Abstract
Background
Early and intensive targeted treatment with disease modifying anti-rheumatic drugs (DMARDs) has been shown to lead to substantial reductions in disease activity and radiograph damage in patients with early rheumatoid arthritis (RA). The aim of this quasi-experimental study was to compare the first-year radiographic progression rates between a treat-to-target (T2 T) strategy with initial combination therapy (strategy II, started in 2012) versus an initial step-up monotherapy (strategy I, started in 2006).
Methods
A total of 128 patients from strategy II was individually matched with 128 patients from strategy I on sex, age (± 5 yrs.) and baseline disease activity (± 0.5 on the DAS28). Differences in radiographic progression (Sharp/van der Heijde) scores (SHS) and the number of patients experiencing a minimal clinically important difference (MCID; ≥ 5 SHS points) between both strategies were tested with Mann Whitney U and chi-square tests. Next, linear and logistic regression analyses were performed to examine which baseline variables were associated with radiographic progression scores and the probability of experiencing an MCID within 1 year.
Results
Patients with initial combination therapy had slightly higher baseline disease activity scores and pain scores, but better mental health scores. Patients with initial monotherapy had significantly more, and more frequently clinically relevant, radiographic progression after 1 year. Experiencing a MCID was independently associated with fewer tender joints (p = 0.050) and higher erythrocyte sedimentation rate (p = 0.015) at baseline.
Conclusion
Treating early RA patients with initial combination therapy results in better radiographic outcomes than initial monotherapy in daily clinical practice.
Trial registration
Netherlands Trial Register NTR578, 12 January 2006.
Similar content being viewed by others
Background
Rheumatoid arthritis (RA) is characterized by joint inflammation leading to joint destruction and related to a decrease in functional capacity, work disability, and reduced quality of life [1]. Prevention of structural damage is an important goal in the treatment of RA. In the last years, research has shown that early intensive treatment improves both the short- and long-term outcomes of RA [2,3,4,5,6]. The benefits of early use of (combinations of) disease modifying anti-rheumatic drugs (DMARDs) and biological agents [7,8,9,10,11,12], in combination with protocolled treatment aimed at a predefined goal (treat-to-target (T2T)) [13,14,15], has led to a change in traditional treatment paradigms. More specifically, early and intensive targeted treatment with DMARDs has been shown to lead to substantial reductions in disease activity [5, 6] and radiologic damage in patients with early RA [8, 13, 16,17,18,19,20,21].
However, in some early RA patients joint damage progresses even during DMARD use. In fact, even if DMARDs are initiated ‘very early’ in the disease, some patients may still develop erosions and progressive joint damage [2, 4, 22, 23]. Previous research has shown that in 7–17% of patients with RA in prolonged clinical remission, progression of joint damage still occurs [24, 25]. Van der Kooij et al. (2009) showed that 10–33% of patients in drug-free remission still showed progressive joint damage over a period of 4 years follow-up [26]. Whether the complete absence of arthritis activity prevents further joint damage in all patients, is still a matter of debate.
Previously, we demonstrated that implementation of a step-up T2T strategy in RA in daily clinical practice led to limited radiographic damage during a follow-up of 3 years [27, 28]. We also showed that while a T2T strategy with initial combination therapy was not superior to a T2T strategy with step-up therapy in the proportion of patients in remission at 12 months follow-up (77% versus 72%, respectively), the strategy with initial combination therapy did result in a significantly shorter time until remission. At 6 months, mean disease activity scores were lower in patients with initial combination therapy than in those with step-up therapy [6]. This is in line with clinical trials showing that initial combination therapy results in more rapid improvements in disease activity, daily functioning and quality of life than initial monotherapy [9, 13, 29, 30]. However, whether initial combination therapy and the subsequent shorter time to remission, as compared to initial step-up monotherapy, also results in better radiological outcomes has not been well studied in real-life clinical practice. Therefore, the aim of the present study was to compare the first-year radiological progression rates between a T2T strategy with initial combination therapy versus a T2T strategy with initial step-up monotherapy within the Dutch RhEumatoid Arthritis Monitoring (DREAM) registry.
Methods
Data selection and study design
This study used data from the ongoing DREAM T2T remission induction strategies I (initial step-up monotherapy) and II (initial combination therapy), two observational, multicenter strategies which were established in 2006 and 2012, respectively [5, 6, 27]. In both strategies, adults ≥18 years with a clinical diagnosis of RA and a disease duration (time from the diagnosis to the start of therapy) < 1 year were enrolled consecutively immediately after a clinical diagnosis of RA. For this study, data were used from two participating hospitals; Medisch Spectrum Twente in Enschede and Isala in Zwolle, both in The Netherlands. Patients included from 2006 to 2012 were used for strategy I, and patients included from 2012 to 2013 were used for strategy II. Both treatment strategies were in line with clinical practice and comply with current guidelines for treatment of RA. Exclusion criteria for both strategies were use of prednisolone ≥10 mg/day or previous or current treatment with disease-modifying antirheumatic drugs (DMARDs). The Medical Ethics Committees of the Medisch Spectrum Twente, Enschede and Isala, Zwolle hospitals determined, in accordance with Dutch Law on medical-scientific research with humans, that no ethical approval was required because all data were collected in the course of regular daily clinical practice. Nonetheless, patients were completely informed and informed consent was obtained from each patient.
At the time of the current analysis, 137 patients had a follow-up of at least 1 year in strategy II. For the aim of this quasi-experimental study, a total of 128 patients from strategy II could be individually matched with 128 patients from strategy I on sex, age (± 5 yrs.) and baseline disease activity (± 0.5 on the DAS28).
Treat to target protocol
Patients in both strategies were treated according to a T2T strategy with protocolized treatment adjustments aiming at remission (DAS28 < 2.6), details of which have previously been published [5, 6, 27]. Briefly, the main differences between both strategies were; time moments of evaluation, and the medication that was started immediately after diagnosis (mono/step-up therapy versus combination therapy). In strategy I, patients were evaluated at 0, 8, 12, 20, 24, 36, and 52 weeks and every 3 months thereafter. In strategy II, patients were evaluated at months 0, 2, 4, 6 and every 3 months thereafter.
In strategy I, treatment protocol was an initial monotherapy of 15 mg/week methotrexate (MTX), with folic acid taken at the second day after MTX. In case of insufficient response (DAS28 ≥ 2.6) at the subsequent time-points, the following per protocol treatment steps were advised: after 2 months, MTX dosage was increased to 25 mg/week; after 3 months sulfasalazine (SSZ) 2000 mg/day was added; in week 20 SSZ dosages was increased to 3000 mg/day. Tumor necrosis factor inhibitor (TNFi) was prescribed at week 24 for patients with persistent moderate disease activity (DAS28 remained ≥3.2). If remission is achieved with DMARDs and/or TNFi, while maintaining remission for at least 6 months, medications were tapered and if possible discontinued starting with the TNFi and subsequently with the DMARDs.
In strategy II, treatment protocol was an initial combination therapy of MTX 20 mg/week and hydroxychloroquine (HCQ) 200 mg twice daily. As bridging therapy, an optional intramuscular triamcinolone injection to a maximum dosage of 120 mg could be given. After 1 month, MTX dosage was increased to 25 mg/week, independent of disease activity. After 2 months, in case of persistent disease activity (DAS28 ≥ 2.6), MTX dosage was further increased to 30 mg/week and an extra optional intramuscular triamcinolone injection could be administered. TNFi was prescribed at 4 months for patients with persistent moderate disease activity (DAS28 ≥ 3.2). If sustained remission for at least 6 months remission was achieved with DMARDs and/or TNFi, medications were tapered and if possible discontinued starting with the TNFi and subsequently with the DMARDs.
Assessments
At each assessment, data were collected on various clinical and patient-reported outcome measures, including measures of disease activity, health related quality of life, physical functioning, and laboratory measures. Disease activity was assessed by trained rheumatology nurses using the Disease Activity Score for 28 joints (DAS28), consisting of a 28 swollen and tender joint count, the erythrocyte sedimentation rate (ESR) and a 100 mm visual analog scale (VAS) on general health (“Considering all the ways your arthritis affects you, how are you doing now?”, where 0 = “very good” and 100 = “very bad”) [31]. The Health Assessment Questionnaire Disability Index (HAQ-DI) was used to assess physical function [32]. Furthermore, patients rated their pain in the past week on a 100 mm VAS (0 = “no pain” and 100 = “unbearable pain”) and completed the Short Form Health Survey with 36 items (SF-36) in order to assess their current physical and mental health status [33]. Radiographs of hands and feet were obtained at baseline and annually thereafter. Radiographs were evaluated by two trained readers together, according to the modified Sharp/van der Heijde score (SHS) method [34], and a consensus score was obtained. Readers were not blinded to treatment strategy or assessment time point of the radiographs. A patient was classified as having erosive disease if the Sharp/van der Heijde erosion score was ≥1. Clinically relevant radiographic progression (minimal clinically important difference; MCID) was defined as an increase of ≥5 in the total SHS score [34].
Statistical analysis
Descriptive statistics for categorical and continuous variables were reported as frequencies, percentages, means and standard deviations (SD). If continuous variables were not normally distributed, the median with the corresponding interquartile range (IQR) was reported. To test for any baseline differences between both strategies, we performed independent t-tests for normally distributed variables, Mann-Whitney U tests for non-normally distributed variables and chi-square tests for categorical variables. As only two time points were examined (baseline and 12 months), radiographic progression was calculated for observed values only. Differences between both strategies in one-year radiographic progression and the proportion of patients experiencing an increase of ≥5 SHS points (MCID) were tested using Mann Whitney U test and chi-square test. Group differences in progression between strategies were additionally visualized with a cumulative probability plot [35]. Next, univariate and multivariate linear and logistic regression analyses were performed to examine which other baseline variables were associated with radiographic progression and experiencing MCID within 1 year and to test for possible interactions with treatment strategy. Continuous variables were mean centered to allow for meaningful interpretation of main effects in addition to the interaction. The linearity assumption of continuous variables in the linear regression analyses was checked with scatterplots. Variables significantly (p < 0.05) associated with progression or with a significant interaction term in univariate analysis were entered as a covariate into a multivariate linear and logistic regression analysis model. To avoid multicollinearity, Pearson correlations were calculated between the independent variables to check for multicollinearity problems (r > 0.5). The explained variance of the final linear and logistic models was examined using (Nagelkerke’s pseudo) R2. The final logistic model was additionally tested for goodness of fit using the Hosmer and Lemeshow test. All statistical calculations were performed using version 22 of the SPSS statistical package for Windows.
Results
Patient characteristics
A total of 256 patients was enrolled in the study, 128 patients for each T2T strategy. Baseline characteristic of patients in both strategies were generally similar (Table 1). Patients had active disease, with a mean DAS28 of 4.8 in the T2T strategy with initial combination therapy (strategy II) versus a mean DAS28 of 4.5 in the T2T strategy with initial monotherapy (strategy I). Most patients were female and the majority was anti-CCP positive. Patients starting with initial combination therapy had slightly higher baseline disease activity scores and pain scores, but better mental health scores. Patients receiving an injection of triamcinolone had higher baseline DAS28 scores than those that did not receive an injection in both strategy I (5.6 ± 1.5 versus 4.5 ± 1.1; p = 0.038) and strategy II (5.1 ± 1.1 versus 4.6 ± 0.9; p = 0.011). Approximately 18% of the patients received MTX at baseline subcutaneously, whereas 82% of the patients received MTX orally. At 12 months the majority of the patients in both strategies received conventional synthetic DMARDs (csDMARDs) only. In strategy I, almost 4% of the patients were prescribed a biological DMARD (bDMARD), versus almost 9% in strategy II. In strategy II there were slightly more patients in whom DMARD use was fully discontinued (8% versus 2%). Median dose of triamcinolone administered to patients at baseline in strategy I was 80 mg. 75% (3/4) of the patients received 80 mg triamcinolone and 25% (1/4) of the patients received 120 mg triamcinolone. The median dose of triamcinolone administered to patients at baseline in strategy II was 120 mg. 93% (62/67) of the patients received 120 mg triamcinolone and 7% (5/67) of the patients received 80 mg triamcinolone at baseline.
Within the first year, there were significantly more patients with registered complications in strategy II (n = 45 [35%]) than in strategy I (n = 15 [12%]; p < 0.01). In total, there were 27 complications registered in strategy I compared to 83 complications in strategy II. Complication in strategy I consisted of: malaise = 14; gastrointestinal = 2; lab abnormality = 4; skin/hair disorder/allergy = 3; infection = 2; cardiovascular event = 1; other = 1. Complication in strategy II consisted of: malaise = 38; gastrointestinal = 7; lab abnormality = 8; skin/hair disorder/allergy = 12; eye complaints = 8; pulmonary abnormality = 1; infection = 1; cardiovascular event = 2; other = 6.
Radiographic progression
Baseline radiographs were available for 250 patients (124 patients in strategy II and 126 patients in strategy I). One-year follow-up radiographs were available for 222 patients (104 patients in strategy II and 118 patients in strategy I). Baseline and follow-up SHS scores are presented separately for both treatment strategies in Fig. 1.
At baseline, median SHS scores were not significantly different (p = 0.119) between both strategies. Median baseline erosion scores tended to be slightly higher in strategy I (p = 0.012) with 43% (54/126) of the patients versus 30% (37/124) of the patients in strategy II having at least one erosion, while joint space narrowing (JSN) scores were not different between strategies with 71% (90/126) of the patients in strategy I versus 68% (84/124) of the patients in strategy II showing JSN.
At one-year follow-up, the median SHS score was significantly higher in patient treated with initial monotherapy (p = 0.001). The median SHS score increased from 3.0 (IQR 1.0–7.0) at baseline to 5.5 (IQR 3.0–12.0) in strategy I and from 2.0 (IQR 1.0–5.75) to 3.0 (IQR 1.0–9.0) in strategy II. One-year erosion scores were significantly different (p < 0.001) between both strategies, while JSN scores were not (P = 0.117). In strategy I, median erosion scores increased from 0.0 (IQR 0.0–1.0) to 2.0 (IQR 0.0–4.0) and median JSN scores increased from 2.0 (IQR 0.0–5.0) to 3.0 (IQR 1.0–7.0). In strategy II, median erosion scores increased from 0.0 (IQR 0.0–1.0) to 1.0 (0.0–2.0), while JSN scores increased from 1.0 (IQR 0.0–4.75) to 2.0 (IQR 1.0–6.0).
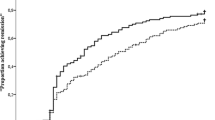
Median progression was significantly higher in strategy I (2.0; IQR 1.0–4.0) than in strategy II (1.0; IQR 0.0–3.0; p < 0.001). This difference was similar for both anti-CCP negative (2.0 [IQR 1.0–3.0] vs. 1.0 [IQR 0.0–2.0]; p = 0.023) and anti-CCP positive patients (3.0 [IQR 1.0–5.0] vs. 1.0 [QR 0.0–3.0]; p < 0.001). The difference in individual radiographic progression scores between both strategies was also visible in the cumulative probability plot (Fig. 2). Most notably, the proportion of patients with no radiographic progression at all was substantially higher strategy II (initial combination therapy) (43%) than in strategy I (initial monotherapy) (14%).
Also, significantly more patients treated with initial monotherapy had clinically relevant progression (≥ 5 SHS points) after 1 year (26/118 [20%]) than patients treated with initial combination therapy (10/104 [8%]; p = 0.012).
Univariate associations with progression
Fewer tender joints (p = 0.033), higher ESR (p = 0.033), higher age (p = 0.042), and no triamcinolone injection (p = 0.007) were significantly associated with more radiographic progression within the first year in the total sample (Table 2). There were no significant interactions with strategy in the linear regression analyses. With respect to clinically relevant radiographic progression, fewer tender joints (p = 0.016), higher ESR (p = 0.038), positive anti-CCP (p = 0.040) and lower BMI (p = 0.039) were significantly associated with experiencing a MCID. Moreover, there was a significant strategy interaction with swollen joint count scores and SF-36 PCS scores. Swollen joint count scores were not associated with experiencing a MCID in strategy II (OR = 0.90; CI 95% 0.77–1.07; p = 0.227) but were positively associated in strategy I (OR = 1.10; CI 95% 1.00–1.22; p = 0.050). SF-36 PCS scores were not associated with reaching a MCID in strategy I (OR = 0.98 CI 95% 0.92–1.04 p = 0.503) but were positively associated with reaching a MCID in strategy II (OR = 1.11; CI 95% 1.00–1.22; p = 0.040).
Multivariate analyses
Strategy I with initial monotherapy remained significantly associated with more radiographic progression and experiencing a MCID after controlling for covariates and strategy interactions in multivariate analyses (Tables 3 and 4). None of the covariates remained significantly associated with continuous radiographic progression scores (Table 3). For clinically relevant progression, fewer tender joints (p = 0.050) and higher ESR (p = 0.015) remained significantly associated with experiencing a MCID (Table 4).
Discussion
The aim of this study was to compare one-year radiographic outcomes of two treat-to-target strategies, with initial mono- versus combination therapy, in early RA patients in daily clinical practice. These two early RA strategies in the DREAM registry confirm that, overall, treat-to-target strategies result in limited short-term radiographic progression. We observed an even more favorable outcome among patients with early RA who were treated with initial combination therapy (strategy II), as compared to patients who were treated with initial monotherapy (strategy I). A substantially larger number of patients within strategy II showed no radiographic progression at all and only a small portion of patients showed clinically relevant progression.
Fewer painful joints and a higher erythrocyte sedimentation rate (ESR) at baseline turned out to be predictive of clinically relevant progression, independent of treatment strategy. Although high ESR is an established risk factor for progression [36,37,38], it was surprising that patients with fewer tender joints ended up with more structural progression. Although the exact reason for this finding is unknown, it could suggest that patients with a higher pain threshold may receive less than optimal treatment (e.g., less frequent glucocorticoid administrations or other treatment intensifications). Consequently, this finding deserves further study. Better physical health at baseline was predictive of clinically relevant progression in the strategy with initial combination therapy only. Treatment strategy remained the strongest independent predictor for the occurrence of radiographic progression after controlling for other potential predictors.
Previously, we demonstrated that patients treated according with initial combination therapy showed a more rapid improvement in disease activity than patients treated with step-up monotherapy [6]. Early and intensive treatment of RA is advocated, in order to prevent structural joint damage in the early phase of the disease and thereby prevent loss of function resulting from joint destruction and active arthritis [39]. Treatment with traditional DMARDs alone or in combination [9, 20] with glucocorticoids [40] has been shown to retard the progression of joint damage. In our study, the increase of median SHS score (progression) after 1 year of follow-up was significantly lower in patients who had been treated with initial combination therapy compared to patients who had been treated with initial step-up monotherapy. Our results are comparable to those of the FIN-RACO trial which showed that the short-term and long-term increase in median Larsen score was significantly lower in patients who were treated with combination DMARDs compared to patients receiving DMARD monotherapy during the first 2 years [41, 42]. Also, the COBRA study, although this study was not aiming at remission, compared step-down combination therapy with prednisolone, methotrexate, and sulfasalazine (SSZ) to SSZ monotherapy and showed that after 1 year the rate of progression of joint damage was lower in the combination therapy group and less persistent over 4–5 years of follow-up [20].
Similar results were demonstrated in the BeST study [21], where radiological results showed that patients who had been treated with initial combination therapy including prednisone had less progression of radiographic joint damage than patients treated with sequential mono-therapy. The BeST study also showed that the number of patients without any progression of radiographic joint damage was higher in the combination therapy group. In contrast to these studies, the tREACH trial found no difference in radiographic progression between initial triple DMARD therapy and intramuscular glucocorticosteroids versus initial triple DMARD therapy and oral glucocorticosteroids versus initial MTX monotherapy and oral glucocorticosteroids [43]. In the tREACH trial, all treatment groups used glucocorticoids, which might result in early control of the inflammatory disease, which in turn might lead to less short-term progression of damage. Our study in daily clinical practice confirmed that in early RA, starting with a combination therapy of multiple DMARDs has several positive outcomes. In general, it is assumed that rapid aggressive treatment may slow long-term progression [20]. From this perspective we might surmise that starting early RA treatment with a single DMARD would be a missed opportunity in a considerable number of patients.
The identification of possible prognostic factors of radiographic progression is relevant for tailoring treatment and for supporting the current treatment strategy. The strength and reliability of known prognostic factors may vary according to the outcome measure of interest. A systematic review by Carpenter (2016) indicated that RF, anti-CCP, along with increased markers of inflammation (ESR or CRP) were strongly associated with radiographic progression [44]. The ESPOIR study identified anti-CCP and baseline ESR as predictors of structural outcome [45]. Van der Heijde (1992) mentioned high disease activity measured as high ESR, CRP or DAS and a positive RF were all significantly associated with radiographic damage after 2 years of follow-up [46]. Our study cannot confirm all of these associations; but fewer tender joints and higher ESR were independently associated with radiographic progression in the total sample.
Glucocorticoids were part of strategy II, as bridging therapy, and were allowed in strategy I to reach remission. Glucocorticoids have previously been shown to retard radiographic progression [40, 47]. In the total sample, use of glucocorticoids at baseline was univariately associated with less radiographic progression. Looking at the initial combination strategy group only, however, there was no significant difference in radiographic progression between those patients who did or did not receive a baseline glucocorticoid injection (data not shown). Moreover, the association between corticosteroid use and radiographic progression did not remain significant in the multivariate model that included strategy group. Consequently, the current study, albeit not specifically designed to answer this question, could not confirm that glucocorticoids retard short-term radiographic progression. It is possible that this association is confounded by indication, as patients who received an injection of triamcinolone had higher baseline DAS28 scores in both strategies.
The major strength of this study is the use of real life data from consecutive patients, recently diagnosed with RA, who were being treated according to a state-of-the-art T2T remission induction protocol. Results from this study demonstrated similar or even better radiographic outcomes than several T2T RCTs. Since RCTs generally have more strict inclusion criteria and more controlled protocols, it is important that treatment outcomes are also examined in real-life settings. This study is one of the first to directly compare the radiographic outcomes of different T2T strategies in early RA patients in daily clinical practice. Another strength of the study is that the Sharp/van der Heijde method was used instead of the Larsen method for scoring radiographic progression. The Sharp van der Heijde index may be considered as the best tool for evaluating patients with early RA because of its sensitivity in detecting signs of early disease and the possibility of expressing anatomical damage progression quantitatively [48]. Although this study was not powered a-priori for the current comparison of radiographic outcomes, a post-hoc power analysis indicated that with the sample size of 128 patients per strategy we had >80% power to detect a small to moderate difference (d = 0.39) in progression between both strategies using a 2-sided Mann-Whitney u test with an alpha of 0.05.
The major limitation of this study is that it is a quasi-experimental study of two strategies separated over time. The first strategy started in 2006 with an initial step-up mono-therapy, the second strategy in 2012 started with an initial combination therapy. Also, both strategies differed not only with respect to initial DMARD therapy (step-up vs. combination), but also with respect to the use of glucocorticoids and MTX starting dose. Another limitation is the follow-up period of 1 year. Finally, an important limitation is that radiographs in both strategies were not scored in a randomized and blinded fashion, as is usually done in true clinical trials.
Although it is not a randomized trial, we still think that the design and results of the study allow us to compare between the two strategies. Both strategy cohorts consisted of very similar populations of all consecutive newly diagnosed RA patients, treated in the same hospitals by the same rheumatologists. Although early radiographic progression is strongly indicative of future radiographic progression, longer follow-up is needed to investigate whether initial combination therapy also shows long-term beneficial effects on radiographic progression. Long-term follow-up of the COBRA and FIN-RACO trials suggested a difference in progression of joint damage after 1–2 years between combination and monotherapy [49, 50], while the BeSt study did not after 1–5 years [51]. In the long-term follow-up of strategy I within the DREAM registry, patients with early joint damage demonstrated more continued radiographic progression [52]. Because patients in strategy II showed less early radiographic progression, initial combination therapy might also prevent the destruction of joints on the long-term.
Conclusion
Patients treated with initial monotherapy had significantly more first-year radiographic progression than patients treated with initial combination therapy in daily clinical practice.
Abbreviations
- Anti-CCP:
-
Anti-cyclic citrullinated peptide
- BMI:
-
Body mass index
- CRP:
-
C-reactive protein
- DAS28:
-
Disease activity score for 28 joints
- DMARD:
-
Disease modifying anti-rheumatic drug
- DREAM:
-
Dutch RhEumatoid Arthritis Monitoring registry
- ESR:
-
Erythrocyte sedimentation rate
- HAQ:
-
Health Assessment Questionnaire Disability Index
- HCQ:
-
Hydroxychloroquine
- IQR:
-
interquartile range
- JSN:
-
Joint space narrowing
- MCID:
-
Minimal clinically important difference
- MCS:
-
Mental component summary
- MTX:
-
Methotrexate
- PCS:
-
Physical component summary
- RA:
-
Rheumatoid arthritis
- RF:
-
Rheumatoid factor
- SD:
-
Standard deviations
- SF36:
-
Short Form Health Survey with 36 items
- SHS:
-
Sharp/van der Heijde score
- SJC:
-
Swollen joint count
- SSZ:
-
Sulfasalazine
- T2 T:
-
Treat-to-target
- TJC:
-
Tender joint count
- TNFi:
-
Tumor necrosis factor inhibitor
- VAS:
-
Visual analog scale
References
Sokka T. Long-term outcomes of rheumatoid arthritis. Curr Opin Rheumatol. 2009;21:284–90.
Lard LR, Visser H, Speyer I, vander Horst-Bruinsma IE, Zwinderman AH, Breedveld FC, et al. Early versus delayed treatment in patients with recent-onset rheumatoid arthritis: comparison of two cohorts who received different treatment strategies. Am J Med. 2001;111:446–51.
Quinn MA, Emery P. Window of opportunity in early rheumatoid arthritis: possibility of altering the disease process with early intervention. Clin Exp Rheumatol. 2003;21:S154–7.
Nell VPK, Machold KP, Eberl G, Stamm TA, Uffmann M, Smolen JS. Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis. Rheumatology (Oxford). 2004;43:906–14.
Steunebrink LMM, Vonkeman HE, ten Klooster PM, Hoekstra M, van Riel PLCM, van de Laar MAFJ. Recently diagnosed rheumatoid arthritis patients benefit from a treat-to-target strategy: results from the DREAM registry. Clin Rheumatol. 2016;35:609–15.
Steunebrink LMM, Versteeg GA, Vonkeman HE, Ten Klooster PM, Kuper HH, Zijlstra TR, et al. Initial combination therapy versus step-up therapy in treatment to the target of remission in daily clinical practice in early rheumatoid arthritis patients: results from the DREAM registry. Arthritis Res Ther. 2015;18:60.
Finckh A, Liang MH, van Herckenrode CM, de Pablo P. Long-term impact of early treatment on radiographic progression in rheumatoid arthritis: a meta-analysis. Arthritis Rheum. 2006;55:864–72.
Boers M, Verhoeven AC, Markusse HM, van de Laar MA, Westhovens R, van Denderen JC, et al. Randomised comparison of combined step-down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone in early rheumatoid arthritis. Lancet. 1997;350:309–18.
Möttönen T, Hannonen P, Leirisalo-Repo M, Nissilä M, Kautiainen H, Korpela M, et al. Comparison of combination therapy with single-drug therapy in early rheumatoid arthritis: a randomised trial. FIN-RACo trial group. Lancet. 1999;353:1568–73.
Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000;343:1586–93.
St Clair EW, van der Heijde DMFM, Smolen JS, Maini RN, Bathon JM, Emery P, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum. 2004;50:3432–43.
Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previo. Arthritis Rheum. 2006;54:26–37.
Grigor C, Capell H, Stirling A, McMahon AD, Lock P, Vallance R, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet. 2004;364:263–9.
Verstappen SMM, Jacobs JWG, van der Veen MJ, Heurkens AHM, Schenk Y, ter Borg EJ, et al. Intensive treatment with methotrexate in early rheumatoid arthritis: aiming for remission. Computer assisted Management in Early Rheumatoid Arthritis (CAMERA, an open-label strategy trial). Ann Rheum Dis. 2007;66:1443–9.
Smolen JS, Aletaha D, Bijlsma JWJ, Breedveld FC, Boumpas D, Burmester G, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69:631–7.
van Jaarsveld CH, Jacobs JW, van der Veen MJ, Blaauw AA, Kruize AA, Hofman DM, et al. Aggressive treatment in early rheumatoid arthritis: a randomised controlled trial. On behalf of the rheumatic research foundation Utrecht, The Netherlands. Ann Rheum Dis. 2000;59:468–77.
Stenger AA, Van Leeuwen MA, Houtman PM, Bruyn GA, Speerstra F, Barendsen BC, et al. Early effective suppression of inflammation in rheumatoid arthritis reduces radiographic progression. Br J Rheumatol. 1998;37:1157–63.
Emery P, Marzo H, Proudman S. Management of patients with newly diagnosed rheumatoid arthritis. Rheumatology (Oxford). 1999;38(Suppl 2):27–31.
Abu-Shakra M, Toker R, Flusser D, Flusser G, Friger M, Sukenik S, et al. Clinical and radiographic outcomes of rheumatoid arthritis patients not treated with disease-modifying drugs. Arthritis Rheum. 1998;41:1190–5.
Landewé RBM, Boers M, Verhoeven AC, Westhovens R, van de Laar MAFJ, Markusse HM, et al. COBRA combination therapy in patients with early rheumatoid arthritis: long-term structural benefits of a brief intervention. Arthritis Rheum. 2002;46:347–56.
Goekoop-Ruiterman YPM, de Vries-Bouwstra JK, Allaart CF, van Zeben D, Kerstens PJSM, JMW H, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum. 2008;58:S126–35.
van Aken J, Lard LR, le Cessie S, Hazes JMW, Breedveld FC, Huizinga TWJ. Radiological outcome after four years of early versus delayed treatment strategy in patients with recent onset rheumatoid arthritis. Ann Rheum Dis. 2004;63:274–9.
Maillefert JF, Combe B, Goupille P, Cantagrel A, Dougados M. Long term structural effects of combination therapy in patients with early rheumatoid arthritis: five year follow up of a prospective double blind controlled study. Ann Rheum Dis. 2003;62:764–6.
Cohen G, Gossec L, Dougados M, Cantagrel A, Goupille P, Daures JP, et al. Radiological damage in patients with rheumatoid arthritis on sustained remission. Ann Rheum Dis. 2007;66:358–63.
Molenaar ETH, Voskuyl AE, Dinant HJ, Bezemer PD, Boers M, Dijkmans BAC. Progression of radiologic damage in patients with rheumatoid arthritis in clinical remission. Arthritis Rheum. 2004;50:36–42.
van der Kooij SM, Goekoop-Ruiterman YPM, De Vries-Bouwstra JK, Güler-Yüksel M, Zwinderman AH, PJSM K, et al. Drug-free remission, functioning and radiographic damage after 4 years of response-driven treatment in patients with recent-onset rheumatoid arthritis. Ann Rheum Dis. 2009;68:914–21.
Vermeer M, Kuper HH, Hoekstra M, Haagsma CJ, Posthumus MD, Brus HLM, et al. Implementation of a treat-to-target strategy in very early rheumatoid arthritis: results of the Dutch rheumatoid arthritis monitoring remission induction cohort study. Arthritis Rheum. 2011;63:2865–72.
Vermeer M, Kuper HH, Moens HJB, Drossaers-Bakker KW, van der Bijl AE, van Riel PLCM, et al. Sustained beneficial effects of a protocolized treat-to-target strategy in very early rheumatoid arthritis: three-year results of the Dutch rheumatoid arthritis monitoring remission induction cohort. Arthritis Care Res (Hoboken). 2013;65:1219–26.
den Uyl D, ter Wee M, Boers M, Kerstens P, Voskuyl A, Nurmohamed M, et al. A non-inferiority trial of an attenuated combination strategy (‘COBRA-light’) compared to the original COBRA strategy: clinical results after 26 weeks. Ann Rheum Dis. 2014;73:1071–8.
Goekoop-Ruiterman YPM, de Vries-Bouwstra JK, Allaart CF, van Zeben D, PJSM K, Hazes JMW, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum. 2005;52:3381–90.
Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8.
Bruce B, Fries JF. The health assessment questionnaire (HAQ). Clin Exp Rheumatol. 2005;23:S14–8.
Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83.
van der Heijde D. How to read radiographs according to the sharp/van der Heijde method. J Rheumatol. 1999;26:743–5.
Landewé R, van der Heijde D. Radiographic progression depicted by probability plots: presenting data with optimal use of individual values. Arthritis Rheum. 2004;50:699–706.
Jansen LM, van der Horst-Bruinsma IE, van Schaardenburg D, Bezemer PD, Dijkmans BA. Predictors of radiographic joint damage in patients with early rheumatoid arthritis. Ann Rheum Dis. 2001;60:924–7.
Smolen JS, Van Der Heijde DMFM, St Clair EW, Emery P, Bathon JM, Keystone E, et al. Predictors of joint damage in patients with early rheumatoid arthritis treated with high-dose methotrexate with or without concomitant infliximab: results from the ASPIRE trial. Arthritis Rheum. 2006;54:702–10.
Syversen SW, Gaarder PI, Goll GL, Ødegård S, Haavardsholm EA, Mowinckel P, et al. High anti-cyclic citrullinated peptide levels and an algorithm of four variables predict radiographic progression in patients with rheumatoid arthritis: results from a 10-year longitudinal study. Ann Rheum Dis. 2008;67:212–7.
Smolen JS, Landewé R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73:492–509.
Kirwan JR, Bijlsma JWJ, Boers M, Shea BJ. Effects of glucocorticoids on radiological progression in rheumatoid arthritis. Cochrane Database Syst Rev. 2007:CD006356.
Korpela M, Laasonen L, Hannonen P, Kautiainen H, Leirisalo-Repo M, Hakala M, et al. Retardation of joint damage in patients with early rheumatoid arthritis by initial aggressive treatment with disease-modifying antirheumatic drugs: five-year experience from the FIN-RACo study. Arthritis Rheum. 2004;50:2072–81.
Mäkinen H, Kautiainen H, Hannonen P, Möttönen T, Leirisalo-Repo M, Laasonen L, et al. Sustained remission and reduced radiographic progression with combination disease modifying antirheumatic drugs in early rheumatoid arthritis. J Rheumatol. 2007;34:316–21.
de Jong PH, Hazes JM, Han HK, Huisman M, van Zeben D, van der Lubbe PA, et al. Randomised comparison of initial triple DMARD therapy with methotrexate monotherapy in combination with low-dose glucocorticoid bridging therapy; 1-year data of the tREACH trial. Ann Rheum Dis. 2014;73:1331–9.
Carpenter L, Nikiphorou E, Sharpe R, Norton S, Rennie K, Bunn F, et al. Have radiographic progression rates in early rheumatoid arthritis changed? A systematic review and meta-analysis of long-term cohorts. Rheumatology (Oxford). 2016;55(6):1053–9
Combe B, Logeart I, Belkacemi MC, Dadoun S, Schaeverbeke T, Daurès JP, et al. Comparison of the long-term outcome for patients with rheumatoid arthritis with persistent moderate disease activity or disease remission during the first year after diagnosis: data from the ESPOIR cohort. Ann Rheum Dis. 2015;74:724–9.
van der Heijde DM, van Riel PL, van Leeuwen MA, van ‘t Hof MA, van Rijswijk MH, van de Putte LB. Prognostic factors for radiographic damage and physical disability in early rheumatoid arthritis. A prospective follow-up study of 147 patients. Br J Rheumatol. 1992;31:519–25.
Kavanaugh A, Wells AF. Benefits and risks of low-dose glucocorticoid treatment in the patient with rheumatoid arthritis. Rheumatology (Oxford). 2014;53:1742–51.
Giovagnoni A, Valeri G, Burroni E, Amici F. Rheumatoid arthritis: follow-up and response to treatment. Eur J Radiol. 1998;27(Suppl 1):S25–30.
van Tuyl LHD, Boers M, Lems WF, Landewé RB, Han H, van der Linden S, et al. Survival, comorbidities and joint damage 11 years after the COBRA combination therapy trial in early rheumatoid arthritis. Ann Rheum Dis. 2010;69:807–12.
Rantalaiho V, Korpela M, Laasonen L, Kautiainen H, Järvenpää S, Hannonen P, et al. Early combination disease-modifying antirheumatic drug therapy and tight disease control improve long-term radiologic outcome in patients with early rheumatoid arthritis: the 11-year results of the Finnish rheumatoid arthritis combination therapy trial. Arthritis Res Ther. 2010;12:R122.
Klarenbeek NB, Güler-Yüksel M, van der Kooij SM, Han KH, Ronday HK, Kerstens PJSM, et al. The impact of four dynamic, goal-steered treatment strategies on the 5-year outcomes of rheumatoid arthritis patients in the BeSt study. Ann Rheum Dis. 2011;70:1039.
Versteeg LGA, Steunebrink LMM, Kuper IH, Vonkeman HE, ten Klooster PM, van der Bijl AE. Van de LM. Early radiological progression in rheumatoid arthritis leads to more long-term joint damage in daily clinical practice; six year radiological outcomes of a strict treat-to-target cohort in the Netherlands [abstract]. Arthritis Rheumatol. 2016;68
Acknowledgements
We would like to thank all the patients, rheumatology nurses, and rheumatologists who participated in our study.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
LS, GA, HV, PTK, MH, MvdL significantly participated in the preparation of the manuscript. LS drafted the first version of the manuscript. GA, HV, PTK, MH, and MvdL revised it critically for important intellectual content. LS performed the statistical analysis. All authors participated in the interpretation of the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Medical Ethics Committees of the Medisch Spectrum Twente, Enschede and Isala, Zwolle hospitals determined, in accordance with the ‘Medical Research involving Human subjects’ act in the Netherlands, that no ethical approval was required because all data were collected in the course of regular daily clinical practice. Nonetheless, patients were fully informed, and prior written informed consent was obtained from each patient.
Consent for publication
Not applicable.
Competing interests
There was no involvement of study sponsors, none of the authors have financial, commercial, or other associations that might pose a conflict of interest in connection with the work.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Steunebrink, L.M.M., Versteeg, L.G.A., Vonkeman, H.E. et al. Radiographic progression in early rheumatoid arthritis patients following initial combination versus step-up treat-to-target therapy in daily clinical practice: results from the DREAM registry. BMC Rheumatol 2, 1 (2018). https://doi.org/10.1186/s41927-018-0009-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41927-018-0009-8