto, Amgen Inc., Thousand Oaks, CA, USA) is the first ADA biosimilar approved by the European Medicines Agency and the US Food and Drug Administration for the treatment of IMIDs, including RA, PsA, and AS, and has been available in Europe since October 2018 and in the US since January 2023. The regulatory approval of ABP 501 was based on the “totality-of-the-evidence” (TOE), which included rigorous assessment of analytical structure and function, toxicity in nonclinical studies, and clinical pharmacology, efficacy, and safety in clinical trials in comparison to its reference product (RP) [12]. Biosimilarity of ABP 501 to its RP in terms of efficacy, safety, and immunogenicity has been demonstrated in two randomized controlled, double-blind, comparative clinical trials in patients with RA [13] and patients with plaque psoriasis [14, 15], respectively. However, due to the strict inclusion criteria, relatively short duration, and small sample sizes of randomized clinical trials, the results of these trials may not be generalizable to routine clinical practice. There remains a paucity of real-world data about biosimilar use, especially in indications such as PsA and AS, where clinical trial data were not available and approval was granted on the basis of extrapolation. Therefore, we designed this retrospective observational study to evaluate patterns of use of the ADA biosimilar ABP 501 to treat patients with inflammatory arthritis (RA, PsA, AS) using German and French pharmacy claims databases.
Methods
Study Design and Data Source
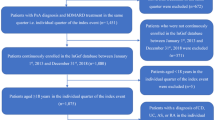
This retrospective cohort analysis used the IQVIA German and French pharmacy claims Longitudinal Prescription Data (LRx) databases, which contained data up to 30 April 2021 (at data lock). Supplemental Fig. 1 presents the study design schema.
IQVIA LRx, a longitudinal pharmacy administrative claims database, gathers data from retail pharmacies; each patient is assigned an anonymized unique patient ID and followed over time to allow evaluation of the treatment journey through medication dispensing and reimbursement data. Specifically, the German LRx database, created in 2008, contains data from ~ 84% of Germany statutory health insurance patients nationally [16]. The French LRx database, created in 2012, collects data from more than 9600 French retail pharmacies and has a national coverage of ~ 45%. Retail pharmacies covered by the French LRx are representative of the geographical and age distribution of the French population [17], which allows extrapolation of data to the overall population of France. As German LRx and French LRx are two independently collected datasets, representing region-specific patient information from two independent health delivery systems with potentially different prescribing practices and reimbursement criteria, analyses were conducted separately for patients in German and French LRx; no pooled analyses were conducted.
IQVIA LRx, as a fully de-identified database, does not require patient consent or approval from an institutional review board or ethics committee to access de-identified information about patient treatment histories.
Study Population
This analysis included adult patients 18 years or older who had documented evidence of RA, PsA, or AS and received at least one prescription of ABP 501 between October 2018 (initial market availability) and March 2020. Patients must have had at least 365 days of continuous observation of overall pharmacy records in the LRx database before ABP 501 initiation, allowing evaluation of their baseline characteristics and treatment history; and at least 365 days of follow-up after ABP 501 initiation, allowing evaluation of ABP 501 treatment persistence, adherence, and patterns of switching from ABP 501 during the follow-up period.
Diagnoses were not documented directly in the IQVIA LRx pharmacy claims database and were imputed in German LRx using machine learning models developed based on treatment histories of patients and validated in electronic medical records (Supplemental Information 1) and in French LRx using a mix of rule-based and machine learning approaches (Supplemental Information 2). Those diagnostic algorithms have been successfully applied to previously published studies using the LRx data [16, 18,19,20,21].
Study Outcomes and Variables
Treatment Persistence
Treatment persistence, which reflects the cumulative probability of ABP 501 continuation during the follow-up period, was evaluated using Kaplan–Meier analysis. ABP 501 therapy was considered to have been discontinued when 1) no additional ABP 501 prescription was filled within the permissible treatment gap (up to 120 days) following the end of the previous prescription; 2) patients were switched to another targeted therapy (detailed drug list in Supplemental Table 1); or 3) patients reached the end of their observation period such as the end of LRx database coverage (ie, “censored” patients). A sensitivity analysis of treatment persistence was conducted using a shorter treatment gap of up to 90 days.
Treatment Adherence
Treatment adherence was measured using the medical possession ratio (MPR), calculated using the equation:
The numerator is the sum of the durations of all ABP 501 prescriptions from the date of initiation up to 365 days of follow-up for each patient and the denominator is 365 days. The MPR was truncated at a maximum value of 1.0 to prevent false inflation of the population average. Patients were considered to be adherent to ABP 501 if MPR was ≥ 80% of covered days.
Initial Switching Patterns
The initial pattern of switching to another targeted therapy after discontinuation of ABP 501 was described as switching to either ADA RP, another ADA biosimilar (excluding ABP 501), a non-ADA TNFi, a non-TNFi biologic, or a Janus Kinase inhibitor (JAKi) (detailed drug list in Supplemental Table 1). Patients were considered to have switched when they initiated a new targeted therapy, either while still having an active ABP 501 prescription or within the predefined treatment gap of up to 120 days after the end of the previous prescription.
Covariates
Patient characteristics including age, sex, treating specialty, treatment setting, prior treatment history, and concomitant treatment during baseline (defined as within 12 months before initiation of ABP 501) were reported. Treatment specialty or setting referred to the specialty or setting that accounted for the greatest number of ABP 501 prescriptions or, if there were an equal number of prescriptions in both, the specialty or setting of the initial prescriber. Lists of drugs for prior and concomitant treatment are detailed in Supplemental Table 1.
When evaluating baseline characteristics and outcome measures, patients were stratified by prior use of ADA products (including ADA RP and ADA biosimilars) into two mutually exclusive categories: 1) patients naïve to ADA, who had not previously been treated with an ADA product, and 2) patients experienced with ADA, who had been treated with an ADA product during the baseline period, prior to receiving treatment with ABP 501.
Statistical Analysis
As this was a descriptive analysis, there were no a priori hypotheses or statistical comparisons between groups. Analyses were conducted separately for each disease cohort, in Germany and France, respectively. Summary statistics, including mean and standard deviation, were reported for continuous variables. The frequency and percentage were calculated for categorical variables. Kaplan–Meier curves were provided for time-to-event outcomes (persistency analysis). For French LRx analysis, results below a threshold of 10 patients were reported as “ < 10”, according to French data privacy protection guidelines. The statistical software SAS Version 9.4 (SAS Institute, Cary, US) was used to perform statistical analyses.
Results
Patient Characteristics and Medication Use at Baseline
Patient baseline characteristics are presented in Table 1. The majority of patients were treated by rheumatologists, in the office-based practices for German patients and hospital-based practices for French patients. Across indications, prior use of csDMARDs and glucocorticoids during baseline was commonly observed in both German and French patients, especially in patients with RA or PsA and patients naïve to ADA. Prior exposure to biologics and JAKi was also observed during baseline, mainly in patients naïve to ADA. While receiving ABP 501 therapy, some German and French patients also received concomitant csDMARDs and/or glucocorticoids (Table 1), which was mostly commonly seen in patients with RA.
Treatment Persistence
Overall treatment persistence appeared to be within a similar range across indications and countries, though it varied slightly. The median persistence on ABP 501 was 9.4 months (95% confidence interval [CI]: 8.6–10.3), 10.2 months (95% CI: 9.0–11.7), and 12.1 months (95% CI: 11.0–13.1) in German patients with RA, PsA, and AS, respectively, and 11.7 (95% CI: 9.9–13.3), 7.1 (95% CI: 5.8–8.4), and 10.8 (95% CI: 9.6–11.9) months in French patients with RA, PsA, and AS, respectively (Fig. 1). At the end of 12 months after initiating ABP 501, the persistence rate was 44%, 47%, and 51%, respectively, in German patients and 50%, 32%, and 46%, respectively, in French patients with RA, PsA, and AS (Fig. 1).
When analyzing by prior exposure to ADA products, in Germany, median persistence in patients experienced with ADA was numerically longer than that in patients naïve to ADA (RA, 13.1 [95% CI: 11.3–14.9] and 8.0 [95% CI: 7.0–8.7]; PsA, 13.6 [95% CI: 12.1–15.4] and 7.7 [95% CI: 6.6–8.7]; AS, 14.2 [95% CI: 12.4–15.1] and 9.9 [95% CI: 8.7–11.6] months, respectively); while in France, median persistence in patients naïve to ADA and patients experienced with ADA seemed to be more comparable (RA, 10.5 [95% CI: 9.0–13.0] and 12.9 [95% CI: 10.2–15.9]; PsA, 7.2 [95% CI: 6.0–8.6] and 6.2 [95% CI: 4.1–11.1]; AS, 11.3 [95% CI: 9.6–13.0] and 10.1 [95% CI: 7.9–11.8] months, respectively) (Fig. 1).
A sensitivity analysis of persistence was conducted using a shorter treatment gap of up to 90 days (Supplemental Fig. 2). Compared with the primary analysis results, persistence rates for ABP 501 were slightly shorter across all indications and for both countries, but differences did not appear to be substantial, and patterns remained similar.
Treatment Adherence
Across the three indications, over 40% of patients in Germany were adherent to ABP 501 therapy during the first 12 months of treatment initiation, and adherent patients ranged from 31.3 to 37.7% in France (Supplemental Table 2).
Patterns of Switching from ABP 501
Among patients who did not persist on ABP 501 at the end of 12 months, though varied across indications and between countries, 25–32% patients overall discontinued ABP 501 (without switching to any other targeted therapies during available follow-up), 12–31% resumed ABP 501 but with a treatment gap (> 120 days), and 43–63% switched from ABP 501 to another targeted therapy (Supplemental Table 3). Of those who switched to another targeted therapy, the patterns of switching differed between patients naïve to ADA and patients experienced with ADA across all three indications in both Germany and France. Patients naïve to ADA switched most frequently to other targeted therapies including non-ADA TNFi, non-TNFi biologics, or JAKi (for RA only), while patients experienced with ADA switched most frequently back to ADA RP (Table 2).
Discussion
To our knowledge, this is the first claim-based analysis evaluating treatment patterns of biosimilar ABP 501 among patients with RA, PsA, and AS. Our findings showed there were no substantial differences in ABP 501 treatment persistence and switching patterns between Germany and France and among patients across all three rheumatologic disease indications. These real-world data support the use of ABP 501 biosimilar for indications approved based on data extrapolation (PsA and AS) and across different countries with different healthcare systems.
Treatment persistence and adherence are important factors when treating inflammatory arthritis and, in the absence of a direct measure, may serve as a surrogate composite marker of effectiveness, safety, and treatment satisfaction. Observational studies have evaluated persistence on and adherence to treatment with TNFi RP in patients with rheumatologic diseases and have reported findings that vary widely. One-year persistence and adherence rates ranged from ~ 50% to 80% in systematic literature reviews and retrospective claims analysis of patients with RA, psoriasis, or PsA who received treatment with ADA RP before 2017 in the United States and Europe [22]. In our current analysis, we observed that, across 3 studied rheumatologic indications, about 50% of German and French patients persisted on biosimilar ABP 501 after 12 months. Our findings were generally comparable to those reported for ADA RP in real-world settings [22]. Considering the rapidly evolving treatment landscape for RA, PsA, and AS over recent years, including market availability of several TNFi biosimilars in European countries, it is not unexpected that the persistence and adherence rate of the TNFi biosimilars, including ABP 501, might be slightly lower than that of its RP. In addition, our study period overlapped with the COVID-19 pandemic, which could have influenced the persistence and other treatment pattern outcomes of the study [23].
For patients who switched from ABP 501 to another targeted therapy, we observed different switching patterns between patients naïve to ADA and patients experienced with ADA regardless of indication and country. Patients naïve to ADA switched most frequently to other targeted therapies, while patients experienced with ADA switched most frequently back to ADA RP. This finding may, at least partially, indicate the nocebo effect. Nocebo effects are psychological, physiological, and neurobiological phenomena associated with actual or perceived harms that occur because of patients’ negative expectations, and not due to the known pharmacologic actions of treatment [24,25,26,27]. A prospective observational study reported frequent early nocebo complaints following non-medical biosimilar switch (including infliximab and ADA RP to biosimilar switches) in Canadian patients with inflammatory bowel disease, although biomarker levels and their clinical efficacy were well maintained [28]. Unfortunately, reasons for switching and patient clinical status were unknown in our current study as they were not documented in the LRx claims databases. Future studies are warranted to elucidate underlying reason(s) for biosimilar discontinuation/switching and evaluate potential nocebo effect, if any, in patients with rheumatologic diseases. Providing evidence-based communication to patients and their treating physicians should help to improve treatment persistence and adherence and result in more positive treatment outcomes for patients.
Our study has several limitations. The patient diagnoses were imputed based on models trained using patient treatment history and electronic medical records, which may result in misclassification of disease types. However, the imputation model has been well validated and applied in previously published studies using the German and French LRx databases [16, 18,19,20,21]. Even though there may be a chance to misclassify patients between PsA and RA or AS, the model accuracy to predict the three rheumatologic diseases together can reach 95%. The second potential limitation is the coverage of IQVIA LRx retail pharmacy claims database, which does not include medications dispensed in a hospital setting and may not portray complete treatment histories in some patients. Since only ~ 1% of all ABP 501 prescriptions were dispensed in hospital pharmacies in both Germany and France, this limitation may not be of much significance for most of our analyses. However, analysis of switching patterns may be impacted by this limitation as any follow-up medications received out of the coverage, such as in hospital pharmacies (e.g., infliximab for French patients), would not be captured. Also, the baseline clinical characteristics of patients are not recorded in the LRx claims databases; potential differences in baseline clinical characteristics should be considered when interpreting our findings. Finally, as mentioned earlier, the timeframe of our study included the COVID-19 pandemic period, which could have influenced treatment pattern outcomes studied in our analysis.
Conclusion
In summary, the real-world treatment patterns of ABP 501 did not appear to be substantially different between indications for which clinical trial data were unavailable and approval was granted based on extrapolation (PsA, AS) and an indication for which comparative clinical trial data were available (RA), supporting the use of this ADA biosimilar for all approved indications. Across three rheumatologic disease indications, about half of the patients persisted with and adhered to ABP 501 therapy in Germany and France after 12 months of treatment. Among patients who switched from ABP 501 to another targeted therapy (including biologics and JAKi), switching patterns varied between patients naïve to ADA (who switched most frequently to other targeted therapies) and patients experienced with ADA (who switched most frequently back to ADA RP), regardless of indication and country, suggesting a possible nocebo effect. Future studies are needed to understand reasons for switching in the real-world setting upon ADA biosimilars entering the US market starting in January 2023.
Data Availability
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: https://wwwext.amgen.com/science/clinical-trials/clinical-data-transparency-practices/clinical-trial-data-sharing-request/
References
Di Matteo A, Bathon JM, Emery P. Rheumatoid arthritis. Lancet. 2023;402(10416):2019–33.
Gravallese EM, Firestein GS. Rheumatoid arthritis-common origins, divergent mechanisms. N Engl J Med. 2023;388(6):529–42.
Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med. 2017;376(10):957–70.
Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390(10089):73–84.
Taurog JD, Chhabra A, Colbert RA. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med. 2016;374(26):2563–74.
Burmester GR, Pope JE. Novel treatment strategies in rheumatoid arthritis. Lancet. 2017;389(10086):2338–48.
Van den Bosch F, Coates L. Clinical management of psoriatic arthritis. Lancet. 2018;391(10136):2285–94.
Baraliakos X, Kiltz U, Kononenko I, Ciurea A. Treatment overview of axial spondyloarthritis in 2023. Best Pract Res Clin Rheumatol. 2023;37(3):101858.
Kay J, Schoels MM, Dörner T, Emery P, Kvien TK, Smolen JS, et al. Consensus-based recommendations for the use of biosimilars to treat rheumatological diseases. Ann Rheum Dis. 2018;77(2):165–74.
Bridges SL Jr, White DW, Worthing AB, Gravallese EM, O’Dell JR, Nola K, et al. The science behind biosimilars: entering a new era of biologic therapy. Arthritis Rheumatol. 2018;70(3):334–44.
QuintilesIMS. The Impact of Biosimilar Competition in Europe 2017 [Available from: https://www.medicinesforeurope.com/wp-content/uploads/2017/05/IMS-Biosimilar-2017_V9.pdf. Accessed November 2, 2023
Markus R, McBride HJ, Ramchandani M, Chow V, Liu J, Mytych D, et al. A review of the totality of evidence supporting the development of the first adalimumab biosimilar ABP 501. Adv Ther. 2019;36(8):1833–50.
Cohen S, Genovese MC, Choy E, Perez-Ruiz F, Matsumoto A, Pavelka K, et al. Efficacy and safety of the biosimilar ABP 501 compared with adalimumab in patients with moderate to severe rheumatoid arthritis: a randomised, double-blind, phase III equivalence study. Ann Rheum Dis. 2017;76(10):1679–87.
Papp K, Bachelez H, Costanzo A, Foley P, Gooderham M, Kaur P, et al. Clinical similarity of biosimilar ABP 501 to adalimumab in the treatment of patients with moderate to severe plaque psoriasis: a randomized, double-blind, multicenter, phase III study. J Am Acad Dermatol. 2017;76(6):1093–102.
Papp K, Bachelez H, Costanzo A, Foley P, Gooderham M, Kaur P, et al. Clinical similarity of the biosimilar ABP 501 compared with adalimumab after single transition: long-term results from a randomized controlled, double-blind, 52-week, phase III trial in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2017;177(6):1562–74.
Richter H, Dombrowski S, Hamer H, Hadji P, Kostev K. Use of a German longitudinal prescription database (LRx) in pharmacoepidemiology. Ger Med Sci. 2015;13:14.
Vilcu AM, Blanchon T, Sabatte L, Souty C, Maravic M, Hanslik T, et al. Cross-validation of an algorithm detecting acute gastroenteritis episodes from prescribed drug dispensing data in France: comparison with clinical data reported in a primary care surveillance system, winter seasons 2014/15 to 2016/17. BMC Med Res Methodol. 2019;19(1):110.
Ilgin YSA, Chauvin F, Leheyda N, Wolk A, Witte M, Subramani S. Statistical models to predict indication of respiratory patients between asthma and COPD. Value in Health. 2016;19:PA381.
Larger E, Alexandre-Heymann L, Pilet S, Raoul T, Perray L, Maravic M. Polypharmacy in diabetes: a nation-wide, pharmacy-based, observational study. Diabetes Epidemiol Manage. 2022;8:100088.
Joumaa H, Sigogne R, Maravic M, Perray L, Bourdin A, Roche N. Artificial intelligence to differentiate asthma from COPD in medico-administrative databases. BMC Pulm Med. 2022;22(1):357.
Jin R, Kruppert S, Scholz F, Bardoulat I, Karzazi K, Kricorian G, et al. Treatment persistence and switching patterns of ABP 501 in European patients with inflammatory bowel disease. Therap Adv Gastroenterol. 2024;17:17562848231222332.
Murage MJ, Tongbram V, Feldman SR, Malatestinic WN, Larmore CJ, Muram TM, et al. Medication adherence and persistence in patients with rheumatoid arthritis, psoriasis, and psoriatic arthritis: a systematic literature review. Patient Prefer Adherence. 2018;12:1483–503.
George MD, Venkatachalam S, Banerjee S, Baker JF, Merkel PA, Gavigan K, et al. Concerns, healthcare use, and treatment interruptions in patients with common autoimmune rheumatic diseases during the COVID-19 pandemic. J Rheumatol. 2021;48(4):603–7.
Colloca L, Miller FG. The nocebo effect and its relevance for clinical practice. Psychosom Med. 2011;73(7):598–603.
Colloca L, Finniss D. Nocebo effects, patient-clinician communication, and therapeutic outcomes. JAMA. 2012;307(6):567–8.
Häuser W, Hansen E, Enck P. Nocebo phenomena in medicine: their relevance in everyday clinical practice. Dtsch Arztebl Int. 2012;109(26):459–65.
Chavarria V, Vian J, Pereira C, Data-Franco J, Fernandes BS, Berk M, et al. The placebo and nocebo phenomena: their clinical management and impact on treatment outcomes. Clin Ther. 2017;39(3):477–86.
Wetwittayakhlang P, Karkout K, Tselekouni P, Aljabri R, Bessissow T, Afif W, et al. P417 frequent early nocebo complaints but maintained clinical efficacy, biomarker and therapeutic drug levels following mandatory biosimilar switch in patients with inflammatory bowel disease: preliminary data from a prospective observational cohort study. J Crohn’s Colitis. 2023;17(Supplement_1):i546.
Acknowledgements
Medical Writing/Editorial Assistance.
Pranali Pathare (BioScience Communications, New York, NY) provided writing and editorial support, which was funded by Amgen Inc.
Funding
Amgen Inc., Thousand Oaks, Calif., USA, sponsored this study and its publication, including the journal’s Rapid Service Fee.
Author information
Authors and Affiliations
Contributions
Contributed to the concept or design of the study: Ran Jin, Silvia Kruppert, Florian Scholz, Isabelle Bardoulat, Khalil Karzazi, Francois Morand, Greg Kricorian, David Collier, and Jonathan Kay. Contributed to acquisition of data: Silvia Kruppert, Isabelle Bardoulat, and Khalil Karzazi. Contributed to analysis or interpretation of data: Ran Jin, Silvia Kruppert, Florian Scholz, Isabelle Bardoulat, Khalil Karzazi, Francois Morand, Greg Kricorian, David Collier, and Jonathan Kay. Drafted the article or revised it critically for important intellectual content: Ran Jin, Silvia Kruppert, Florian Scholz, Isabelle Bardoulat, Khalil Karzazi, Francois Morand, Greg Kricorian, David Collier, and Jonathan Kay. Approved the version of the article to be published: Ran Jin, Silvia Kruppert, Florian Scholz, Isabelle Bardoulat, Khalil Karzazi, Francois Morand, Greg Kricorian, David Collier, and Jonathan Kay.
Corresponding author
Ethics declarations
Conflict of Interest
Ran Jin and Greg Kricorian are employees and stockholders of Amgen. David Collier is a stockholder and former employee of Amgen. Silvia Kruppert, Florian Scholz, Isabelle Bardoulat, Khalil Karzazi, and Francois Morand are employees of IQVIA Real World Solutions. Jonathan Kay has received research grants (paid to UMass Chan Medical School) from Aker BioMarine AS, Biogen, and Galapagos NV and is a consultant to Alvotech Swiss AG, Boehringer Ingelheim GmbH, Bristol Myers Squibb Co., Fresenius Kabi, Novartis Pharmaceuticals Corp., Organon, Pfizer Inc., Samsung Bioepis, Teijin Pharma Ltd., Viatris Inc., and UCB Inc.
Ethical Approval
This retrospective cohort analysis used fully de-identified IQVIA Longitudinal Prescription Data databases and therefore does not require patient consent for information on treatment histories or approval from an institutional review board or ethics committee. Consent from participants for publication is not applicable since no patient identifying information is included.
Additional information
Prior Presentation: Preliminary results were presented as a poster at the AAD Annual Meeting: March 17–21, 2023; New Orleans, LA, USA. Poster 43187.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Jin, R., Kruppert, S., Scholz, F. et al. Treatment Persistence and Switching Patterns of Adalimumab Biosimilar ABP 501 in European Patients with Rheumatologic Diseases. Rheumatol Ther 11, 523–537 (2024). https://doi.org/10.1007/s40744-024-00647-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-024-00647-4