Abstract
Introduction
Biologic disease-modifying anti-rheumatics drugs (bDMARDs) and targeted synthetic DMARDs (tsDMARDs) are important treatments for rheumatoid arthritis (RA). As more of these drugs become available, there is a greater need to assess their real-world adherence and drug survival.
Methods
Treatment-naïve and treatment-experienced patients with RA who initiated treatment with bDMARDs and tofactinib during 2015–2018 in a large Israeli health maintenance organization were included. Adherence and time to treatment suspension were recorded. Odds for adherence were estimated using a multivariable logistic regression model. Risk for treatment suspension was estimated using a mixed-effect Cox proportional hazard model.
Results
The analysis included 753 eligible patients (61.8% treatment-naïve) treated with 1287 treatment episodes (tofacitinib 24.2%, tocilizumab 17.5%, etanercept 16.0%, adalimumab 10.4%, abatacept 9.9%, rituximab 9.0%, golimumab 6.9%, certolizumab pegol 3.6%, infliximab 1.9%, and sarilumab 0.5%). Good adherence was measured for almost all drugs, yet over 50% of all treatment episodes were suspended. Older age was associated with reduced risk for treatment suspension while higher number of primary care visits and higher Charlson’s comorbidity score were associated with increased risk. Compared to etanercept, treatment with adalimumab, certolizumab, or rituximab was associated with increased risk for treatment suspension (HR 1.68 95% CI 1.27–2.22, HR 1.62 95% CI 1.00–2.60, and HR 2.72 95% CI 2.02–3.67, respectively).
Conclusion
Treatment choice primarily depends on disease activity and prognosis. Real-world data, showing differences in drug survival of bDMARDs and tsDMARD, can also be used in the variety of considerations when choosing treatment. Future studies could separate patients with RA into subgroups, which would also account for potential drug survival differences and enable personalized therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
As more biologic and non-biologic therapies for rheumatoid arthritis (RA) are being introduced, including oral-administered therapies, there is a need for real-world studies that examine the long-term adherence and drug survival of various modes of action in both treatment-naïve and treatment-experienced patients with RA. |
The aim of this study was to assess adherence and persistence to biologic and non-biologic therapies among patients with RA, and to examine patients' socio-demographic characteristics, line of therapy, and other related factors that may affect adherence and persistence. |
What was learned from the study? |
Differences in drug survival and risk for treatment suspension between various biologic and non-biologic therapies available for patients with RA were observed in this real-world study. |
Although treatment choice depends on the individual patients' characteristics, in particular disease activity and prognosis, real-world data can also be used in the variety of considerations when choosing the appropriate treatment. Future studies, could separate patients with RA into subgroups, which would also account for potential drug survival differences and enable personalized therapy. |
Introduction
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease that primarily affects the lining of the synovial joints [1] that over time can cause cartilage damage, bone erosion, disability, and poor quality of life [2]. RA is characterized by an abundant infiltration of inflammatory cells into the synovial tissue which leads to the release of multiple pro-inflammatory cytokines and matrix-degrading enzymes, facilitating progressive joint destruction [3, 4].
The pharmacologic treatment options available for patients with RA target various pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α and interleukin (IL)-6, that play critical roles in RA development [4]. These treatment options include the following groups of disease-modifying anti-rheumatic drugs (DMARDs): conventional synthetic DMARDs (csDMARDs), primarily methotrexate, recommended for naïve patients with moderate-to-high disease activity, and biologic DMARDs (bDMARDs) and targeted synthetic DMARDs (tsDMARDs), recommended for those with an inadequate response to csDMARDs [5]. bDMARDs that target specific components of the immune system are an important treatment option for RA [4]. These therapies include a variety of drugs that target various components of the immune system: TNF-α, IL-6 receptor, IL-1 receptor, CD20 and T cell antigen-presenting cell communication. In addition to these therapies, targeted tsDMARDs that impede Janus kinase activation (JAK inhibitors) are a recent non-biologic therapy that is often compared to bDMARDs, and also indicated for patients with RA with moderate to severe disease [5].
Treatment adherence is defined as the extent to which patients take medications prescribed by their healthcare providers. Among patients with RA, adherence has been reported to range between 30 and 80% [6,7,8]. Various parameters were found to affect long-term adherence, such as patients’ demographics, frequency of administration, type of drug, and treatment experience. Monthly administration of subcutaneous biologic therapies in patients with RA was found to yield higher rates of adherence compared to both weekly or every other week administration [9].
Persistence to treatment is often measured as the proportion of patients who did not suspend their treatment in a defined time frame from treatment initiation and is used to assess the drug survival [10, 11] that serves as an indirect measure to assess the safety and effectiveness of drugs in the real world [12]. Drug survival of bDMARDs has been reported to be affected by various factors, including sex, disease duration, concomitant use of csDMARDs, treatment-experience, and the specific bDMARD used [13,14,15]. In recent years, many studies have examined the drug survival of bDMARDs and tsDMARDs in patients with RA, yet these studies vary in terms of follow-up period, the variety of drugs included, the patients with RA included in terms of treatment-experience and disease severity, and the different healthcare settings [13, 16,17,18].
As more biologic and non-biologic therapies are being approved and marketed for RA [19], there is a greater need for real-world studies that examine long-term treatment patterns and comparative effectiveness between different modes of action in both treatment-naïve and treatment-experienced patients with RA. Therefore, we aimed to assess adherence and persistence to biologic and non-biologic therapies among patients with RA, and to examine patients’ socio-demographic characteristics, treatment experience, and other related factors that may affect adherence and persistence.
Methods
This retrospective cohort study was conducted using the computerized database of Maccabi Healthcare Services (MHS), a large health maintenance organization in Israel with 2.5 million members, accounting for 25% of the Israeli population. The MHS central database integrates data from the MHS central laboratory, physician diagnoses, hospitalizations, medical procedures, socio-demographic data, and all prescription drugs dispensed throughout the MHS pharmacy network.
The study was conducted in accordance with the protocol, applicable regulations, and guidelines governing clinical study conduct and the ethical principles that have their origin in the Declaration of Helsinki. The independent ethics committee and institutional review board, the MHS Institutional Review Board (IRB), approved the study protocol and related documents. MHS’s IRB waived the requirement to obtain any informed consent for this secondary analysis of existing data.
Study Population and Follow-Up
According to the Israeli regulatory guidelines, patients with RA are eligible for lower co-payment for bDMARDs or tsDMARDs if they fulfill certain criteria. Before purchasing these drugs, members are required to receive an authorization from the MHS drugs authorization center to make sure they comply with the guidelines. The study population included patients who first purchased at least one of the following drugs between January 1, 2015 and December 31, 2017 for the indication of RA: TNF-α inhibitors [adalimumab (ADA), infliximab (IFX), golimumab (GLM), etanercept (ETN), and certolizumab pegol (CTZ)]; IL-6 inhibitors [tocilizumab (TCZ) and sarilumab (SAR)]; IL-1 inhibitor [anakinra (ANA)]; CTLA4-Ig [abatacept (ABA)]; anti-CD20 [rituximab (RTX)]; and JAK inhibitor [tofacitinib (TOF)]. The first purchase was defined as the index date for the study. Included patients had to be adults (age ≥ 18 years) and MHS members for at least 12 months before and after the index date for the study. Patients were followed until the earliest of the following dates: death, leaving MHS, or the end of the follow-up period (December 31, 2018). We included treatment-naïve patients, starting their first bDMARD or tsDMARD, as well as treatment-experienced patients at the study index date.
Treatment Related Variables
For all patients, we followed all lines of therapy used during the study follow-up period and numbered them. For those defined as treatment-experienced when entering the study, we determined the lines of therapy used during the study follow-up period, using data on bDMARDs and tsDMARD dispensed up to 10 years before entering the study.
Adherence was assessed for each line of therapy, using the proportion of days covered (PDC) method [20,21,22,23] and categorized into: non-adherent (PDC < 40%), moderate adherence (40% ≤ PDC < 80%), and high adherence (PDC ≥ 80%).
Treatment suspension was defined as the first gap of 120 days or more after the last supply day (for RTX, which is administered every 6 months, the gap was set at 180 days or more). These patients were further classified as: switching (starting a new line of therapy), restarting (purchased the same drug after a gap of at least 120 days, or 180 days for RTX), or discontinued (permanent suspension, without switching or restarting). Time to treatment suspension was defined as the time between the drug index date (the index date of every new drug, i.e., line of therapy, used during follow-up), and the last supply day. Patients who did not suspend their treatment were censored at the end of follow-up.
Other Variables
All drugs were categorized by mode of administration [injection (INJ) or oral (PO)], dosing schedule (daily, once every 1–2 weeks, once every 4–11 weeks, and once every 12 weeks or more). Injectable drugs were additionally categorized as sub-cutaneous (SC) or intra-venous (IV) (Supplementary Material Table S1). All other variables were defined per drug index date. Socio-demographic variables included age, sex, and socioeconomic status (SES), categorized into low, medium, and high. Disease duration was defined as from the first RA diagnosis by a physician to drug index date. Concomitant use of csDMARDs (methotrexate, hydroxychloroquine, leflunomide, sulphasalazine) was defined as at least one purchase in the 90 days before or after the index date. Corticosteroid use was defined according to the number of times oral corticosteroids were purchased during the 180 days before the index date. Hospitalizations were defined during the 180 days before the index date and categorized into none and at least one. Visits to the primary care physician (PCP) were defined by the number of visits during the 180 days before the index date. Additional comorbidities were defined according to the following MHS registries [24]: cardio-vascular diseases, hypertension, diabetes, obesity, chronic obstructive pulmonary disease (COPD), osteoporosis, chronic kidney disease, and cancer. Depression and anxiety were defined according to at least one purchase of antidepressants and/or anti-anxiety drugs in the 180 days before the index date. Charlson’s Comorbidity Index (CCI) was calculated on the index date [25].
Statistical Analysis
The baseline characteristics of the study population at study index date and the distribution of drug related variables by adherence categories and treatment experience are presented using descriptive statistics. Chi-square test for categorical variables and t test or Mann–Whitney U test for continuous variables were performed to determine significant differences between treatment-naïve and treatment-experienced patients.
Odds ratios (ORs) and 95% confidence intervals (CIs) for the association between various treatment related variables and being adherent (PDC ≥ 0.8) were evaluated using generalized estimating equations to fit repeated measures logistic regression models, since some patients used more than one line of therapy during follow-up. All models were adjusted for age group (≤ 45, 46–64, and ≥ 65 years), sex, socio-economic status, disease duration, concomitant use of csDMARDs, CCI, visits to the PCP, and line of therapy.
Proportions of treatment suspension in all lines of therapy during the study follow-up were calculated by line and drug class. Kaplan–Meier survival analysis was used for time to treatment suspension and the log-rank test was used to evaluate difference in drug persistence between drug types. To account for repeated measures, a multivariable mixed-effect Cox proportional hazard regression model was used to estimate hazard ratios (HR) and 95% CIs for treatment suspension. As a sensitivity analysis, a multivariable Cox proportional hazards regression model with all lines of therapy was also performed. Both models were adjusted for the potential confounders [26,27,28,29]: age group (≤ 45, 46–64 and ≥ 65), sex, SES, disease duration, concomitant use of csDMARDs, visits to the PCP, CCI, line of therapy, and individual drug.
All statistical tests were two-sided. P values less than 0.05 were considered statistically significant. Analyses were performed by the IBM-SPSS v.25.0 (IBM, Amrock, NY, USA) and R ® Foundation for Statistical Computing, Vienna, Austria).
Results
We identified 753 eligible patients, treated with 1287 treatment courses (Fig. 1). Table 1 shows the baseline characteristics of the study population at study index date. A total of 465 (61.8%) patients were treatment-naïve and the rest (n = 288, 38.2%) were treatment-experienced (entered the study starting second line of therapy or beyond). The proportion of females was similar in both groups (79.6% among treatment-naïve vs. 84.0% among treatment-experienced). Treatment-naïve patients were slightly younger (54.27, SD ± 14.69 vs. 56.65, SD ± 13.66; P = 0.021), had shorter disease duration (12.0 months, IQR 0–72 vs. 101.5, IQR 48–173; P < 0.01), were more likely to use csDMARDs (81.5% vs. 51.0%; P < 0.001), and less likely to be diagnosed with diabetes, chronic kidney disease, and osteoporosis (P < 0.05).
Figure 2 shows the disposition of patients according to line of therapy. Among patients using their first line, the most common drug class used was TNF-α inhibitors (53.1%), followed by the JAK inhibitor (21.5%), IL-6 inhibitors (14.6%), anti-CD20 (5.6%), and CTLA4-Ig (5.2%). Among patients using second line and above (including treatment-naïve patients who switched during follow-up), TNF-α inhibitors were the most frequently used drug in the second line (34.4%) and third line (28.5%), but not for fourth line or beyond, where the JAK inhibitor was most frequently used (31.8%).
Adherence During Follow-Up
Table 2 shows the proportions of patients in each adherence category by treatment-experience status and different drug-related factors. In all lines of therapy, the proportion of adherent (PDC ≥ 0.8) patients was similar, ranging between 63.9 % and 67.4%. The proportion of adherent patients was higher in those using injectable drugs compared to those using the orally administered drug, regardless of treatment-experience status. In both sub-groups, the proportion of adherent patients was highest for drugs taken once every 4–11 weeks (73.2% in all lines). Adherence by drug varied between the two sub-groups: in treatment-naïve patients, GLM had the highest proportion of adherent patients (79.1%), followed by ETN (70.0%), while RTX had the lowest (34.6%). In treatment-experienced patients, the highest proportion of adherent patients was observed in those treated with IFX (94.7%) and the lowest for CTZ (50.0%).
Table 3 shows the results of four adjusted logistic regression models that tested the association between the following drug-related factors and being adherent: mode of administration (INJ vs. PO), mode of administration and dosing schedule (in injectable drugs) and individual drug. Being treated with injectable drugs was associated with increased odds for adherence (OR 1.54, 95% CI 1.21–1.97). TNF-α inhibitors were the most frequently used group during the study follow-up period therefore ETN, which was the most frequently used of them, was set as the reference drug. Being treated with CTZ, TCZ, or TOF was associated with decreased odds of being adherent, compared to being treated with ETN (OR 0.37, 95% CI 0.21–0.67; OR 0.53, 95% CI 0.36–0.79; OR 0.49, 95% CI 0.34–0.71, respectively).
Treatment Suspension and Drug Survival During Follow-Up
More than 50% of all lines of therapy during follow-up were suspended (Fig. 3A–D). The proportions of treatment suspension were similar in all lines of therapy (from 54.1% among those on their ≥ fourth line of therapy to 54.5% among those on their second line). In all lines of therapy, most treatment suspensions were the result of switching to a different drug. When analyzing the drug classes patients switched to according to their previous drug class, we observed that, among first line patients who switched to second line therapy (Fig. 3A), 16.1% switched to TNF-α inhibitors, 10.1% switched to IL-6 inhibitors, 7.3% switched to the JAK inhibitor, and the rest switched to CTLA4-Ig and anti-CD20. The proportions of switching and the drugs switched to were similar in second line patients switching to a third line (Fig. 3B). Among third line patients who switched to a fourth line, compared to those who switched to second and third lines, there was a smaller proportion of patients switching to TNF-α inhibitors, IL-6 inhibitors, and the JAK inhibitor, and more switching to CTLA4-Ig and anti-CD20 (Fig. 3C).
Proportions of treatment suspension (switching, restarting, and discontinuing) in all lines of therapy by line and drug class (1287 courses). *Less than 50 patients; patients can be included in more than one course of treatment; results of Chi-square test revealed no statistically significant difference in the proportion of patients switching, restarting and discontinuing between lines of therapy (P > 0.05); switching- starting a new therapy line, with any of the drugs included in the study; restarting: purchased the same drug after a gap of at least 120/180 days; discontinuing: stopping treatment without switching/ restarting. Anti-CD20 anti-CD20 monoclonal antibody, CTLA4-Ig cytotoxic T-lymphocyte–associated antigen 4 immunoglobulin, IL interleukin, JAK Janus kinase, STCM Super T-cell Media, TNF tumor necrosis factor
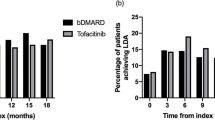
According to the Kaplan–Meier analysis for drug survival (Fig. 4A–B; SAR and CTZ had relatively short follow-up periods and are only shown in the figure for illustrative purposes), treatment-naïve patients showed higher survival rates compared to treatment-experienced patients. In treatment-naïve patients, TCZ had the highest survival rate at 12 months, while at 24 and 36 months it was second to GLM. The survival rates of ETN were similar to those of TCZ. Lower survival rates were observed for TOF, ABA, and GLM throughout the study follow-up period. In treatment-experienced patients, TCZ, ETN, TOF, ABA, and GLM had the highest survival rates at 12 and 24 months. At 36 months, GLM, TOF, ABA, and TCZ had similar survival rates (between 20% and 30%). In both treatment-naive and treatment-experienced patients, ADA, IFX, and RTX showed the lowest survival.
Multivariable Analysis
The HRs and 95% CIs obtained from the mixed-effect Cox proportional hazard model and the simple Cox model, used as a sensitivity analysis, were almost identical. The likelihood ratio test of the mixed-effect Cox model was 98.91 while the integrated log-likelihood of the simple Cox model was 98.68, suggesting that the random effect of multiple lines of therapy used by some patients did not affect the model. The results of the mixed-effect model are presented in Table 4. Older age was associated with a reduced risk for treatment suspension (HR 0.79, 95% CI 0.62–0.98, P = 0.031) while more visits to the PCP was associated with increased risk (HR 1.01, 95% CI 1.00–1.03, P = 0.013), as was higher CCI score (HR 1.06, 95% CI 1.01–1.11, P = 0.009). Compared to patients treated with ETN, treatment with ADA, CTZ, or RTX was associated with increased risk for treatment suspension (HR 1.68, 95% CI 1.27–2.22, P < 0.001; HR 1.62, 95% CI 1.00–2.60, P = 0.048; HR 2.72, 95% CI 2.02–3.67; P < 0.001, respectively).
Discussion
In this retrospective cohort study, we evaluated the adherence, persistence, and factors associated with treatment suspension for nine bDMARD and TOF, in both treatment-naïve and treatment-experienced patients with RA.
In all lines of therapy, over half of all patients were adherent to treatment, reflecting that most patients purchased the therapy month after month until suspension or end of follow-up, with the only exception of RTX in treatment-naïve patients where the proportion of adherent patients was 34.6%. Good adherence to bDMARDs was also observed by Machado-Alba et al. [30]. Nevertheless, unlike adherence, persistence rates were lower, with over 60% of all lines of therapy being suspended during follow-up. Most treatment suspensions were due to switching to a new drug with the exception of RTX, in which most suspensions were the result of restarting or discontinuing. These results for RTX could be explained by the long-lasting effect that RTX has in some patients, with some physicians choosing to prolong the dosing interval, waiting for a flare to occur [31]. Overall, treatment suspension and switching, in particular, could be due to emergence of adverse events or loss of effectiveness that historically account for the majority of all suspensions [32,33,34].
Although the exact etiology of RA is still unclear, it is certain that genetic and environmental factors affect the development of RA [19]. While many patients achieve remission or low disease state following treatment with the available range of DMARDs [5], there is still a significant proportion of patients who could experience partial response or no response as well as adverse effects [35]. Further research, to understand the pathogenetic roles of the various molecules involved in RA, could facilitate discovery of potential therapeutic targets and approaches [4, 19].
Since more and more advanced treatments for RA are being introduced, it is important to compare our results of drug survival to those of recent studies that examined a similar range of drugs in similar populations of patients. In our study, the highest survival in treatment-naïve patients was observed for TCZ. These results are similar to the ones found by Ebina et al. [33], who showed lower incidence of treatment suspension due to adverse events for TCZ in biologic-naïve patients over a similar time period, while the highest survival rate was recorded for ABA. Stamm et al. [34], who measured drug survival in both bio-naïve and bio-experienced patients, revealed that the highest survival was for TCZ and ABA and lowest for IFX. In our study, ABA showed higher survival rates in treatment-experienced patients while IFX had the second-lowest rate. The low survival of IFX might be explained by its relatively high rate of efficacy loss [32, 36], and by the fact that it was used by a very small number of patients, less than 3% in each sub-group, possibly reflecting both patient and physician preferences, and potentially patients who are treated with IFX might be those with worse prognosis. We also compared our results to those of a previous study on ADA, which utilized the MHS databases [37]. We observed a decrease in the median time to treatment suspension, from 16 months to about 8 months. This decrease might be explained by the prescription habits of bDMARDs, which might have changed from the previous follow-up period of 2008–2013, in which a limited variety of bDMARDs for RA were available. Although this issue is still controversial [38], previous studies [36, 39, 40] have shown that the year of treatment initiation can influence the treatment suspension rate, since rheumatologists are more prone to change treatment if more alternatives are available [41]. Furthermore, the emergence of the treat-to-target paradigm in 2010 [42, 43], defined as a treatment strategy in which the physician treats the patient aggressively enough to reach and maintain explicitly specified and sequentially measured goals, such as remission or low disease activity [44], might have an effect on treatment decisions, promoting switching to a new line of therapy rather than waiting on a less than optimal clinical outcome.
The results of the multivariable model suggest that the biologic therapy type was the factor most strongly associated with treatment suspension, as previously suggested [36]. Patients treated with ADA, CTZ, or RTX had a significantly increased risk for treatment suspension compared to those treated with ETN. While all other drugs included in the study also had higher risk for treatment suspension versus ETN, these results did not reach statistical significance. Older age (≥ 65) was associated with lower risk for treatment suspension, as previously described [45,46,47], while higher number of visits to the PCP and higher CCI score were both associated with a modestly increased risk for treatment suspension, both probably reflecting worse disease severity or higher disease burden [47]. Previous studies showed an association between comorbidities and CCI and the risk for treatment suspension [48, 49]. However, in the current study, preliminary univariate analysis revealed no statistically significant differences in comorbidities between those who suspended treatment and those who did not; therefore, only CCI was included in the model. Other factors that are often found to affect treatment suspension, such as disease duration [18], concomitant use of csDMARDs [36], and line of therapy [50], did not reach statistical significance in our study.
Our study has several limitations. Although the multivariable analysis for drug survival was adjusted for various factors, confounding of unmeasured factors not assessed in our study cannot be excluded, such as individual disease severity, comorbidities not included in the CCI score or their severity, previous treatment experience with csDMARDs, concomitant use of corticosteroids and non-steroidal anti-inflammatory drugs, patients’ preference, and treatment guidelines, all of which may affect drug survival [28, 29, 33, 36, 46]. Despite the lack of data on disease severity, all the drugs included in the study are indicated for patients with moderate-to-severe disease, and, as mentioned in the Methods section, during the drug authorization process, patients' data were reviewed by a pharmacist to make sure they complied with the guidelines. We cannot rule out a channeling bias wherein patients with higher disease activity and/or other indicators of a more severe disease (positive rheumatoid factor and/or anti-CCP titers) are prescribed certain drugs in lieu of others. We were also unable to determine the reason for treatment suspension, since these are not recorded in MHS databases, yet we were able to further classify treatment suspensions, allowing us to carefully speculate on the reason for treatment suspension. We might have overestimated the proportion of non-adherence and treatment suspension in RTX patients, since the intervals between each dose are not fixed but rather individually adjusted. We found increased odds for adherence among those treated with injectable drugs compared to TOF, yet, since TOF was the only orally administered drug included in the study, additional studies with various orally-administered tsDMARDs are needed to more completely assess the impact of mode of administration on adherence. The study included only patients who started a new line of therapy during 2015–2017, while those who started treatment before 2015 and remained treated were not included in the study, which may affect the survival estimate for some drugs. Nevertheless, we followed-up all treatment-naïve patients with RA from 2015 to 2017, and the results for this group represent the current real-world treatment patterns of bDMARDs and tsDMARD.
The current study also has some relevant strengths: we were able to include in the study various modes of action and molecules available in the market for RA, thus reflecting not only the real-world adherence and survival of these drugs but also the current preferences of physicians and patients. Additional strengths include the completeness of data without loss to follow-up and being able to assess the treatment experience with bDMARDs and tsDMARD of each patient, using data up to 10 years before follow-up initiation.
Conclusions
The results of our study show differences in drug survival and risk for treatment suspension between various bDMARDs and tsDMARD available for patients with RA. While the treatment choice depends on the individual patients’ characteristics, in particular disease activity and prognosis, data from a real-world study that addresses potential drug survival differences can also be used among other considerations when choosing the appropriate treatment. Future studies, which could discover potential therapeutic targets, will be able to assist physicians to distinguish between subgroups of patients with RA, and to establish precision medicine strategies to realize personalized therapy.
References
Silman AJ, Pearson JE. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res. 2002;4(Suppl 3):265–72.
Conforti A, Di Cola I, Pavlych V, et al. Beyond the joints, the extra-articular manifestations in rheumatoid arthritis. Autoimmun Rev. 2021;20(2): 102735.
Chadha S, Behl T, Bungau S, et al. Mechanistic insights into the role of pyroptosis in rheumatoid arthritis. Curr Res Transl Med. 2020;68(4):151–8.
Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6:15.
Fraenkel L, Bathon JM, England BR, et al. 2021 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol (Hoboken, NJ). 2021;73(7):1108–23.
van den Bemt BJ, van den Hoogen FH, Benraad B, Hekster YA, van Riel PL, van Lankveld W. Adherence rates and associations with nonadherence in patients with rheumatoid arthritis using disease modifying antirheumatic drugs. J Rheumatol. 2009;36(10):2164–70.
van den Bemt BJ, van Lankveld WG. How can we improve adherence to therapy by patients with rheumatoid arthritis? Nat Clin Pract Rheumatol. 2007;3(12):681.
Koncz T, Pentek M, Brodszky V, Ersek K, Orlewska E, Gulacsi L. Adherence to biologic DMARD therapies in rheumatoid arthritis. Expert Opin Biol Ther. 2010;10(9):1367–78.
Calvo-Alén J, Monteagudo I, Salvador G, et al. Non-adherence to subcutaneous biological medication in patients with rheumatoid arthritis: a multicentre, non-interventional study. Clin Exp Rheumatol. 2017;35(3):423–30.
Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15(8):565–74 (Discussion 575–567).
Sikka R, Xia F, Aubert RE. Estimating medication persistency using administrative claims data. Am J Manag Care. 2005;11(7):449–57.
Iannone F, Sinigaglia L, Favalli EG, et al. Drug survival of adalimumab in patients with rheumatoid arthritis over 10 years in the real-world settings: high rate remission together with normal function ability. Clin Rheumatol. 2016;35(11):2649–56.
Lin CT, Huang WN, Tsai WC, et al. Predictors of drug survival for biologic and targeted synthetic DMARDs in rheumatoid arthritis: analysis from the TRA Clinical Electronic Registry. PLoS One. 2021;16(4): e0250877.
Choi S, Ghang B, Jeong S, et al. Association of first, second, and third-line bDMARDs and tsDMARD with drug survival among seropositive rheumatoid arthritis patients: cohort study in A real world setting. Semin Arthritis Rheum. 2021;51(4):685–91.
Naffaa ME, Hassan F, Golan-Cohen A, et al. Factors associated with drug survival on first biologic therapy in patients with rheumatoid arthritis: a population-based cohort study. Rheumatol Int. 2021;41(11):1905–13.
Egeberg A, Rosenø NAL, Aagaard D, et al. Drug survival of biologics and novel immunomodulators for rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, and psoriasis—a nationwide cohort study from the DANBIO and DERMBIO registries. Semin Arthritis Rheum. 2022;53: 151979.
Emery P, Vlahos B, Szczypa P, et al. Longterm drug survival of tumor necrosis factor inhibitors in patients with rheumatoid arthritis. J Rheumatol. 2020;47(4):493–501.
Choquette D, Bessette L, Alemao E, et al. Persistence rates of abatacept and TNF inhibitors used as first or second biologic DMARDs in the treatment of rheumatoid arthritis: 9 years of experience from the Rhumadata® clinical database and registry. Arthritis Res Ther. 2019;21(1):138.
Huang J, Fu X, Chen X, Li Z, Huang Y, Liang C. Promising therapeutic targets for treatment of rheumatoid arthritis. Front Immunol. 2021;12: 686155.
Lam WY, Fresco P. Medication adherence measures: an overview. Biomed Res Int. 2015;2015: 217047.
Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–7.
Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10(1):3–12.
Tang KL, Quan H, Rabi DM. Measuring medication adherence in patients with incident hypertension: a retrospective cohort study. BMC Health Serv Res. 2017;17(1):135.
Arbelle JE, Chodick G, Goldstein A, Porath A. Multiple chronic disorders—health care system’s modern challenge in the Maccabi Health Care System. Isr J Health Policy Res. 2014;3:29.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
Ebina K, Hashimoto M, Yamamoto W, et al. Drug tolerability and reasons for discontinuation of seven biologics in 4466 treatment courses of rheumatoid arthritis-the ANSWER cohort study. Arthritis Res Ther. 2019;21(1):91.
Narongroeknawin P, Chevaisrakul P, Kasitanon N, et al. Drug survival and reasons for discontinuation of the first biological disease modifying antirheumatic drugs in Thai patients with rheumatoid arthritis: analysis from the Thai Rheumatic Disease Prior Authorization registry. Int J Rheum Dis. 2018;21(1):170–8.
Brodszky V, Bíró A, Szekanecz Z, et al. Determinants of biological drug survival in rheumatoid arthritis: evidence from a Hungarian rheumatology center over 8 years of retrospective data. CEOR. 2017;9:139–47.
Leon L, Rodriguez-Rodriguez L, Rosales Z, et al. Long-term drug survival of biological agents in patients with rheumatoid arthritis in clinical practice. Scand J Rheumatol. 2016;45(6):456–60.
Machado-Alba JE, Machado-Duque ME, Gaviria-Mendoza A, Reyes JM, Gamboa NC. Use of healthcare resources in a cohort of rheumatoid arthritis patients treated with biological disease-modifying antirheumatic drugs or tofacitinib. Clinical Rheumatol. 2021;40(4):1273–81. https://doi.org/10.1007/s10067-020-05432-6.
Wendler J, Burmester GR, Sörensen H, et al. Rituximab in patients with rheumatoid arthritis in routine practice (GERINIS): six-year results from a prospective, multicentre, non-interventional study in 2,484 patients. Arthritis Res Ther. 2014;16(2):R80.
Gomides APM, de Albuquerque CP, Santos ABV, et al. Real-life data of survival and reasons for discontinuation of biological disease-modifying drugs ‘in’ rheumatoid arthritis. Int J Clin Pharm. 2020. https://doi.org/10.1007/s11096-020-01171-5.
Ebina K, Hirano T, Maeda Y, et al. Drug retention of 7 biologics and tofacitinib in biologics-naïve and biologics-switched patients with rheumatoid arthritis: the ANSWER cohort study. Arthritis Res Ther. 2020;22(1):142.
Stamm TA, Reichardt B, Zwerina J, Ritschl V, Nell-Duxneuner V. Use of biological disease modifying antirheumatic drugs in rheumatoid arthritis in Austria from 2008 to 2011: a retrospective analysis of 72% of the population. Wien Klin Wochenschr. 2018;130(7–8):230–7.
Mittal N, Mittal R, Sharma A, Jose V, Wanchu A, Singh S. Treatment failure with disease-modifying antirheumatic drugs in rheumatoid arthritis patients. Singapore Med J. 2012;53(8):532–6.
Silvagni E, Bortoluzzi A, Carrara G, Zanetti A, Govoni M, Scirè CA. Comparative effectiveness of first-line biological monotherapy use in rheumatoid arthritis: a retrospective analysis of the RECord-linkage On Rheumatic Diseases study on health care administrative databases. BMJ Open. 2018;8(9): e021447.
Gendelman O, Weitzman D, Rosenberg V, Shalev V, Chodick G, Amital H. Characterization of adherence and persistence profile in a real-life population of patients treated with adalimumab. Br J Clin Pharmacol. 2018;84(4):786–95.
Acurcio FA, Machado MAA, Moura CS, et al. Medication persistence of disease-modifying antirheumatic drugs and anti-tumor necrosis factor agents in a cohort of patients with rheumatoid arthritis in Brazil. Arthritis Care Res. 2016;68(10):1489–96.
Souto A, Maneiro JR, Gómez-Reino JJ. Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: a systematic review and meta-analysis of drug registries and health care databases. Rheumatology (Oxford). 2016;55(3):523–34.
Gabay C, Riek M, Scherer A, Finckh A. Effectiveness of biologic DMARDs in monotherapy versus in combination with synthetic DMARDs in rheumatoid arthritis: data from the Swiss Clinical Quality Management Registry. Rheumatology (Oxford). 2015;54(9):1664–72.
Scirè CA, Caporali R, Sarzi-Puttini P, et al. Drug survival of the first course of anti-TNF agents in patients with rheumatoid arthritis and seronegative spondyloarthritis: analysis from the MonitorNet database. Clin Exp Rheumatol. 2013;31(6):857–63.
Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69(4):631–7.
Smolen JS, Breedveld FC, Burmester GR, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis. 2016;75(1):3–15.
Solomon DH, Bitton A, Katz JN, Radner H, Brown EM, Fraenkel L. Review: treat to target in rheumatoid arthritis: fact, fiction, or hypothesis? Arthritis Rheumatol (Hoboken, NJ). 2014;66(4):775–82.
Marras C, Monteagudo I, Salvador G, et al. Identification of patients at risk of non-adherence to oral antirheumatic drugs in rheumatoid arthritis using the Compliance Questionnaire in Rheumatology: an ARCO sub-study. Rheumatol Int. 2017;37(7):1195–202.
Mahlich J, Sruamsiri R. Persistence with biologic agents for the treatment of rheumatoid arthritis in Japan. Patient Prefer Adherence. 2016;10:1509–19.
Calip GS, Adimadhyam S, Xing S, Rincon JC, Lee W-J, Anguiano RH. Medication adherence and persistence over time with self-administered TNF-alpha inhibitors among young adult, middle-aged, and older patients with rheumatologic conditions. Semin Arthritis Rheum. 2017;47(2):157–64.
D’Amico ME, Silvagni E, Carrara G, et al. Role of comorbidities on therapeutic persistence of biological agents in rheumatoid arthritis: results from the RECord-linkage On Rheumatic Disease study on administrative healthcare databases. Scand J Rheumatol. 2021;50(5):333–42.
Letarouilly JG, Salmon JH, Flipo RM. Factors affecting persistence with biologic treatments in patients with rheumatoid arthritis: a systematic literature review. Expert Opin Drug Saf. 2021;20(9):1087–94.
Jones G, Hall S, Bird P, et al. A retrospective review of the persistence on bDMARDs prescribed for the treatment of rheumatoid arthritis in the Australian population. Int J Rheum Dis. 2018;21(8):1581–90.
Acknowledgements
Funding
AbbVie Inc. (North Chicago, Illinois, USA) funded the research and both the journal’s rapid service and open access fees. AbbVie and Maccabi participated in the study design, research, analysis, data collection, interpretation of the data, review and approval of the manuscript and publication.
Medical Writing, Editorial, and Other Assistance
Editorial support was provided by Amish Vora, PharmD, and Kayla Smull of ICON (Blue Bell, PA, USA) and was funded by AbbVie
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. No honoraria or payments were made for authorship.
Author Contributions
All authors (Vered Rosenberg, Gabriel Chodick, Zhenyi Xue, Freddy Faccin and Howard Amital) contributed to the study conception and design. Material preparation, data collection and analysis were performed by Howard Amital, Vered Rosenberg and Gabriel Chodick. The first draft of the manuscript was written by Vered Rosenberg and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Prior Presentation
These analyses were presented at the American College of Rheumatology/ARP – 2020 Annual Scientific Meeting held virtually from 5 November through 9 November 2020.
Disclosures
Freddy Faccin is a full-time AbbVie employee and may own AbbVie stock and/or options. Zhenyi Xue is a former AbbVie employee. Vered Rosenberg and Gabriel Chodick have nothing to declare. Howard Amital received consultant fees from AbbVie for this project. After completion of the manuscript, Gabriel Chodick changed his affiliation within Maccabi Healthcare Services, and his new affiliation is: Maccabi Healthcare Services, Tel Aviv, Israel. His other affiliation, of Tel Aviv university, remains the same.
Compliance with Ethics Guidelines
The study was conducted in accordance with the protocol, applicable regulations, and guidelines governing clinical study conduct and the ethical principles that have their origin in the Declaration of Helsinki. The independent ethics committee and institutional review board, MHS Institutional Review Board, approved the study protocol and related documents (approval number 0108-18-BBL, December 18 2018). MHS's IRB waived the requirement to obtain any informed consent for this secondary analysis of existing data.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available because the data that support the findings of this study originate from Maccabi Healthcare Services and restrictions apply to the availability of these data. Due to restrictions, these data can be accessed only by request to the authors and/or Maccabi Healthcare Services.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Rosenberg, V., Chodick, G., Xue, Z. et al. Real-World Data of Adherence and Drug Survival of Biologics in Treatment-Naïve and Treatment-experienced Adult Patients with Rheumatoid Arthritis. Adv Ther 40, 4504–4522 (2023). https://doi.org/10.1007/s12325-023-02607-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02607-w