Abstract
Introduction
The aim of this work is to compare real-world outcomes of patients with rheumatoid arthritis (RA) receiving adalimumab (ADA) bio-originator (non-switchers) to those who had switched from ADA bio-originator to an ADA biosimilar (switchers) on the basis of the hypothesis that these outcomes would differ.
Methods
Data were drawn from the Adelphi RA Disease Specific Programme™, a point-in-time survey of physicians and their patients in Europe (France, Germany, Italy, Spain, UK) in 2020. Physicians completed a questionnaire for their next ten adult patients with RA, followed by four additional patients who had switched from ADA bio-originator to an ADA biosimilar (switchers). Physician- and patient-reported outcomes (PROs) for switchers and non-switchers were compared by propensity score matching.
Results
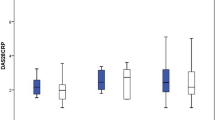
Three hundred and three rheumatologists provided data for 160 non-switchers and 225 switchers, 140 patients provided data; 51 non-switchers, 89 switchers. According to physician-reported disease activity, non-switchers were more likely to improve on their current ADA treatment than switchers (68%, n = 108 vs. 26%, n = 59 p < 0.001) and less likely to worsen (1%, n = 2 vs. 9%, n = 20; p < 0.01). Physician-reported patient adherence was significantly lower amongst switchers versus non-switchers (0.66 vs. 0.78, respectively; p = 0.04). More non-switchers than switchers were reported by their physicians to be consistent in taking their RA medicine (p < 0.001). Compared with non-switchers, PRO measures indicated quality of life was worse (EQ-5D Visual Analogue Scale: 62.9 vs. 71.9; p < 0.001) and activity impairment was greater (Work Productivity Activity Index: 31.0 vs. 24.4; p = 0.02) for switchers, with trends for poorer health status and greater pain.
Conclusions
Non-medical switching in RA treatment may lead to unforeseen outcomes that should be considered by health decision-makers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
This analysis of the Adelphi Rheumatoid Arthritis (RA) Disease-Specific Programme evaluated the impact on patient outcomes of switching patients with RA from the ADA bio-originator to an ADA biosimilar in France, Germany, Italy, Spain, and the UK, as compared to patients who remained on the ADA bio-originator treatment, in a real-world setting. |
Our hypothesis was that patient outcomes would differ between the two groups, which would lead to a need for improvement in patient care within the group reporting poorer outcome(s). |
What was learned from the study? |
Results showed that rheumatologists and their patients with RA report poorer outcomes in terms of disease severity, treatment adherence, and quality of life in patients who switched to adalimumab biosimilar when compared, using propensity score matching, to patients who remained on ADA bio-originator. |
Rheumatologists should be aware of the impact of non-medical switching for economic reasons on patient outcomes, and consider how to best minimize the potential negative impact. |
Understanding of the nocebo effect by rheumatologists and effective communications about non-medical switching (NMS) to patients should be included in rheumatologist–patient interactions prior to the switch. |
Introduction
Rheumatoid arthritis (RA) is a chronic, inflammatory, autoimmune condition characterized by inflammation of the synovial joints [1, 2]. It is one of the most prevalent chronic inflammatory diseases, with prevalence in Europe ranging from 0.19 to 0.82% [3]. RA is a highly heterogeneous disease with a complex pathophysiology; symptoms such as tender/swollen joints and fatigue prevail in the early stages, while cartilage destruction, bone erosion, and serious systemic manifestations such as vasculitis, lung disease, and hematologic abnormalities may evolve particularly in poorly managed disease [1, 2]. The disease causes significant negative impact to patients’ physical and mental health-related quality of life (QoL) [4]. Ultimately, progressive articular damage and systemic manifestations may result in functional loss and increased mortality [1, 2].
The main aim of pharmacological therapy for patients with RA is clinical remission, or at least low disease activity, with a treat-to-target strategy using disease-modifying antirheumatic drugs (DMARD) [5]. Importantly, this strategy prioritizes relief of symptoms, normalization of physical function and QoL, and inhibition of the occurrence or progression of structural joint damage [5]. However, the heterogeneity of RA extends to response to DMARDs, which can fail or produce only partial responses in some patients [1].
The tumor necrosis factor (TNF) inhibitor adalimumab (ADA) was the first fully human monoclonal antibody approved for use in RA (AbbVie Inc., Humira ®, Europe, 2003 [6], USA, 200 [7]). Since the originator product patent expired in 2016 (USA; Europe, 2018) [8], seven ADA biosimilars have been approved for use in RA [9, 10]. Biosimilars are biotherapeutic products that are highly similar in pharmacokinetic, safety, immunogenic, and efficacy profiles to the licensed biotherapeutic reference product (bio-originator), and could reduce the treatment cost, thereby improving access to optimal therapy [9, 11]. EULAR recommends lower-cost biosimilar DMARDs (bsDMARDs) when efficacy and safety have been proven to be highly similar to those of the bio-originator [5, 12].
Since biosimilars manufacturers with varying manufacturing processes will not attain identical drug substance and drug product attributes to the bio-originator [13], biosimilars are not considered identical to their bio-originator [14]. As such, concerns about switching between a bio-originator and its biosimilar remain [14].
The increasing economic burden of RA and of the availability of biosimilars has resulted in physicians switching some patients to biosimilars [3, 9, 11]. Reportedly, approximately 35% of European patients had been switched from the ADA bio-originator to an ADA biosimilar by the end of 2019 [15]. The switching of patients with well-tolerated and effective therapy from the bio-originator to a biosimilar for economic reasons is known as non-medical switching [11].
Switching trials have shown that switching from the ADA bio-originator to a currently approved biosimilar does not significantly impact safety, immunogenicity, or efficacy [10]. Subtle differences were considered to be due to methodological differences rather than the biosimilar properties [10]. However, some unsuccessful switches have been attributed to patients’ perceptions (the “nocebo” effect [16]), and reduced efficacy and safety [14]. Furthermore, the manner and content of communications to patients about the reasons for non-medical switching may greatly influence the outcome [17]. The lower biosimilar retention rates in open-label switch studies compared to double-blind switch trials suggests that the nocebo effect might also play a role in biosimilar retention rates [18].
This paper reports data from a multinational, prospective, point-in-time survey of physicians and their consulting patients with RA. The objective of this analysis was to compare physician- and patient-reported outcomes of patients with RA receiving the ADA bio-originator with those of patients who switched from the ADA bio-originator to an ADA biosimilar in a real-world setting.
Methods
Survey Design
Data were drawn from the Adelphi Rheumatoid Arthritis Disease-Specific Programme™ (DSP) conducted in 2020 in France, Germany, Italy, Spain, and the United Kingdom. DSPs are large, multinational, point-in-time surveys conducted in real-world clinical practice of physicians and their consulting patients. A complete description of the methods of the DSP has been previously published and validated [19,20,21].
Participants
A geographically representative sample of physicians was recruited to participate in the DSP. Physicians were eligible to participate in the survey if they were personally responsible for and actively involved in treatment decisions and management of patients with RA, and consulted at least three patients with RA in a typical month.
Patients were eligible for inclusion if they were over 18 years of age, had a physician-confirmed diagnosis of RA and visited the physician. Patients included in this analysis are a combination of a sub-set of these consecutively sampled patients, and a deliberately captured additional set of patients (the over-sample). These patient samples were chosen to assess the effects of switching from ADA originator to an ADA biosimilar. The consecutively sampled patients were the next ten prospectively consulting RA patients seen by their physician. Those who were either currently being prescribed ADA bio-originator (referred to as non-switchers) or currently being prescribed ADA biosimilar therapy, having switched from ADA bio-originator therapy (referred to as switchers), were included for analysis. Physicians selected which ADA biosimilar the patient was taking from a list of available brands at time of data collection. The over-sample included switchers only. Both consecutively sampled patients and the over-sample of switchers had to fulfil the inclusion criteria. Patients who had received advanced therapy prior to initiation of ADA bio-originator were removed from the sample to remove any confounding effects of previous advanced therapy use. Switcher patients were not requested to have a minimal time period since switch from ADA originator to an ADA biosimilar.
“Advanced therapy” was used to collectively refer to the following: targeted synthetic DMARDs (tsDMARD; e.g., tofacitinib, baricitinib, upadacitinib), biologic originator DMARD (boDMARD; e.g., abatacept, adalimumab, certolizumab-pegol, etanercept, golimumab, infliximab, rituximab, sarilumab, and tocilizumab), and biosimilar DMARD (bsDMARD; e.g., those for etanercept and infliximab).
Data Collection
Physicians (rheumatologists) completed a patient record form (PRF) for their next ten consecutive patients with RA who visited their clinic for routine care. The PRF contained questions on demographics, treatment history, and clinical outcomes such as satisfaction and adherence. Completion of the PRF was through consultation of existing patient clinical records and, consistent with decisions made in routine clinical practice, the judgement and diagnostic skills of the respondent physician. Thereafter, the same rheumatologists provided data for the over-sample by completing up to a further four PRFs on a prospective basis.
Disease activity, satisfaction, and patient adherence were collected as physician-reported outcomes. Disease severity was assessed by physicians’ subjective opinion as mild, moderate, or severe across key timepoints from initiation of current ADA treatment to the time of data collection. Patients were considered to have either improved, worsened, or remained stable based on physician-perceived severity (mild, moderate, severe) across these timepoints. Disease severity change was measured for two time frames: firstly, from initiation of first ADA treatment to time of data collection, secondly, from initiation of current ADA treatment to time of data collection.
Physicians invited the patients for whom PRF was completed to complete a patient-reported self-completion form (PSC). The PSC collected data on patient demographics, compliance, treatment satisfaction, and QoL. The PSC also asked about the impact of RA on patients’ lives, using the EuroQol- 5 Dimension (EQ-5D) [22] and the Work Productivity and Activity Impairment (WPAI) questionnaire [23]. Patients’ pain and fatigue were assessed using the Health Assessment Questionnaire (HAQ) [24] and the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F) [25, 26], respectively.
All physician-reported and patient-reported questions and scoring are detailed in Supplementary Table S1.
Ethical Approval
The survey did not require Ethics or Institutional Review Board review due to its non-interventional, observational nature of data collection. Informed consent from physicians and patients was required before their participation. The survey was conducted in accordance with the Western Institutional Review Board (protocol number 21-ADRW-104). Where patients provided data directly, they signed an informed consent form prior to participation in this study.
Data collection was conducted in accordance with national market research and privacy regulations, including European Pharmaceutical Market Research Association (EphMRA), and the US Department of Health and Human Services National Institutes of Health, Health Insurance Portability and Accountability Act (HIPAA).
All responses captured on the data collection forms were anonymized to preserve respondent confidentiality. Responses were anonymized before aggregated reporting, the identity of the physicians was blinded, and no patient identifiers were collected.
This study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments.
Statistical Analysis
Data were summarized using descriptive analyses using Stata 17 [27]. Means and standard deviations (SD) were calculated for continuous variables, and frequency and percentages calculated for categorical variables. For descriptive data, numbers and percentages were shown in each category. Where propensity score matching was conducted, relevant scores (means or percentages) for each group are shown with an associated p value. Sample n numbers are not shown, as these values represent the scores of a matched patient population, not a true population.
Initially, bivariate comparisons of the two patient populations, the 186 non-switchers and the 284 switchers, were used to compare clinical and QoL outcomes. These analyses showed that the baseline characteristics were significantly different between the two populations. Key differences across the two populations included: a longer time since diagnosis of switchers (an additional 6.8 months), current ADA therapy duration was shorter for switchers (13.2 months less), fewer switchers with lower physician-reported disease activity based on DAS28 score estimates (remission [DAS28 < 2.6], low [DAS28 2.6–3.2], moderate [DAS28 3.3–5.1], and high disease activity [DAS28 > 5.1]) at diagnosis (p = 0.03), and more switchers with lower physician-reported disease activity currently (p = 0.202) compared with non-switchers.
To control for these differences, non-switchers and switchers were compared using propensity score matching (PSM), confounding for patient age, sex, body mass index, time since first ADA therapy (months), time since diagnosis (months), and the change in physician-perceived severity categories (mild, moderate, severe) over the duration of all ADA therapy (from initiation of ADA originator to the time of data collection). The data were matched in two ways: physician-reported patient data were matched according to physician-reported patient characteristics, and patient-reported data were matched according to patient-reported characteristics. The propensity score was estimated using a logistic regression model. Each patient in the non-switcher group was matched 1:1 to a patient in the switcher group, with replacement (i.e., the same patient being used as a match more than once) and allowing for ties (i.e., when switchers and non-switchers had identical propensity scores, so patients from one group are matched to more than one patient from the other group). For all variables, standard mean differences (SMDs) were within the – 10% and 10% limits, indicating good matching of the non-switcher and switcher groups on both patient and physician-reported characteristics. After matching, there were 160 non-switcher and 225 switcher patients with physician-reported data. The unmatched patient base was higher (n = 284) than that of the matched patient group (n = 225) because not all patients had data for each matching variable.
For all analyses conducted, a p value of < 0.05 was deemed statistically significant.
Results
Physician and Patient Demographics
As part of the wider Adelphi RA DSP sample, 303 rheumatologists provided information for a total of 526 patients; 242 (209 ADA non-switchers; 33 switchers) patients consecutively sampled and 284 from the over-sample (switchers). Patients were from France (n = 124, 23.6%), Germany (n = 121, 23.0%), Italy (n = 95, 18.1%), Spain (n = 104, 19.8%), and the UK (n = 82, 15.6%).
Of the total physician-reported patient data sample, 385 patients were eligible for PSM; 160 non-switchers and 225 switchers. Of the patient-reported data population, a total of 140 eligible matched patients provided their data; 51 non-switchers and 89 switchers.
Physicians switched 284 patients from the ADA bio-originator, and in a multi-choice question, reported mainly doing so for financial reasons (n = 190, 67%), formulary driven switch (n = 67, 24%), insurance restrictions (n = 48, 17%) and fewer administrative hurdles (n = 23, 8%). (Supplementary Table S4). Switchers had received their current treatment (ADA biosimilar) for a mean duration of 11.2 months (n = 225).
Physician-reported Matched Patient Characteristics
After PSM, SMDs between non-switcher and switcher groups were between – 10 and 10, indicating they had been well matched (Supplementary Table S2). Non-switchers had a mean age of 52.1 years and 36% were male, mean time since first ADA therapy was 43.9 months, and mean time since diagnosis was 90.1 months. Switchers had a mean age of 52.3 years and 36% were male, mean time since first ADA therapy was 46.2 months, and mean time since diagnosis was 89.2 months.
Physician-reported Matched Group Differences
PSM requires binary comparisons when outcomes are categorical, so all PSM scores of categorical variables are reported on a scale of 0–1. Physicians reported that patient adherence was significantly lower amongst switchers compared to non-switchers (0.66 vs 0.78; p = 0.04; where 1 indicates greater adherence, Fig. 1).
Physician-reported results of patients with RA who were receiving adalimumab: Non-switcher versus switcher patients. Scale: 0.0–1.0 (binary scale). RA rheumatoid arthritis. Non-switcher patients prescribed adalimumab bio-originator who were not switched to an adalimumab biosimilar, NMS, patients previously prescribed adalimumab bio-originator who had been switched to an adalimumab biosimilar. Physician-reported results from the patient-record form. Non-switcher and NMS patients were compared using propensity score matching. *Significance at p < 0 05. Red text denotes negative result
When asked to report their satisfaction with the treatment of patients’ RA at time of data collection, physicians reported being generally satisfied with treatment in both groups. Physicians reported satisfaction with treatment more frequently when reporting on switchers’ treatment than non-switchers’, however this was not statistically significant. Physician-reported disease activity status at time of data collection was also similar between switchers and non-switchers (Fig. 1).
There were significant between-group differences in the numbers of patients whose disease severity had worsened, was stable, or had improved from initiation of their current therapy to time of data collection (Fig. 2). When asked to describe the patient’s current clinical condition, physicians reported a greater proportion of switchers had worsened from initiation of their current ADA therapy to time of data collection than non-switchers (9 vs. 1%, respectively; p < 0.01), and that non-switchers were more likely to improve over the course of their current treatment than switchers (68 vs. 26%, respectively; p < 0.001). This higher proportion of patients with worsening disease, and a lower proportion of patients with improving disease meant switchers were significantly more likely to remain stable (i.e., neither improve nor worsen) throughout their current ADA treatment than non-switchers (p < 0.001) (Fig. 2).
Physician-reported results of % disease severity change in patients with RA who were receiving adalimumab: Non-switcher versus switcher patients. RA rheumatoid arthritis. Non-switcher patients prescribed adalimumab bio-originator who were not switched to an adalimumab biosimilar. NMS patients previously prescribed adalimumab bio-originator who had been switched to an adalimumab biosimilar. % disease severity change from initiation of current therapy to time of data collection. Physician-reported results from the patient-record form. Non-switcher and NMS patients were compared using propensity score matching. *Significance at p < 0.05
PSM scoring confirmed group differences in disease severity from initiation of their current therapy to the time of data collection reported by physicians (Fig. 3).
Physician-reported results of disease severity change in patients with RA who were receiving adalimumab: Non-switchers versus switchers. RA rheumatoid arthritis. Non-switcher patients prescribed adalimumab bio-originator who were not switched to an adalimumab biosimilar. NMS patients previously prescribed adalimumab bio-originator who had been switched to an adalimumab biosimilar. Binary severity change from initiation of current therapy to time of data collection. Severity rated as mild, moderate, or severe. Physician-reported results from the patient-record form. Non-switcher and NMS patients were compared using propensity score matching. *Significance at p < 0 05. Red text denotes negative result
Patient Self-reported Matched Patient Characteristics
Non-switchers and switchers were well matched following propensity score matching (Supplementary Table S3). Non-switchers had a mean age of 53.2 years and 32% were male, mean time since first ADA therapy was 50.8 months, and mean time since diagnosis was 106.3 months. Switchers had a mean age of 53.0 years and 35% were male, mean time since first ADA therapy was 47.6 months, and mean time since diagnosis was 108.3 months.
Patient-reported Matched Group Differences
Group comparisons showed that both non-switchers and switchers were satisfied with their treatment, with a trend for greater satisfaction in the non-switchers (0.77 vs. 0.64; p = 0.11; where 1 indicates satisfaction, Fig. 4). Switchers also reported forgetting to take their medication “sometimes” or “once in a while” more frequently than non-switchers (Fig. 4). The majority of patients in both treatment groups reported that they almost never refused to take the medication suggested by their doctor (0.04 vs. 0.05; p = 0.70; where 1 indicates refusal, Fig. 4).
Self-reported treatment satisfaction and adherence reported by patients with RA receiving adalimumab: Non-switcher versus switcher patients. RA rheumatoid arthritis. Non-switcher patients prescribed adalimumab bio-originator who were not switched to an adalimumab biosimilar. NMS patients previously prescribed adalimumab bio-originator who had been switched to an adalimumab biosimilar. Patient-reported results from the patient self-completion form. Non-switcher and NMS patients were compared using propensity score matching. *Significance at p < 0 05. Red text denotes negative result
Rating questions from “all the time” to “never” (score 1–5, respectively; Fig. 4), significantly more non-switchers “almost never” forgot their RA medicine (4.84 vs. 4.57; p = 0.02) or stopped their medication if they felt worse (4.84 vs. 4.54; p < 0.001) compared with switchers. Additionally, comparing non-switchers and switchers, most patients “almost never” stopped their medication if they felt better (4.69 vs. 4.59; p = 0.45). There was no difference between non-switchers and switchers when asked ‘How often do you not get your RA medicine because they cost too much money?’ (a 1–5 scale, where 1 = all the time, 5 = never; 4.84 vs. 4.84; p = 1.0).
The findings from validated patient-reported outcome tools are shown in Fig. 5. The EQ-5D VAS showed that QoL was significantly worse for switchers compared with non-switchers (62.9 vs. 71.9; p < 0.01), while the EQ-5D Index showed that switchers reported a poorer health status than non-switchers (0.85 vs. 0.88; p = 0.31). From the WPAI questionnaire, switchers had significantly greater activity impairment due to their RA than non-switchers (31.0 vs. 24.4; p = 0.02). The HAQ disability index pain scale results showed a trend for switchers to report greater pain. The FACIT-F showed fatigue levels were similar regardless of treatment group.
Self-reported treatment outcomes reported by patients with RA receiving adalimumab: Non-switcher versus switcher patients. EQ5D EuroQol-5 dimension, FACIT Fatigue, functional assessment of chronic illness therapy – fatigue, HAQ health assessment questionnaire, RA rheumatoid arthritis, VAS visual analogue scale, WPAI work productivity and activity impairment. Non-switcher patients prescribed adalimumab bio-originator who were not switched to an adalimumab biosimilar. NMS patients previously prescribed adalimumab bio-originator who had been switched to an adalimumab biosimilar. Patient-reported results from the patient self-completion form. Non-switcher and NMS patients were compared using propensity score matching. EQ5D calculated using the German tariff. *Significance at p < 0 05. Red text denotes negative result
Discussion
This analysis of the Adelphi RA DSP compared real-world patient outcomes between patients with RA who had switched from the ADA bio-originator to an ADA biosimilar, and patients who remained on the ADA bio-originator. The results of our PSM analysis showed poorer outcomes in disease severity, treatment adherence, and quality of life in the patients who had switched to ADA biosimilar treatment.
Patients who switched were more likely to show worsening disease severity throughout their current treatment than non-switcher patients. Indeed, two-thirds of non-switchers continued to improve. However, most switchers had mild disease at initiation of their current treatment, compared to most non-switchers whose disease was moderate/severe. Thus, for switchers there was less scope for improvement and more scope for worsening, which may explain our results. However, this is not true of all switchers—some fell into the ‘stable’ category and remained ‘moderate’. Disease severity remained stable for most switchers from initiation of biosimilar treatment to time of data collection, suggesting that physicians were employing non-medical switching for economic reasons rather than for clinical benefit once patients had achieved an adequately stable disease state on the ADA bio-originator. It is unusual in clinical practice to switch from an originator to a biosimilar for reasons of "clinical benefit" in terms of disease activity control, except for the case of a poorly tolerated biologic (e.g., injection site pain with a citrated formula). As such, most switches are likely not due to medical reasons.
The subset of switchers experiencing an increase in disease severity during their biosimilar treatment present an increase in clinical burden. Reportedly, healthcare professionals find it acceptable to initiate newly diagnosed patients on biosimilars without giving them the choice [28]. Studies have demonstrated that cost savings are achievable by switching to biosimilars [29]. However, these savings would be partially or wholly offset if either: significantly more switchers have disease worsening and significantly less switchers have disease improvement versus non-switchers, or, in a significant proportion of switchers the originator was offered at the same price as the biosimilar and/or the logistics of the switch was costly [30]. While bsDMARDs have shown equivalence to their bio-originators for key outcome measures in clinical trials, many physicians have no choice but to use particular bsDMARD. This is because it is mandated that not all non-medical switch cases are obliged by a third party, and in some instances is a purely medical decision [31]. The dislike of such non-medical switches is likely due to theoretical concerns about increasing immunogenicity due to sequential exposure to different post-translational modifications [31].
Many studies of biosimilars in patients with immune-mediated diseases have concluded that most treatment failures following non-medical switching are due to a nocebo effect [32]. Thus, the physician reported increase of disease activity reported in our analysis could be a result of the nocebo effect, whereby the patient’s negative perception of the biosimilar results in worsening of symptoms unrelated to the treatment’s pharmacological effects, as reported in other studies in RA [33, 34]. Further research is required to determine if the presumed nocebo effect is a true effect, and whether similar results would have been found using data from another point in time or collected in a different fashion. Nonetheless, any negative impact from a nocebo response may impact a patient’s treatment journey and ultimately potential cost savings. It has been suggested that nocebo has a role in treatment persistence. Lower biosimilar retention rates are reported in open-label studies of patients with rheumatic and musculoskeletal diseases switching from bio-originator to biosimilar treatments than in switch randomized controlled trials [18]. Switching treatment and nocebo response to biosimilar therapy may negatively impact medication adherence and lead to poorer clinical outcomes [12, 32]. Mitigating the nocebo effect requires increased awareness among prescribers and patients of the strict regulatory evaluation, similar safety and efficacy of biosimilars to their reference product [12], and enhanced prescriber–patient communications [17].
Many studies have shown that disease control has a positive impact on patient-reported outcomes, and therefore recommend that functional improvement is included alongside clinical outcomes when considering successful treatment outcomes [35]. In this analysis, the between-group differences in disease severity may have impacted patients’ QoL. Switchers reported a significantly reduced QoL and work activity impairment following the switch to an ADA biosimilar compared with their ADA non-switcher counterparts. These findings are also consistent with the reduced level of patient-reported satisfaction with ADA biosimilar than with ADA bio-originator.
This survey and analysis are not without limitations. Patients with more active or severe RA may have consulted with their physician more frequently. This survey samples the consulting population, and as such is likely to represent patients who visit their rheumatologist more frequently, for example, more severely affected patients. Similarly, our patient-reported data is representative of the patient population more willing to respond to surveys about their RA, who may share certain characteristics not shared by the overall RA population. The same is true for physician-reported data; whilst minimal inclusion criteria governed the selection of the participating physicians, participation was influenced by willingness to complete the survey. Recall bias may have affected survey responses from both patients and physicians, despite attempts to minimize this effect by use of medical records for recording historical data. Analyses were also limited by available sample size, and therefore it was not possible to stratify the results by patient age, intelligence, country, or duration of current ADA biosimilar therapy, all of which may impact outcomes and may be areas of future research. Whilst the propensity score matching confounded for some patient characteristics (such as patient age), it was not possible to confound for them all. Findings may have been affected by variations in clinical practice across the sampled countries, and may have differed if data were drawn from other countries. The data were collected during the coronavirus disease (COVID-19) pandemic and therefore shifting practice patterns, patient behavior and expectations towards their treatment caused by the pandemic cannot be ruled out.
Conclusions
In conclusion, this analysis of the Adelphi RA DSP demonstrated that the switching of patients with RA from ADA bio-originator to an ADA biosimilar, may have some unforeseen outcomes that should be considered by health decision makers, such as effects on patients’ disease severity, treatment adherence and QoL. Disease severity worsened for a few but remained stable for the majority of switchers throughout the duration of their biosimilar treatment, resulting in relatively poorer outcomes than in the case of non-switchers. While the majority of studies have demonstrated good results following a switch to biosimilar therapies, switching patients for non-medical reasons in real-world practice may result in untoward outcomes, with the nocebo effect potentially playing a significant role [14].
The reasons for and downstream implications of all possible outcomes amongst patients non-medically switched to biosimilars remain an area of further research.
References
McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–19.
Lin YJ, Anzaghe M, Schülke S. Update on the pathomechanism, diagnosis, and treatment options for rheumatoid arthritis. Cells. 2020;9(4):880.
Silva-Fernández L, Macía-Villa C, Seoane-Mato D, Cortés-Verdú R, Romero-Pérez A, Quevedo-Vila V, et al. The prevalence of rheumatoid arthritis in Spain. Sci Rep. 2020;10(1):21551.
Matcham F, Scott IC, Rayner L, Hotopf M, Kingsley GH, Norton S, et al. The impact of rheumatoid arthritis on quality-of-life assessed using the SF-36: a systematic review and meta-analysis. Semin Arthritis Rheum. 2014;44(2):123–30.
Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–99.
EMA. Humira. European public assessment report (EPAR) EMEA/H/C/000481. https://www.ema.europa.eu/en/medicines/human/EPAR/humira (Accessed 01 Dec 2021).
Drugs.com. FDA Approves Humira https://www.drugs.com/newdrugs/fda-approves-humira-adalimumab-rheumatoid-arthritis-77.html (Accessed 01 Dec 2021).
Norman P. Humira: the impending patent battles over adalimumab biosimilars. Pharm Pat Anal. 2016;5(3):141–5.
Huizinga TWJ, Torii Y, Muniz R. Adalimumab biosimilars in the treatment of rheumatoid arthritis: A systematic review of the evidence for biosimilarity. Rheumatol Ther. 2021;8(1):41–61.
Lu X, Hu R, Peng L, Liu M, Sun Z. Efficacy and safety of adalimumab biosimilars: Current critical clinical data in rheumatoid arthritis. Front Immunol. 2021;12: 638444.
Azevedo V, Dörner T, Strohal R, Isaacs J, Castañeda-Hernández G, Gonçalves J, et al. Biosimilars: considerations for clinical practice. Considerations Med. 2017;1:13–8.
Smolen JS, Goncalves J, Quinn M, Benedetti F, Lee JY. Era of biosimilars in rheumatology: reshaping the healthcare environment. RMD Open. 2019;5(1): e000900.
Vulto AG, Jaquez OA. The process defines the product: what really matters in biosimilar design and production? Rheumatology (Oxford) 2017;56(suppl_4):iv14–iv29.
Renton WD, Leveret H, Guly C, Smee H, Leveret J, Ramanan AV. Same but different? A thematic analysis on adalimumab biosimilar switching among patients with juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2019;17(1):67.
Coghlan J, He H, Schwendeman AS. Overview of Humira® biosimilars: Current European landscape and future implications. J Pharm Sci. 2021;110(4):1572–82.
Benedetti F, Lanotte M, Lopiano L, Colloca L. When words are painful: unraveling the mechanisms of the nocebo effect. Neuroscience. 2007;147(2):260–71.
Kaneko K, Prieto Alhambra D, Jacklin C, Bosworth A, Dickinson S, Berry S, et al. The influence of information provided prior to switching from Humira to biosimilar adalimumab on UK patients’ satisfaction: a cross sectional survey by patient organisations. BMJ Open. 2022;12(2): e050949.
Kravvariti E, Kitas GD, Mitsikostas DD, Sfikakis PP. Nocebos in rheumatology: emerging concepts and their implications for clinical practice. Nat Rev Rheumatol 2018;14(12):727–40.
Anderson P, Benford M, Harris N, Karavali M, Piercy J. Real-world physician and patient behaviour across countries: Disease-specific programmes - a means to understand. Curr Med Res Opin. 2008;24(11):3063–72.
Babineaux SM, Curtis B, Holbrook T, Milligan G, Piercy J. Evidence for validity of a national physician and patient-reported, cross-sectional survey in China and UK: the Disease Specific Programme. BMJ Open. 2016;6(8): e010352.
Higgins V, Piercy J, Roughley A, Milligan G, Leith A, Siddall J, et al. Trends in medication use in patients with type 2 diabetes mellitus: a long-term view of real-world treatment between 2000 and 2015. Diabetes Metab Syndr Obes. 2016;9:371–80.
EuroQol Group. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208.
Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–65.
Bruce B, Fries JF. The Health Assessment Questionnaire (HAQ). Clin Exp Rheumatol. 2005;23(5 Suppl 39):S14-18.
FACIT-F group. Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F). https://www.facit.org/measures/FACIT-F (accessed 01 Dec 2021).
Hewlett S, Hehir M, Kirwan JR. Measuring fatigue in rheumatoid arthritis: a systematic review of scales in use. Arthritis Rheum. 2007;57(3):429–39.
StataCorp. Stat Statistical Software: Release 17. College Station, TX: StataCorp LLP; 2021.
Aladul MI, Fitzpatrick RW, Chapman SR. Healthcare professionals’ perceptions and perspectives on biosimilar medicines and the barriers and facilitators to their prescribing in UK: a qualitative study. BMJ Open. 2018;8(11): e023603.
Kim H, Alten R, Avedano L, Dignass A, Gomollón F, Greveson K, et al. The future of biosimilars: Maximizing benefits across immune-mediated inflammatory diseases. Drugs. 2020;80(2):99–113.
Kaplan GG, Ma C, Seow CH, Kroeker KI, Panaccione R. The argument against a biosimilar switch policy for infliximab in patients with inflammatory bowel disease living in Alberta. J Can Assoc Gastroenterol. 2020;3(5):234–42.
Braun J, Kudrin A. Switching to biosimilar infliximab (CT-P13): Evidence of clinical safety, effectiveness and impact on public health. Biologicals. 2016;44(4):257–66.
Fleischmann R, Jairath V, Mysler E, Nicholls D, Declerck P. Nonmedical switching from originators to biosimilars: Does the nocebo effect explain treatment failures and adverse events in rheumatology and gastroenterology? Rheumatol Ther. 2020;7(1):35–64.
Cantini F, Niccoli L, Franchi G, Damiani A, Benucci M. The nocebo effect in rheumatology: An unexplored issue. Isr Med Assoc J. 2020;22(3):185–90.
Scherlinger M, et al. Switching from originator infliximab to biosimilar CT-P13 in real-life: The weight of patient acceptance. Joint Bone Spine. 2018;85(5):561–7.
Smolen JS, Landewé R, Breedveld FC, Damiani A, Benucci M. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73(3):492–509.
Acknowledgements
Adelphi Real World would like to thank all patients and physicians who provided the data which constitute the basis for these analyses.
Funding
This work and journal's Rapid Service Fee was supported by AbbVie Inc., North Chicago, Illinois 60064, USA. Data collection was undertaken by Adelphi Real World as part of an independent survey, entitled the Adelphi Rheumatoid Arthritis DSP. The DSP is a wholly owned Adelphi product, and is the intellectual property of Adelphi Real World. The analysis described here used data from the Adelphi Rheumatoid Arthritis DSP. AbbVie Inc., North Chicago, Illinois 60064, USA, was one of multiple subscribers to the DSP, and were involved in research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. No honoraria or payments were made for authorship.
Medical Writing, Editorial and Other Assistance
Medical writing and editorial assistance were provided by Sue Libretto, PhD, of Sue Libretto Publications Consultant Ltd (Hertfordshire, UK).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Peter Taylor critically revised this work, Yuri Sanchez Gonzalez interpreted the data, critically revised this work and contributed to its conception, Ryan Clark critically revised this work, Freddy Faccin critically revised this work, Oliver Howell contributed to this study’s conception, data acquisition, and analysis, interpreted the data and provided critical revisions.
Disclosures
Peter Taylor has served as a consultant to AbbVie, Biogen, Janssen, Pfizer, Sanofi, Fresenius and UCB. Yuri Sanchez-Gonzalez, Ryan Clark and Freddy Faccin are employees of AbbVie and may own AbbVie stock and/or options. Oliver Howell is an employee of Adelphi Real World and was a paid consultant to AbbVie in connection with the development of this manuscript.
Compliance with Ethics Guidelines
The survey did not require Ethics or Institutional Review Board review due to its non-interventional, observational nature of data collection, but required informed consent from physicians and patients before their participation. The survey was conducted in accordance with the Western Institutional Review Board (protocol number 21-ADRW-104). Where patients provided data directly, they signed an informed consent form prior to participation in this study. Data collection by DSP fieldwork teams was conducted in accordance with national market research and privacy regulations, including European Pharmaceutical Market Research Association (EphMRA), and the US Department of Health and Human Services National Institutes of Health, Health Insurance Portability and Accountability Act (HIPAA). All responses captured on the data collection forms were anonymized to preserve respondent confidentiality. Responses were anonymized before aggregated reporting, the identity of the physicians was blinded, and no patient identifiers were collected. This study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments.
Data Availability
All data relevant to the study are included in the article or uploaded as supplementary information. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Taylor, P.C., Gonzalez, Y.S., Clark, R. et al. Outcomes Following Adalimumab Bio-originator to Biosimilar Switch—A Comparison Using Real-world Patient- and Physician-Reported Data in European Countries. Rheumatol Ther 10, 433–445 (2023). https://doi.org/10.1007/s40744-022-00526-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-022-00526-w