Abstract
Filgotinib is an oral preferential Janus kinase 1 inhibitor that demonstrated significant reductions in radiographic progression, with an acceptable tolerability and safety profile, vs placebo in patients with rheumatoid arthritis (RA) and an inadequate response to methotrexate (MTX-IR; FINCH 1) and vs MTX in MTX-naïve patients with RA (FINCH 3). International treatment guidelines identify multiple poor prognostic factors (PPFs) associated with worse disease outcomes among patients with RA. However, questions remain both about the clinical utility of considering PPFs and about which PPFs should drive treatment decisions. Additionally, the role of radiographic findings in clinical practice continues to be discussed and to evolve. This review examines radiographic results from post hoc analyses of phase 3 trials of filgotinib that examined subgroups with 4 PPFs or with baseline estimated rapid radiographic progression (e-RRP). In MTX groups, there were trends toward greater progression among patients with 4 PPFs or e-RRP, suggesting these subgroups may comprise a higher-risk population. Results show general consistency for the efficacy of filgotinib 200 mg plus MTX vs placebo plus MTX/MTX monotherapy on radiographic assessments, including change from baseline in modified total Sharp score and proportions without radiographic progression, even among MTX-IR or MTX-naïve patients with 4 PPFs or e-RRP who may be at higher risk of bone damage. Multivariate analysis identified multiple factors associated with baseline e-RRP status. This summary of the current understanding of benefits associated with filgotinib on radiographic progression and the relevance of baseline factors to these benefits may help inform treatment decisions for patients facing high risk of radiographic progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Poor prognostic factors (PPFs) are used in practice to predict disease course; however, there are questions regarding the utility of PPFs to predict outcomes, including radiographic progression. |
In the post hoc analyses reviewed in this paper, regardless of baseline predictors of prognosis, filgotinib groups showed additional treatment benefit compared to monotherapy with methotrexate (MTX). |
PPFs were generally associated with greater changes in modified total Sharp scores, with trends toward greatest change in patients with more PPFs across treatment groups. |
Benefits of filgotinib were observed in high-risk subgroups both in MTX-naïve patients and those with inadequate response to MTX. |
Introduction
Rheumatoid arthritis (RA) is a potentially difficult to treat autoimmune disorder, now treated with a variety of classes of disease-modifying antirheumatic drugs (DMARDs), including tumor necrosis factor and Janus kinase (JAK) inhibitors [1,2,3]. Several risk factors for poor prognosis (poor prognostic factors [PPFs]) among patients with RA have been identified, including seropositivity for rheumatoid factor (RF) and/or anti-citrullinated protein (i.e., anti-cyclic citrullinated peptide [anti-CCP]) antibodies [4], high disease activity, high baseline levels of acute reactants (e.g., C-reactive protein [CRP] [5]), and radiographic bone erosion at disease onset [6]. The 2022 European Alliance of Associations for Rheumatology (EULAR) guidelines for management of RA recommend adding a biologic DMARD or a targeted synthetic DMARD for patients with any of these 4 PPFs who do not achieve adequate response with the first conventional synthetic DMARD strategy [6]. Though the EULAR recommends treating to a clinical target, radiographic assessment provides an objective measure of anatomical damage and is a common element in clinical trials. Several publications confirmed that having a high rate of radiographic progression within the first year of RA was a strong predictor of subsequent radiographic progression and functional impairment [7,8,9]. While radiographic damage may accumulate at variable rates [7], pretreatment radiographic progression demonstrated an association with joint damage at follow-ups over 6 years, even among patients treated to the point of low clinical disease activity [8].
Filgotinib is an oral preferential JAK-1 inhibitor that, in combination with conventional synthetic DMARDs, has been shown to have clinical and radiographical efficacy in multiple populations of patients with RA [10]. In phase 3 trials in patients with RA and one or more of the PPFs mentioned above, filgotinib + methotrexate (MTX) demonstrated significant reductions in radiographic progression as measured by modified total Sharp score (mTSS) vs placebo + MTX among patients with inadequate response (IR) to MTX (MTX-IR; FINCH 1 [NCT02889796]) [11] and vs MTX in MTX-naïve patients (FINCH 3 [NCT02886728]) [12], with an acceptable tolerability and safety profile [10]. Supplementary Material Fig. 1 shows designs of these trials. An additional, smaller phase 3 trial in patients with IR/intolerance to one or more prior biologic DMARDs evaluated clinical endpoints, finding benefits for filgotinib vs placebo (FINCH 2 [NCT02873936]) [13].
In practice, PPFs are used as signals to begin or change therapeutic strategies; PPFs have also been used to predict disease outcomes, such as joint damage, remission, and functional limitation [14,15,16,17]. However, the utility of PPFs to predict outcomes may vary according to the combinations of PPFs included [18], as well as disease duration, treatment, and treatment targets [16, 19]. Radiographic progression, in particular, was questioned as a PPF, as the rate of progression among modern patients is notably more gradual than observed in past decades, and it may not be practical to conduct a trial of sufficient duration to detect treatment effects [18]. Additionally, radiographic progression may not be measured in clinical practice and therefore may have limited real-world application.
The objectives of this review are to consider and discuss the clinical meaningfulness of the 4 PPFs used to define higher-risk patients in analyses of data from filgotinib studies, review post hoc analyses of FINCH 1 and FINCH 3 that evaluated the effects of filgotinib on radiographic progression in patients with the 4 identified PPFs, characterize the effect of baseline progression rate on progression during subsequent filgotinib treatment, and identify any trends in adverse event (AE) rates between patients with 4 PPFs vs < 4 PPFs or different baseline progression rates in light of integrated safety analysis [20].
Data Sources
PubMed was searched to find radiographic assessments in phase 3 trials of filgotinib. Two phase 3 trials and three post hoc analyses were identified; the designs of these trials are shown in Supplementary Material Fig. 1. Post hoc analyses evaluated effects in subgroups with PPFs or with estimated rapid radiographic progression (e-RRP; ≥ 5).
In the FINCH 1 study, eligible MTX-IR patients with RA were randomly assigned in a 3:3:2:3 ratio to one of the following: filgotinib 200 mg (FIL200) or filgotinib 100 mg (FIL100) administered orally once daily, adalimumab (ADA) 40 mg administered subcutaneously biweekly, or placebo. All patients also received stable background MTX. At week 24, placebo-treated patients were re-randomized (1:1) to FIL200 or FIL100 while continuing the use of background MTX. Patient characteristics have been described in detail [11]. The 1755 patients who were randomized and treated were well matched across treatment groups: 82% were female, 68% were white, the mean age was 53 years, and the mean duration of RA was 7.8 years. Radiographs were performed at screening and weeks 12, 24, and 52 (campaign A included readings at screening, week 12, and week 24 or early termination visit before week 24; campaign B included readings at screening, week 24, week 52, or end of treatment visit if after week 24). The van der Heijde mTSS was assigned by two central readers blinded to treatment, patient information, and chronologic order of the radiographs; the average value was reported.
The FINCH 3 trial evaluated the efficacy and safety of filgotinib in combination with MTX or as a monotherapy, compared with the standard of care MTX monotherapy in MTX-naïve patients (those with active RA with limited or no prior MTX exposure). Patients were randomly assigned in a 2:1:1:2 ratio to receive FIL200 + MTX, FIL100 + MTX, FIL200 monotherapy, or MTX monotherapy. Patient characteristics have been described in detail [12]. The 1249 patients who were randomized and treated were well matched across treatment groups: 77% were female, 66% were white, the mean age was 53 years, and the mean duration of RA was 2.2 years. Radiographs were scored centrally in campaign A (those radiographs taken at baseline and week 24) and campaign B (those radiographs taken at baseline, week 24, and week 52 for patients who had images after week 24) by two blinded central readers and adjudicated by a third, if necessary.
The study protocols were reviewed and approved by all local institutional review boards or ethics committees of participating institutions. The studies were carried out in accordance with the Declaration of Helsinki of 1964 and its later amendments and the International Council for Harmonization Good Clinical Practice guidelines. Informed consent to participate was provided by all participants. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Radiographical Results in Total Randomized Trial Populations
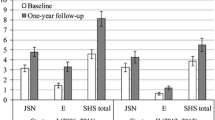
In the primary report of FINCH 1, as shown in Fig. 1a, at week 24, mTSS was reduced vs placebo by both FIL200 and FIL100; erosion score was similarly reduced by both FIL200 and FIL100 at week 24, while no significant differences were seen among groups in joint space narrowing [11]. At week 52, change from baseline in mTSS, erosion score, and joint space narrowing were reduced by FIL200 vs ADA (Fig. 1b). Figure 2 shows mTSS change and its components at weeks 24 and 52 of FINCH 3; at week 52, FIL200 + MTX, FIL100 + MTX, and FIL200 monotherapy were all associated with lower change from baseline in mTSS, joint space narrowing, and erosion score vs MTX monotherapy [12]. More patients in filgotinib treatment groups were free of radiographic progression (defined as mTSS change from baseline ≤ 0) at week 24 vs patients receiving MTX.
Radiographic progression (FINCH 1, MTX-IR patients) through a week 24, and b week 52 [11, 21]. ADA adalimumab, FIL filgotinib, FIL100 FIL 100 mg, FIL200 FIL 200 mg, IR inadequate response, JSN joint space narrowing, LS least squares, mTSS modified total Sharp score, MTX methotrexate, PBO placebo, PPF poor prognostic factor. Data from Combe et al. [11], Springer Nature, and Combe et al. [21], Springer Nature

Reproduced from Westhovens et al. [12] with permission from BMJ Publishing Group Ltd. (link to license), and Aletaha et al. [22] with permission from BMJ Publishing Group Ltd (link to license)
Findings from FINCH 3 (MTX-naïve patients); a change from baseline in mTSS and components in overall population at week 24, b change from baseline in mTSS and components in overall population at week 52, c mTSS change in patients with 4 PPFs vs overall population at week 24, and d mTSS change in patients with 4 PPFs and overall population at week 52 [12, 22]. a, b ***Nominal p < 0.001; **nominal p < 0.01; *nominal p < 0.05; for supportive analysis without adjustment for multiplicity. Error bars represent 95% CI. For mTSS, week 24 includes only data from campaign A and week 52 includes data from campaign A and B. Week 52 n values are not provided for mTSS change from baseline, as the analysis included both campaign A (through week 24) and campaign B (through week 52 including re-reading of baseline and week 24). c, d *Nominal p < 0.05; **nominal p < 0.01; ***nominal p < 0.001. CI confidence interval, ES erosion score, FIL filgotinib, FIL100 FIL 100 mg, FIL200 FIL 200 mg, JSN joint space narrowing, LSM least squares mean, mono monotherapy, mTSS modified total Sharp score, MTX methotrexate, PPF poor prognostic factor.
mTSS Change from Baseline in Subgroups with PPFs
A post hoc analysis of FINCH 1 evaluated the clinical benefits of filgotinib according to the presence or absence of 4 PPFs at baseline (seropositivity for RF or anti-CCP, high-sensitivity CRP [hsCRP] ≥ 6 mg/L, Disease Activity Score in 28 joints with CRP [DAS28(CRP)] > 5.1, and erosions) [21]; on the basis of study inclusion criteria, all patients were to have at least 1 PPF [11] (Table 1).
After 24 weeks of treatment, the change from baseline in mTSS among patients with 4 PPFs was highest in the placebo + MTX group (0.59) and lowest in the FIL200 + MTX group (0.17). Among patients with 4 PPFs, change from baseline in mTSS was significantly reduced by FIL200 + MTX and FIL100 + MTX vs placebo + MTX; among patients with < 4 PPFs, numerically smaller change from baseline vs placebo + MTX was seen (Fig. 1a) [21]. Supplementary Material Table 1 summarizes mTSS change from baseline in these subgroups, as well as mTSS change and proportions without progression in these subgroups and the other subgroups presented in this review. Supplementary Material Fig. 2 shows the mean change from baseline in mTSS among patients with 1, 2, 3, or 4 PPFs. In the placebo + MTX treatment group, change from baseline at week 24 increased numerically with each additional PPF, with the comparison between 2 and 4 PPFs reaching p < 0.001 and between < 4 and 4 PPFs reaching p = 0.007. Change from baseline was highest among those with 4 PPFs at weeks 24 and 52 in all treatment groups except ADA + MTX at week 52. Among patients in the FIL200 + MTX treatment group, change from baseline in mTSS at week 52 was comparable regardless of the number of PPFs. Week 52 change from baseline in the FIL100 + MTX treatment group was highest among patients with 4 PPFs. In the ADA + MTX treatment group, mTSS change from baseline increased numerically with greater numbers of PPFs [21].
In a post hoc analysis of the FINCH 3 MTX-naïve population, efficacy and safety among patients with 4 PPFs (as previously mentioned, except with ≥ 4 mg/L as the threshold for hsCRP) were examined [22] and compared to those in the entire trial population (based on study criteria, all patients were to have at least one PPF) [12]. All p values were considered nominal. Figure 2 shows change in mTSS from baseline in patients with 4 PPFs or the total population at week 24 and week 52 [22]. Compared with the population of all MTX-naïve patients, radiographic progression was numerically greater in patients with all 4 PPFs; at week 52, mTSS change from baseline in the MTX monotherapy arm was 1.45 among patients with 4 PPFs, compared with 0.74 in the total population of MTX-naïve patients. Patients with 4 PPFs had less radiographic progression at week 52 following treatment with all filgotinib regimens relative to MTX monotherapy, similar to results among all MTX-naïve patients.
mTSS Change from Baseline in Patients With or Without High Baseline Radiographic Progression Rate
Baseline radiographic progression rates were calculated for the FINCH 1 and FINCH 3 trial populations by dividing baseline mTSS by duration of RA in years (baseline mTSS/year) [23]. Post hoc analysis was then conducted comparing subgroups with e-RRP (change of ≥ 5) vs estimated nonrapid radiographic progression (e-NRRP; < 5).
Among MTX-IR patients, mTSS change from baseline was greater at week 24 among patients in the e-RRP subgroup vs e-NRRP in the FIL200 + MTX group (0.30 vs 0.04; p = 0.019) and in the placebo + MTX group (0.81 vs 0.19; p = 0.001), with numerically greater change from baseline among e-RRP subgroups vs e-NRRP in other treatment groups [23]. This may be due to the inclusion in FINCH 1 of patients with several years of disease duration; the 24 weeks of escalated treatment were possibly too brief to yield significant suppression of radiographic progression in e-NRRP patients, but the rapid changes seen in radiographs of the e-RRP patient population can be slowed by earlier intervention. This is supported by the observation that in the e-RRP subgroup, patients who switched to filgotinib after 24 weeks of placebo had notably greater progression at week 52 compared to patients who received filgotinib throughout the study. Significant suppression of radiographic progression vs placebo + MTX was seen with both FIL200 + MTX and FIL100 + MTX groups at week 24 in both e-RRP and e-NRRP subgroups (Fig. 3a). At week 52, FIL200 + MTX and FIL100 + MTX groups were also associated with reduced mTSS change from baseline compared with ADA among patients with e-RRP, while changes were comparable across treatments (with a trend toward the lowest change in the FIL200 + MTX group) in the e-NRRP subgroup. Among patients who were originally assigned to placebo + MTX who were then re-randomized to FIL200 + MTX or FIL100 + MTX at week 24, those in the e-RRP subgroups had greater radiographic progression (1.63 or 1.58) at week 52 than did patients in the e-NRRP subgroups (0.25 or 0.59; p < 0.001 or p = 0.022, respectively). Supplementary Material Fig. 3a displays a cumulative percentile of mTSS change at week 52; several individual patient values of high percentile mTSS change can be seen in the ADA + MTX e-RRP subgroup.

Reproduced from Tanaka et al. [23], Springer Nature (link to license)
Change from baseline in mTSS at week 24 and 52 in a MTX-IR and b MTX-naïve patients [23]. All p values are nominal vs PBO or ADA at week 24 and vs ADA at week 52 in FINCH 1 and vs MTX mono in FINCH 3. ADA at week 24 is out of scope for statistical calculation. ADA adalimumab, e-NRRP estimated nonrapid radiographic progression, e-RRP estimated rapid radiographic progression, FIL filgotinib, FIL100 FIL 100 mg, FIL200 FIL 200 mg, IR inadequate response, mono monotherapy, mTSS modified total Sharp score, MTX methotrexate, PBO placebo, W week.
Among the MTX-naïve population, within all treatment arms, mTSS change from baseline at week 24 was numerically greater in e-RRP subgroups compared to e-NRRP subgroups of the same treatment (e.g., 0.67 vs 0.25; p = 0.31 among patients taking MTX) [23]. Among e-RRP patients, only the FIL200 monotherapy group showed significantly reduced mTSS change from baseline at week 24 vs MTX monotherapy (p = 0.015; Fig. 3b). Results among patients with e-NRRP did not show significant benefit for filgotinib groups vs MTX at week 52; this may be due to mTSS change from baseline being notably lower among MTX-naïve patients with e-NRRP receiving MTX at week 52—approximately 1.0 among patients with e-RRP and below 0.5 among those with e-NRRP. At week 52, mTSS change from baseline among patients with e-RRP was lower in all filgotinib treatment groups compared with MTX monotherapy (p < 0.001, p = 0.043, and p = 0.011 for FIL200 + MTX, FIL100 + MTX, and FIL200 monotherapy, respectively). The cumulative percentile change in mTSS (Supplementary Material Fig. 3b) shows the spread of higher-percentile individuals particularly in the MTX group with e-RRP.
Proportions Without Radiographic Progression Among Patients with 4 or < 4 PPFs
Among MTX-IR patients with 4 PPFs, across treatment arms, the proportions of patients with no radiographic progression (based on ≤ 0.5-point change in mTSS) at week 24 were numerically smaller than in the < 4-PPF subgroup (Fig. 4) [21]. Consistently greater proportions (p < 0.05) of patients in the FIL200 + MTX group were without radiographic progression compared with those receiving placebo + MTX in patients with 4 or < 4 PPFs, while proportions in the FIL100 + MTX groups were numerically greater compared with placebo + MTX.

Adapted from Combe et al. [21], Springer Nature (link to license)
Proportions of MTX-IR patients (FINCH 1) with 4 PPFs and with < 4 PPFs with no radiographic progression (change from baseline mTSS ≤ 0.5) at week 24 [21]. All treatment groups also received MTX. ADA adalimumab, FIL filgotinib, FIL100 FIL 100 mg, FIL200 FIL 200 mg, IR inadequate response, mTSS modified total Sharp score, MTX methotrexate, PBO placebo, PPF poor prognostic factor.
Supplementary Material Table 2 shows proportions with no radiographic progression from baseline at week 24 among total MTX-naïve patients and those with 4 PPFs [22]. The proportion of FIL200 + MTX patients with 4 PPFs and no radiographic progression was 16.1% higher vs MTX (p = 0.004), while it was 8.1% higher in all MTX-naïve patients (p = 0.015). Supplementary Material Table 3 shows odds ratios and 95% confidence intervals for no radiographic progression at week 52 following treatment with filgotinib vs MTX monotherapy [22]. Among patients with 4 PPFs, the odds ratio for FIL200 + MTX vs MTX was 2.30 (nominal p = 0.004), compared with 1.93 in all MTX-naïve patients (p = 0.001). Only FIL200 + MTX showed nominally significant higher odds vs MTX monotherapy; FIL100 + MTX and FIL200 monotherapy did not.
Proportions Without Radiographic Progression Among Patients With or Without High Baseline Radiographic Progression Rate
Proportions of MTX-IR patients without radiographic progression were compared between e-RRP and e-NRRP subgroups in each treatment arm (Table 2) [23]. In the placebo + MTX group, at week 24, proportions without progression among e-RRP patients were lower vs those in the e-NRRP patients (76.2% vs 91.6%; p < 0.001), suggesting the baseline estimated yearly progression rate may be associated with radiographic progression under standard of care. The proportions of patients with no radiographic progression between treatment groups in e-RRP and e-NRRP subgroups are also shown in Table 2. Higher proportions of MTX-IR patients in both FIL200 + MTX and FIL100 + MTX groups vs placebo + MTX showed no radiographic progression (p = 0.024 and 0.014) among patients with e-RRP, while among e-NRRP, only FIL200 + MTX showed significantly greater proportions with no radiographic progression vs placebo + MTX (97.0% vs 91.6%; p = 0.012). At week 52, FIL200 + MTX sustained the proportion of e-RRP patients with no progression, while with FIL100 + MTX, the proportion decreased from 88.5% to 76.8%. For those with e-NRRP, proportions with no progression were sustained from week 24 to 52 in all treatment arms, including FIL100 + MTX. The proportions of both arms originally randomized to placebo + MTX with no progression in the e-RRP subgroup were reduced from 76.2% to 65.3% or 69.1%, respectively, for placebo + MTX to FIL200 + MTX or for FIL100 + MTX, while the e-NRRP subgroup proportions without progression were maintained from week 24 to 52.
Among MTX-naïve patients, proportions without radiographic progression in the e-RRP subgroup were higher (nominal p < 0.05) at week 24 in FIL200 + MTX and FIL200 groups vs MTX monotherapy; at week 52, proportions were higher in the FIL200 + MTX group vs MTX monotherapy (Table 3) [23]. In the e-NRRP subgroup, proportions without progression were higher at week 52 in the FIL200 + MTX group vs the MTX monotherapy group. Those patients with e-RRP receiving MTX showed lower proportions of no radiographic progression compared to those with e-NRRP at weeks 24 and 52 (78.6% vs 87.9% and 74.0% vs 84.4%; p = 0.032 and 0.029, respectively). Those receiving FIL200 + MTX showed lower proportions of no radiographic progression in the e-RRP subgroup compared to those with e-NRRP at week 24 (86.9% vs 94.0%, respectively; p = 0.033); no significant difference was observed at week 52 (86.0% vs 91.9%, respectively; p = 0.12). The proportions with no radiographic progression at week 24 in all treatment arms, except FIL200 monotherapy, were largely sustained at week 52.
Supplementary Material Table 4 shows the results of a multivariate analysis conducted to identify baseline factors associated with e-RRP rate (≥ 5) in the FINCH 1 and FINCH 3 populations [23]. Seropositivity was not associated with e-RRP status in either MTX-IR or MTX-naïve populations. Having hsCRP ≥ 30 mg/L at baseline was associated with e-RRP in the MTX-IR population only. Disease activity measures, including Clinical Disease Activity Index and Simple Disease Activity Index, were associated with e-RRP status in the MTX-naïve population but not in the MTX-IR trial population. Other previously identified factors, including baseline erosion score and swollen joint count, showed no association with e-RRP status in either MTX-naïve or MTX-IR populations.
Safety Among Higher-Risk RA Populations
Safety data from the PPF subgroup analysis of FINCH 1 demonstrated that having 4 PPFs was not associated with notably higher incidence of any particular treatment-emergent AE (TEAE) vs those with < 4 PPFs. Incidences of TEAEs of special interest—laboratory abnormalities, serious infections, herpes zoster, major adverse cardiovascular events, venous thromboembolism, malignancy, and gastrointestinal perforation—were < 5% or lower among patients with 4 PPFs or < 4 PPFs [21].
Among the MTX-naïve population, frequencies of TEAEs among patients with 4 PPFs, including all TEAEs, TEAEs of severity grade ≥ 3, serious TEAEs, and TEAEs leading to premature discontinuation, were generally similar to those in the total randomized population and comparable among treatment arms. The frequency of infectious TEAEs was higher among patients with 4 PPFs treated with FIL100 + MTX or FIL200 monotherapy (41.2% and 42.5%, respectively) vs all MTX-naïve patients (36.7% and 35.7%, respectively) and were comparable in patients with 4 PPFs receiving FIL200 + MTX [22]; otherwise, TEAEs of interest were generally similar between patients with 4 PPFs and all MTX-naïve patients.
Rates of TEAEs—including all TEAEs, serious TEAEs, TEAEs leading to permanent discontinuation, and death—were comparable between e-RRP and e-NRRP subgroups in the FIL200 + MTX treatment arm in both MTX-IR and MTX-naïve populations [23]. Proportions with TEAEs in MTX-IR patients were similar between FIL200 + MTX, FIL100 + MTX, and ADA + MTX arms. Among MTX-naïve patients, rates of overall TEAEs in the FIL100 + MTX group were 84.4% and 69.4% in e-RRP and e-NRRP subgroups, respectively. Among MTX-naïve patients, all treatment arms with e-RRP, except FIL200 monotherapy, showed higher rates of infections compared with those with e-NRRP. There were no evident trends for AEs of special interest across treatment groups or between e-RRP and e-NRRP subgroups [23].
In general, safety findings from these post hoc analyses were consistent with the results of an integrated safety analysis of available filgotinib phase 2 and 3 trial data [20]. That analysis found infections were more frequent among filgotinib groups vs placebo; these post hoc analyses did not find a consistent indication that PPFs or higher baseline radiographic progression rates were associated with greater incidence of infections among MTX-IR patients across treatment arms, while infection rates were generally higher among MTX-naïve patients with 4 PPFs or e-RRP status.
Discussion
Interpreting Filgotinib Effects on Radiographical Progression in Higher-Risk Populations
Data presented here from post hoc analyses indicate that previously identified predictive factors may affect the efficacy of MTX monotherapy treatment among patients with MTX-IR or MTX-naïve RA. FIL200 + MTX retained its efficacy vs MTX monotherapy even in higher-risk patients with all 4 PPFs. Furthermore, while estimated radiographic progression rate at baseline may influence treatment efficacy, even among patients with high baseline progression rates, FIL200 + MTX was associated with reduced radiographic change vs MTX monotherapy.
Other factors were previously found to predict radiographic progression. In analysis of abatacept clinical trial data, baseline disease activity measures, such as DAS28(CRP), were significant predictors of radiographically detected structural progression [24]. The EULAR guidelines include several additional PPFs beyond those examined here as prompts for treatment initiation/change, such as moderate-to-high disease activity based on composite measures and failure of two or more conventional synthetic DMARDs [6]. While the FINCH 1 and FINCH 3 trials demonstrated the benefits of filgotinib in patients with at least 1 PPF, the analyses described here indicate that even the presence of 4 PPFs does not prevent FIL200 + MTX from slowing radiographic progression compared with ADA + MTX or MTX monotherapy [22]. Therapy with biologic DMARDs was previously demonstrated to be effective in patients with 1 or 2 PPFs. Certolizumab, a humanized anti-TNF antibody fragment, in combination with MTX was more effective in suppressing radiographic progression than placebo + MTX among patients with any of the several PPFs, including anti-CCP antibody, CRP > 1.0 mg/dL, or high disease activity [25]. In the post hoc analysis of trials of the JAK inhibitor tofacitinib, greater treatment effect on suppression of progression was seen among patients with both baseline erosions and higher CRP level vs patients with a single PPF [18]. In post hoc analyses reviewed here, patients in the FIL100 + MTX group showed greater progression at week 52 in the presence of 4 vs < 4 PPFs, while FIL200 + MTX was similarly effective at suppressing progression regardless of the number of PPFs.
Limitations
FINCH 1 and FINCH 3 are subject to the usual limitations of generalizability attendant to randomized clinical trials. As discussed by Combe et al. [11], FINCH 1 excluded patients with prior bDMARD failure; hence, observations cannot be extrapolated to patients who had been previously treated with bDMARDs. While FINCH 1 included a “placebo” group that demonstrated an unexpectedly strong response (nearly 50% achieved study endpoints; note that this group received background MTX therapy as did all trial participants), FINCH 3 did not include a placebo group, which may have introduced expectation bias. Both FINCH 1 and 3 enrolled only patients with moderate-to-severe disease; effects cannot be assumed to be similar in patients with less severe disease. And finally, in FINCH 3, the low progression rate of structural damage compromised differentiation of benefit between filgotinib and MTX.
The findings we report here, based on post hoc analyses of clinical trials, may not necessarily translate to effects in the real world. Results from real-world studies have found several other predictive factors that may affect adherence (a proxy for efficacy and/or safety) to JAK inhibitor therapy, including prior anti-interleukin-6 receptor use, age, prednisolone use, and male sex [26]. History of radiographic results at the start of treatment may not be routinely available, limiting the usefulness of past radiographic progression rate as a predictor of treatment efficacy in clinical practice. We were not able to review the erosion score and joint space narrowing scores in the higher-risk subgroups, limiting our ability to interpret the mechanism of filgotinib’s effects.
One analysis described here employed the rate of progression before treatment as a predictive factor. While this appears to play a role in subsequent progression, calculation of progression before treatment presents its own difficulties and may represent a limitation of the analysis. The method of calculating the e-RRP makes it dependent on the timing of diagnosis of RA, and the time of past diagnosis likely does not correspond precisely to disease onset. While the duration of high/moderate disease activity prior to the trial can contribute to a poor prognosis, disease activity at baseline may not necessarily reflect duration. The analyses described here found notably higher progression rates with ADA + MTX and MTX monotherapy treatment among those patients with higher baseline progression compared with those who had lower baseline progression, while filgotinib ameliorated this progression [23]. Yet, because pretreatment progression rate is difficult to quantify, further work is needed to determine whether it can be considered a reliable predictor of progression or treatment response.
The sensitivity of radiographic changes during a relatively limited duration in a clinical trial makes the understanding of different treatment effects challenging. Nevertheless, the observations in different subgroups over time, particularly while initiating a new therapy, offer an opportunity to understand the degrees of inhibition of radiographic progression associated with different treatments.
Conclusions
The post hoc analyses reviewed here extended the knowledge base for radiographic benefits of filgotinib in patients with RA. As shown in the primary publications in MTX-IR and MTX-naïve populations, filgotinib groups showed additional treatment benefit vs monotherapy with MTX in patients with baseline predictors of prognosis (PPF and e-RRP), suggesting a broad patient population may realize benefit with filgotinib. Progression was slowed by FIL200 + MTX vs comparators even in high-risk populations; the analyses reviewed here help to describe both those patient populations likely to show greater progression when receiving standard of care therapy and those with the most to gain via the introduction of filgotinib.
Data Availability
Gilead Sciences shares anonymized individual patient data upon request or as required by law or regulation with qualified external researchers based on submitted curriculum vitae and reflecting non conflict of interest. The request proposal must also include a statistician. Approval of such requests is at Gilead Science’s discretion and is dependent on the nature of the request, the merit of the research proposed, the availability of the data, and the intended use of the data. Data requests should be sent to datarequest@gilead.com.
References
Roodenrijs NMT, van der Goes MC, Welsing PMJ, et al. Difficult-to-treat rheumatoid arthritis: contributing factors and burden of disease. Rheumatology (Oxford). 2021;60(8):3778–88.
Nash P, Kerschbaumer A, Dorner T, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis. 2021;80(1):71–87.
Tanaka Y, Luo Y, O’Shea JJ, Nakayamada S. Janus kinase-targeting therapies in rheumatology: a mechanisms-based approach. Nat Rev Rheumatol. 2022;18(3):133–45.
Liu J, Gao J, Wu Z, et al. Anti-citrullinated protein antibody generation, pathogenesis, clinical application, and prospects. Front Med (Lausanne). 2021;8:802934.
Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754.
Smolen JS, Landewe RBM, Bergstra SA, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 2023;82(1):3–18.
Ten KPM, Versteeg LGA, Oude VMAH, et al. Radiographic progression can still occur in individual patients with low or moderate disease activity in the current treat-to-target paradigm: real-world data from the Dutch Rheumatoid Arthritis Monitoring (DREAM) registry. Arthritis Res Ther. 2019;21(1):237.
Versteeg GA, Steunebrink L, Vonkeman HE, Ten KPM, Van DBAE, Van DLM. Early radiological progression remains associated with long-term joint damage in real-world rheumatoid arthritis patients treated to the target of remission. Scand J Rheumatol. 2022;51(2):87–96.
van den Broek M, Dirven L, de Vries-Bouwstra JK, et al. Rapid radiological progression in the first year of early rheumatoid arthritis is predictive of disability and joint damage progression during 8 years of follow-up. Ann Rheum Dis. 2012;71(9):1530–3.
Tanaka Y, Kavanaugh A, Wicklund J, McInnes IB. Filgotinib, a novel JAK1-preferential inhibitor for the treatment of rheumatoid arthritis: an overview from clinical trials. Mod Rheumatol. 2022;32(1):1–11.
Combe B, Kivitz A, Tanaka Y, et al. Filgotinib versus placebo or adalimumab in patients with rheumatoid arthritis and inadequate response to methotrexate: a phase III randomised clinical trial. Ann Rheum Dis. 2021;80(7):848–58.
Westhovens R, Rigby WFC, van der Heijde D, et al. Filgotinib in combination with methotrexate or as monotherapy versus methotrexate monotherapy in patients with active rheumatoid arthritis and limited or no prior exposure to methotrexate: the phase 3, randomised controlled FINCH 3 trial. Ann Rheum Dis. 2021;80(6):727–38.
Genovese MC, Kalunian K, Gottenberg JE, et al. Effect of filgotinib vs placebo on clinical response in patients with moderate to severe rheumatoid arthritis refractory to disease-modifying antirheumatic drug therapy: the FINCH 2 randomized clinical trial. JAMA. 2019;322(4):315–25.
Tanaka Y, Takeuchi T, Kato D, et al. Post hoc analysis of clinical characteristics of patients with radiographic progression in a Japanese phase 3 trial of peficitinib and methotrexate treatment (RAJ4). Mod Rheumatol. 2023;33(1):73–80.
Takeuchi T, Soen S, Ishiguro N, et al. Predictors of new bone erosion in rheumatoid arthritis patients receiving conventional synthetic disease-modifying antirheumatic drugs: analysis of data from the DRIVE and DESIRABLE studies. Mod Rheumatol. 2021;31(1):34–41.
Albrecht K, Zink A. Poor prognostic factors guiding treatment decisions in rheumatoid arthritis patients: a review of data from randomized clinical trials and cohort studies. Arthritis Res Ther. 2017;19(1):68.
Visser K, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, et al. A matrix risk model for the prediction of rapid radiographic progression in patients with rheumatoid arthritis receiving different dynamic treatment strategies: post hoc analyses from the BeSt study. Ann Rheum Dis. 2010;69(7):1333–7.
Landewe RB, Connell CA, Bradley JD, et al. Is radiographic progression in modern rheumatoid arthritis trials still a robust outcome? Experience from tofacitinib clinical trials. Arthritis Res Ther. 2016;18(1):212.
Robustillo VM, Rodriguez MJ. Are useful prognostic factors in rheumatoid arthritis? Reumatol Clin. 2011;7(5):339–42 (In Spanish).
Winthrop KL, Tanaka Y, Takeuchi T, et al. Integrated safety analysis of filgotinib in patients with moderately to severely active rheumatoid arthritis receiving treatment over a median of 1.6 years. Ann Rheum Dis. 2022;81(2):184–92.
Combe BG, Tanaka Y, Buch MH, et al. Efficacy and safety of filgotinib in patients with high risk of poor prognosis who showed inadequate response to MTX: a post hoc analysis of the FINCH 1 study. Rheumatol Ther. 2023;10(1):53–70.
Aletaha D, Westhovens R, Gaujoux-Viala C, et al. Efficacy and safety of filgotinib in methotrexate-naive patients with rheumatoid arthritis with poor prognostic factors: post hoc analysis of FINCH 3. RMD Open. 2021;7(2):e001621.
Tanaka Y, Atsumi T, Aletaha D, et al. Benefit of filgotinib, a JAK1 preferential inhibitor, in rheumatoid arthritis patients with previous rapid radiographic progression: post hoc analysis of two trials. Rheumatol Ther. 2022;10(1):161–85.
Keystone EC, Ahmad HA, Yazici Y, Bergman MJ. Disease activity measures at baseline predict structural damage progression: data from the randomized, controlled AMPLE and AVERT trials. Rheumatology (Oxford). 2020;59(8):2090–8.
Atsumi T, Yamamoto K, Takeuchi T, et al. The first double-blind, randomised, parallel-group certolizumab pegol study in methotrexate-naive early rheumatoid arthritis patients with poor prognostic factors, C-OPERA, shows inhibition of radiographic progression. Ann Rheum Dis. 2016;75(1):75–83.
Ebina K, Hirano T, Maeda Y, et al. Factors affecting drug retention of Janus kinase inhibitors in patients with rheumatoid arthritis: the ANSWER cohort study. Sci Rep. 2022;12(1):134.
Acknowledgements
Medical Writing, Editorial, and Other Assistance
Medical writing support was provided by Rob Coover, MPH, of AlphaScientia, a Red Nucleus company, and funded by Eisai Co., Ltd., and Gilead Sciences K.K.
Funding
Funding for this analysis and the Rapid Service Fee was provided by Eisai Co., Ltd., and Gilead Sciences K.K. The sponsors participated in the planning, execution, and interpretation of the research.
Author information
Authors and Affiliations
Contributions
Yoshiya Tanaka, Tsutomu Takeuchi, Bernard G. Combe, Daniel Aletaha, and Vijay Rajendran were involved in the conception and design of the study/analyses. Yoshiya Tanaka, Tsutomu Takeuchi, Tatsuya Atsumi, Bernard G. Combe, Daniel Aletaha, Toshihiko Kaise, and Vijay Rajendran contributed to the conception of the review and interpretation. All authors commented on previous versions of the manuscript, and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Yoshiya Tanaka received speaking fees and/or honoraria from AbbVie, Amgen, Astellas, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Chugai, Eisai, Eli Lilly, Gilead, Mitsubishi-Tanabe, and YL Biologics and received research grants from AbbVie, Asahi-Kasei, Boehringer-Ingelheim, Chugai, Corrona, Daiichi Sankyo, Eisai, Kowa, Mitsubishi-Tanabe, and Takeda. Tsutomu Takeuchi reports receiving grant/research support from AbbVie, Asahi Kasei, Astellas, Chugai, Daiichi Sankyo, Eisai, Mitsubishi-Tanabe, Shionogi, Takeda, and UCB Japan; serving as a consultant for Astellas, Chugai, and Eli Lilly Japan; and serving on a speakers’ bureau for AbbVie, Ayumi Pharmaceutical Corporation, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Dainippon Sumitomo Eisai, Eli Lilly Japan, Mitsubishi-Tanabe, Novartis, Pfizer Japan, Sanofi, and Gilead Sciences, Inc. Tatsuya Atsumi received research grants, speaking fees, and/or consultancy fees from Gilead Sciences K.K.; Eisai Co., Ltd.; AbbVie Inc.; Alexion Inc.; Asahi-Kasei Co.; Astellas Pharma Inc.; AstraZeneca plc.; Bayer Yakuhin; Bristol-Myers Squibb Co.; Chugai Pharmaceutical Co., Ltd.; Daiichi Sankyo Co., Ltd.; Eli Lilly Japan K.K.; GlaxoSmithKline K.K.; Janssen Pharmaceutical K.K.; Kyowa Kirin Co., Ltd.; Novartis Pharma K.K.; Nippon Boehringer Ingelheim Co., Ltd.; Mitsubishi Tanabe Pharma Co.; Ono Pharmaceutical Co. Ltd.; Otsuka Pharmaceutical Co., Ltd.; Pfizer Inc.; Taiho Pharmaceutical Co., Ltd.; Takeda Pharmaceutical Co., Ltd.; Teijin Pharma Ltd.; and UCB Japan Co. Ltd. Bernard G. Combe receives grant/research support from Pfizer and Roche-Chugai; and received consulting and/or speaker fees from AbbVie, Bristol-Myers Squibb, Celltrion, Eli Lilly, Gilead-Galapagos, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, and Roche-Chugai. Daniel Aletaha reports grants or research support from AbbVie, Merck Sharp & Dohme, Novartis, and Roche; serving as a consultant for Janssen; serving on a speaker’s bureau for Bristol-Myers Squibb, Merck Sharp & Dohme, and UCB; and serving as a consultant and on a speaker’s bureau for AbbVie, Amgen, Celgene, Eli Lilly, Medac, Merck, Novartis, Pfizer, Roche, Sandoz, and Sanofi/Genzyme. Toshihiko Kaise is an employee of Gilead Sciences K.K. and shareholder of Gilead Sciences, Inc. Vijay Rajendran is an employee and shareholder of Galapagos BV.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. The study protocols were reviewed and approved by all local institutional review boards or ethics committees of participating institutions. The studies were carried out in accordance with the Declaration of Helsinki and the International Council for Harmonization Good Clinical Practice guidelines. Informed consent to participate was provided by all participants.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Tanaka, Y., Takeuchi, T., Atsumi, T. et al. Prevention of Radiographic Progression in Higher-Risk Patients with Rheumatoid Arthritis Using Filgotinib in Phase III Studies: Narrative Review of Post Hoc Analyses. Rheumatol Ther 10, 1399–1415 (2023). https://doi.org/10.1007/s40744-023-00590-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-023-00590-w