Abstract
Introduction
One target of rheumatoid arthritis (RA) treatment is to achieve early sustained remission; over the long term, patients in sustained remission have less structural joint damage and physical disability. We evaluated Simplified Disease Activity Index (SDAI) remission with abatacept + methotrexate versus abatacept placebo + methotrexate and impact of de-escalation (DE) in anti-citrullinated protein antibody (ACPA)-positive patients with early RA.
Methods
The phase IIIb, randomized, AVERT-2 two-stage study (NCT02504268) evaluated weekly abatacept + methotrexate versus abatacept placebo + methotrexate. Primary endpoint: SDAI remission (≤ 3.3) at week 24. Pre-planned exploratory endpoint: maintenance of remission in patients with sustained remission (weeks 40 and 52) who, from week 56 for 48 weeks (DE period), (1) continued combination abatacept + methotrexate, (2) tapered abatacept to every other week (EOW) + methotrexate for 24 weeks with subsequent abatacept withdrawal (abatacept placebo + methotrexate), or (3) withdrew methotrexate (abatacept monotherapy).
Results
Primary study endpoint was not met: 21.3% (48/225) of patients in the combination and 16.0% (24/150) in the abatacept placebo + methotrexate arm achieved SDAI remission at week 24 (p = 0.2359). There were numerical differences favoring combination therapy in clinical assessments, patient-reported outcomes (PROs) and week 52 radiographic non-progression. After week 56, 147 patients in sustained remission with abatacept + methotrexate were randomized (combination, n = 50; DE/withdrawal, n = 50; abatacept monotherapy, n = 47) and entered DE. At DE week 48, SDAI remission (74%) and PRO improvements were mostly maintained with continued combination therapy; lower remission rates were observed with abatacept placebo + methotrexate (48.0%) and with abatacept monotherapy (57.4%). Before withdrawal, de-escalating to abatacept EOW + methotrexate preserved remission.
Conclusions
The stringent primary endpoint was not met. However, in patients achieving sustained SDAI remission, numerically more maintained remission with continued abatacept + methotrexate versus abatacept monotherapy or withdrawal.
Trial Registration
ClinicalTrials.gov identifier, NCT02504268.
Video abstract (MP4 62241 KB)
Plain Language Summary
Patients with rheumatoid arthritis (RA) experience inflamed and damaged joints. RA is an autoimmune disease in which proteins called autoantibodies, particularly anti-citrullinated protein autoantibodies, target the patient’s own joint tissue and organs by mistake, leading to symptomatic inflammation. Successful treatment can decrease the disease’s activity to a state known as remission. Patients in remission may experience little or no symptoms and it may be possible for some to then be able to decrease their treatment. Here, we report the results of a large, international study that looked at two treatments, abatacept and methotrexate, in patients with RA and anti-citrullinated protein autoantibodies. The study had two parts. Firstly, to see how many patients had success (remission) with weekly abatacept and/or methotrexate treatment, and secondly, to see if remission was maintained when treatment was either continued or decreased and stopped. The study showed that the number of patients in remission 6 months after treatment started was not greatly different between patients treated with both abatacept and methotrexate and those treated with just methotrexate. Those taking abatacept and methotrexate together had better remission rates 1 year later. More patients also stayed in remission when they continued to receive both abatacept and methotrexate compared with those who were just treated with abatacept or when their abatacept treatment was decreased and stopped. More patients stayed in remission when abatacept was decreased than when it was stopped. The results from this study may help determine possible future treatment reduction and/or withdrawal plans for some patients with RA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Achieving early sustained remission is a target of rheumatoid arthritis (RA) treatment. Over the long term, patients in sustained remission have less structural joint damage and physical disability. |
Following a period of sustained disease remission defined by stringent criteria, treatment dose reduction could be considered, although in general, tapering regimens are not well defined. |
Assessing Very Early Rheumatoid arthritis Treatment-2 (AVERT-2), a randomized, placebo-controlled study, evaluated the efficacy of abatacept + methotrexate (MTX) versus abatacept placebo + MTX and the maintenance of remission during a subsequent dose de-escalation (DE) period. |
What was learned from the study? |
Patient-reported outcomes (PROs) and inhibition of structural damage showed clinically meaningful benefits of abatacept + MTX therapy in anti-citrullinated protein antibody–positive patients with early RA. Numerically more patients maintained Simplified Disease Activity Index remission with improved PROs on continued abatacept + MTX therapy than on abatacept monotherapy or DE and withdrawal; abatacept DE was more effective than withdrawal in maintaining clinical and PRO responses. |
These data provide practice-informing evidence to aid in defining a treatment tapering/withdrawal strategy for patients with RA treated with abatacept and suggest that abatacept-containing DE regimens may be a viable option in some patients without risking damage progression. |
Digital Features
This article is published with digital features, including a video abstract, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.21667850.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by systemic inflammation and joint destruction. The presence of the autoantibodies, rheumatoid factor and anti-citrullinated protein antibody (ACPA), is associated with a less favorable prognosis [1]. Remission, a target of RA treatment, is defined by the American College of Rheumatology (ACR) and the European Alliance of Associations for Rheumatology (EULAR) as a sustained reduction in disease activity measured by a Simplified Disease Activity Index (SDAI) score of ≤ 3.3 or Boolean remission [2, 3]. Achievement of remission has been associated with reduced structural joint damage and physical disability over the longer term [4].
A therapeutic “window of opportunity” may exist where optimal early treatment may induce sustained remission and beneficial long-term outcomes [5– 7]. Use of a treat-to-target approach is advocated by ACR and EULAR; both suggest early use of biologic and targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) when use of conventional synthetic DMARDs (csDMARDs) fails to reach the therapeutic target within 3–6 months [2, 3]. In patients successfully achieving sustained remission by this approach, ACR/EULAR advise that tapering treatment following sustained disease remission may be possible, though they remain cautious about all treatment discontinuation [2, 3]. Tapering can include dose reduction, an increased interval between administration or discontinuation [8]. It has been suggested that drug tapering after remission is possible, but not sustainable, for the majority of patients; no studies have demonstrated sustained drug-free remission for longer than 2 years [9, 10].
Abatacept, a selective co-stimulation modulator that blocks the interaction between CD80/CD86 on antigen-presenting cells and CD28 on T cells, disrupting naive T-cell activation [11], has proven efficacy in the treatment of patients with RA as monotherapy or in combination with csDMARDs [12– 14]. We report the results of Assessing Very Early Rheumatoid arthritis Treatment-2 (AVERT-2), a randomized, placebo-controlled study to evaluate the efficacy and safety of subcutaneous (SC) abatacept + methotrexate (MTX) versus abatacept placebo + MTX in MTX-naive, ACPA-positive patients with early, active RA.
The objectives of the study were: firstly, to investigate the efficacy of weekly SC abatacept + MTX versus abatacept placebo + MTX in achieving stringent remission (SDAI score ≤ 3.3) at 24 weeks in ACPA-positive patients with early, active RA; and secondly, to assess in an exploratory analysis the maintenance of SDAI remission, radiographic progression, patient-reported outcomes (PROs) and safety during a subsequent dose de-escalation (DE) period in those with sustained SDAI remission at week 56. Both the primary endpoint and the exploratory analyses are reported here.
Methods
Study Design
AVERT-2 was a phase IIIb, randomized, double-blind, placebo-controlled study (NCT02504268) in ACPA-positive patients with early RA assessing the primary endpoint at week 24. This two-stage study consisted of a 56-week double-blind, placebo-controlled induction period (IP) assessing if there was a statistical difference in achieving SDAI remission with abatacept + MTX versus abatacept placebo + MTX followed by a 48-week DE period.
IP
In the IP, patients were randomized (3:2) to blinded SC abatacept (125 mg once weekly [QW]) + oral MTX (starting dose 7.5–15 mg/week titrated to ≥ 15 mg [as tolerated and per local practice and regulations] within 8 weeks) or SC abatacept placebo + oral MTX (with titration as above) for 56 weeks (Fig. 1).
Study design. An IP of 56 weeks was followed by a 48-week DE period for patients in sustained SDAI remission (SDAI ≤ 3.3 at both weeks 40 and 52 in the IP) and a 24-week post-treatment follow-up period (all patients). The open-label treatment schedule, which ran throughout the study, was an option for: (1) escape during the IP (between weeks 28 and 52) for patients who, despite a sufficient trial of rescue therapies, were considered non-responders (did not achieve a 20% improvement using the 66/68 Joint Count Assessment in both tender joint count and swollen joint count relative to day 1); (2) patients at the end of the IP (after week 56) who did not achieve sustained remission (SDAI ≤ 3.3 at weeks 40 and 52); or (3) escape during the DE period for patients with SDAI > 11 (at least moderate disease activity). Patients followed the open-label treatment schedule (SC abatacept QW + MTX) until they completed 104 weeks of treatment relative to the initial randomization date. Cohort 1: intention-to-treat population, all randomized patients who received ≥ 1 dose of study drug in the first 56 weeks; cohort 2: patients enrolled following completion of randomization for cohort 1 to ensure an adequate number of patients proceeded to the DE period. aSDAI ≤ 3.3 at both weeks 40 and 52; patients from treatment arm A were randomized into the DE period to one of three treatment arms (C: continuation, D: DE followed by withdrawal, or E: monotherapy) in a ratio of 1:1:1 at week 56. Patients in sustained SDAI remission from treatment arm B continued to receive this treatment in a blinded fashion. bDE completers. ABA abatacept, DE de-escalation, EOW every other week, IP induction period, MRI magnetic resonance imaging, MTX methotrexate, OL open-label period, QW once weekly, SC subcutaneous, SDAI Simplified Disease Activity Index, Wk week. Figure adapted from Emery P, et al. EULAR Congress 2020; 6 June 2020; poster SAT0104 (with permission of the authors)
Per protocol, the primary endpoint was analyzed at week 24 in the first 325 patients randomized globally plus the first 50 patients randomized from Japan (as required by the Japanese regulatory authorities) and consisted of patients who received ≥ 1 dose of study drug in the first 56 weeks of the study (primary analysis population). This primary analysis population was used for the primary endpoint and some secondary endpoints (see Results). Cohort 1 (intention-to-treat population) comprised all randomized patients who received ≥ 1 dose of study drug in the first 56 weeks. Additional patients (cohort 2) who received open-label abatacept + MTX were enrolled following completion of randomization for cohort 1 to ensure an adequate number of patients achieving SDAI remission for inclusion in the DE period.
Dose DE Period
All patients (from cohorts 1 and 2) who completed the initial 56 weeks with abatacept + MTX who had sustained SDAI remission at both weeks 40 and 52 were randomized (1:1:1) at week 56 to one of three blinded abatacept treatment arms in the 48-week DE period: (1) continuation of combination (abatacept QW + MTX for 48 weeks), (2) abatacept stepwise DE and subsequent withdrawal (abatacept every other week [EOW] + MTX for 24 weeks [Part 1] followed by abatacept placebo + MTX for 24 weeks [Part 2]), or (3) abatacept monotherapy (abatacept QW + MTX placebo) (Fig. 1). In a fourth treatment arm, patients with sustained SDAI remission (≤ 3.3) who received abatacept placebo + MTX during the first 56 weeks were not re-randomized, but continued the same treatment in the DE period in a blinded fashion and were followed for monitoring purposes; no comparisons between this arm and the abatacept arms are reported in the DE period (data for these patients are in Supplementary Material Table S1). The DE population comprised those who received ≥ 1 dose of study drug during the DE period.
MTX doses following initial titration remained unchanged during the DE period. Stable doses of oral corticosteroids (≤ 10 mg/day prednisone or its equivalent) were allowed and maintained throughout the study with a single increase to a maximum equivalent of 10 mg/day during the DE period. Between study weeks 56 and 80, a single rescue intervention for RA of intramuscular, intraarticular, or oral steroids was allowed at the investigator’s discretion. A second rescue intervention was allowed between study weeks 80 and 104. For each rescue intervention, the total dose (intramuscular, intraarticular, or oral) was ≤ 80 mg methylprednisolone or its equivalent.
Patients were recruited from 167 sites in 30 countries (Supplementary Material Methods) from September 2015 until September 2019. Randomization and masking details are in the Supplementary Material Methods.
Compliance with Ethics Guidelines
The study was conducted in accordance with the Declaration of Helsinki of 1964 and its later amendments, and the International Conference on Harmonization Good Clinical Practice guidelines. The protocol and patient-informed consent received institutional review board (IRB)/independent ethics committee approval prior to study initiation. The study was governed by both a central IRB (the New England IRB) as well as local and university-based IRBs if required at individual sites. IRB approval numbers per site were not provided and are not available. All patients provided written informed consent prior to enrollment. This informed consent included both the induction and the DE periods.
Patients
Patients aged ≥ 18 years with RA (defined by ACR/EULAR 2010 criteria) [15] were included if they had baseline SDAI scores > 11, disease duration (since diagnosis) ≤ 6 months, were ACPA positive, had high sensitivity C-reactive protein (CRP) > 3 mg/L or erythrocyte sedimentation rate (ESR) ≥ 28 mm/h, had ≥ 3 tender and ≥ 3 swollen joints on a 28-joint count (at screening and day 1), and were DMARD-naive. Exclusion criteria are in the Supplementary Material Methods.
Study Assessments and Endpoints
Efficacy
Clinical efficacy was assessed by the proportions of patients achieving SDAI remission (≤ 3.3), Boolean remission, Disease Activity Score in 28 joints using CRP (DAS28 [CRP]) < 2.6, and ≥ 20%/50%/70% improvement in ACR criteria (ACR20/50/70) during the course of the study. Radiographic progression was evaluated by modified total Sharp score (mTSS; calculated as proportion of non-progressors, defined as a change from baseline ≤ 0.5).
The primary endpoint of the AVERT-2 study was the proportion of patients in SDAI remission at week 24. Secondary endpoints included proportions of patients with: radiographic progression (mTSS; non-progressors change from baseline ≤ 0.5) at week 52, SDAI and Boolean remission at week 52, and DAS28 (CRP) < 2.6 at week 24.
Exploratory endpoints evaluated at the end of the 48-week DE period included proportion of patients with SDAI ≤ 3.3, adjusted mean change in SDAI score from DE period day 1, and radiographic progression (mTSS, non-progressors change from baseline ≤ 0.5). Further endpoints are detailed in the Supplementary Material Methods.
PROs
PROs included the proportion of patients with improvement in Health Assessment Questionnaire-Disability Index (HAQ-DI; responders defined as ≥ 0.30 decrease from baseline [16– 18]) and minimal clinically important difference (MCID; decrease ≥ 0.22 from baseline [19]), the 36-Item Short-Form Health Survey (SF-36) v2.0 Physical Function Scale (PFS) and Mental Component Summary (MCS; PFS and MCS 0–100), Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F; 0–52), and Work Productivity and Activity Impairment–Rheumatoid Arthritis (WPAI-RA; 0–100).
Safety
Safety was assessed by adverse events (AEs), serious AEs (SAEs), discontinuations due to AEs, and AEs of special interest (including those associated with immunomodulatory drugs, such as infections, prespecified autoimmune disorders, malignancies, and injection reactions).
Statistical Analysis
Sample Size Calculation
IP
To have sufficient power (75%) to assess the X-ray secondary endpoint (change in mTSS at week 52), a total sample size of 750 patients was planned for the IP of the study. A sample size of 375 patients (225 in the abatacept + MTX arm plus 150 in the abatacept placebo + MTX arm) was planned for the primary analysis population to assess the primary endpoint (SDAI remission rate at week 24) with > 99% power to detect a treatment difference of 25% between the two treatment groups. This calculation was based on a continuity-corrected chi-squared test with a 5% two-sided alpha level and assuming SDAI remission rates at week 24 of 39% and 14% in the abatacept + MTX and abatacept placebo + MTX treatment groups, respectively.
Dose DE Period
A total sample size of 700 patients receiving abatacept + MTX QW (250 from cohort 2 plus 450 from cohort 1) would allow approximately 280 patients to meet sustained SDAI remission at weeks 40 and 52 and be eligible to be randomized in the DE period. This sample size would allow a 97.5% confidence interval (CI) of the estimate of treatment difference in SDAI remission rate between the arms in each comparison (comparison 1: abatacept EOW + MTX QW [withdrawal arm] versus abatacept QW + MTX QW [combination arm]; comparison 2: abatacept monotherapy versus abatacept QW + MTX QW [combination arm]) to each exclude 0, assuming an 11% delta and 90% SDAI remission rate in the abatacept + MTX QW arm at week 24 of the DE period.
Analyses
Baseline demographics and disease characteristics were analyzed descriptively. The primary endpoint was tested using a logistic regression model with a two-sided alpha equal to 0.05; other binary variables during the IP were also analyzed in this fashion. Point estimates of the adjusted odds ratios (ORs) for the odds of achieving the outcome measure in the abatacept + MTX arm compared with the abatacept placebo + MTX arm and corresponding 95% CIs and p-values were provided. The primary and secondary endpoints were tested in a hierarchical fashion to maintain the overall type I error rate at 5% (detailed in the Supplementary Material Methods). Continuous variables were analyzed using a longitudinal repeated measures model. Missing values were imputed as non-remitter, except if missing between two visits with remission where they were imputed as remitter.
For the DE analysis, all efficacy summaries are presented over time (from week 56 to week 104) and by treatment group. Treatment differences and 97.5% CIs were provided for the three treatment arms of the DE period; no formal statistical analyses were conducted for the DE period. Safety was analyzed descriptively throughout the study.
Results
Patient Disposition and Baseline Characteristics
Cohort 1 comprised 752 patients who were randomized to receive abatacept + MTX (n = 451) or abatacept placebo + MTX (n = 301); 63 (14%) and 68 (23%) discontinued, respectively, by week 52 (Supplementary Material Fig. S1). An additional 242 patients (cohort 2) were treated with open-label abatacept + MTX during the IP (Supplementary Material Fig. S1; Supplementary Material Table S2).
In the DE period, 147 patients in sustained SDAI remission (cohort 1, n = 94; cohort 2, n = 53) were randomized (abatacept QW + MTX continuation, n = 50; DE and withdrawal, n = 50; abatacept monotherapy, n = 47). A total of 37 patients who received abatacept placebo + MTX during the IP continued in the DE period without randomization, and 30 patients discontinued during the DE period (Supplementary Material Fig. S1).
Overall demographic and disease characteristics (Table 1) were similar across treatment groups, whereas at DE period day 1 across randomized arms, ranges of mean scores were 1.87–2.52 (SDAI), 1.63–1.79 (DAS28 [CRP]), 0.18–0.30 (HAQ-DI), and 4.31–8.30 (mTSS). Mean (range) MTX dose at DE day 1 continued unchanged and was 14.9 (7.0–24.9) mg/week in the abatacept + MTX group.
Efficacy
IP
Primary Endpoint (Primary Analysis Population)
The primary endpoint was not met: there was no statistically significant difference between abatacept + MTX compared with abatacept placebo + MTX in the proportion of patients with SDAI ≤ 3.3 at week 24 in the primary analysis population 21.3% (48/225) for abatacept + MTX versus 16.0% (24/150) for abatacept placebo + MTX (adjusted OR [95% CI]: 1.4 [0.8–2.5]; p = 0.2359) (Fig. 2a).
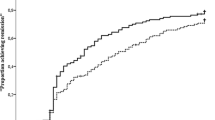
Proportion of patients in SDAI remission (≤ 3.3) in (a) the IP at week 24 (primary endpoint; primary analysis population, n = 375) and in (b) the DE period among randomized patients. For part A, data in table report n (%). For part B, the percentage of patients at week 0 of the DE period represents those in remission at weeks 40 and 52 of the IP; some patients may have lost remission prior to week 0 of the DE period. Numbers of patients in remission during the DE period were as follows: DE week 24, abatacept QW + MTX n = 39, abatacept EOW + MTX n = 37, abatacept QW + MTX PBO n = 30; DE week 48, abatacept QW + MTX n = 37, abatacept PBO + MTX n = 24, abatacept QW + MTX PBO n = 27. The primary analysis population was a subset comprising the first 50 patients from Japan and the first 325 from the rest of the world who were randomized and treated in the IP. Missing values were imputed as non-remitter, except if missing between two visits with remission where they were imputed as remitter. Treatment differences and 95% CIs were based on minimum risk weights. Error bars show 95% CIs. aDe-escalation period weeks 0–48 correspond to study weeks 56–104. ABA abatacept, CI confidence interval, DE de-escalation, EOW every other week, IP induction period, MTX methotrexate, PBO placebo, QW once weekly, SDAI Simplified Disease Activity Index. a Reprinted from ACR/ARHP Annual Scientific Meeting held 19–24 October 2018. The American College of Rheumatology does not guarantee, warrant, or endorse any commercial products or services. Reprinted by Springer Nature. b Adapted from Emery P, et al. EULAR Congress 2020; 6 June 2020; poster SAT0104 (with permission of the authors)
Secondary and Exploratory Endpoints (Primary Analysis Population and Cohort 1)
As the primary analysis was not met, only nominal p-values could be calculated for the subsequent analyses. Nominally significant benefits in favor of abatacept + MTX were observed for all secondary endpoints. At week 24, proportions of patients with DAS28 (CRP) < 2.6 (primary analysis population) were 38.7% for abatacept + MTX and 25.3% for abatacept placebo + MTX (nominal p = 0.0112; Supplementary Material Fig. S2). At week 24, the proportions of patients in Boolean remission with HAQ-DI response and with ACR20/50/70 responses were numerically greater in the abatacept + MTX group than in the abatacept placebo + MTX group (data not shown). At week 52, proportions of patients achieving SDAI remission (primary analysis population) were 29.8% for abatacept + MTX and 15.3% for abatacept placebo + MTX (nominal p = 0.0021; Table 2). Boolean remission was achieved by 21.5% and 11.6% of patients in the abatacept + MTX and abatacept placebo + MTX arms, respectively (cohort 1; nominal p = 0.0006; Table 2). Mean (standard deviation [SD]) changes from baseline in mTSS were 0.5 (2.3) in the abatacept + MTX group and 2.5 (6.2) in the abatacept placebo + MTX group (cohort 1; nominal p < 0.0001); proportions of radiographic non-progressors were 71.8% in the abatacept + MTX group and 49.0% in the abatacept placebo + MTX group (cohort 1; Table 2 and Supplementary Material Fig. S3). The proportions of patients with HAQ-DI MCID (decrease ≥ 0.22) were 77.2% for abatacept + MTX and 69.4% for abatacept placebo + MTX (cohort 1; p = 0.0178; Supplementary Material Table S3). Additional secondary and exploratory endpoints are detailed in Table 2 and Supplementary Material Tables S4 and S5.
Dose DE Period (DE Population)
A total of 74.0% of patients in the abatacept + MTX continuation arm maintained SDAI remission at DE period week 48 (Table 2; Fig. 2b) compared with 48.0% in the abatacept DE and withdrawal arm and 57.4% in the abatacept monotherapy arm. At DE period week 24, 74.0% of patients in the abatacept DE and withdrawal arm maintained SDAI remission prior to withdrawal compared with 78.0% in the abatacept + MTX continuation arm. The adjusted mean changes in SDAI in the DE period were relatively low but higher in the abatacept monotherapy and withdrawal arms compared with the continuation arm (Fig. 3). All SDAI components increased similarly in the DE and withdrawal arm. The proportion of patients with SDAI ≤ 11 was 90.0% in the abatacept + MTX continuation arm, 64.0% in the abatacept DE and withdrawal arm, and 76.6% in the abatacept monotherapy arm at DE period week 48 (Table 2).
Adjusted mean change in SDAI score from de-escalation day 1 in the DE period among randomized patients. Estimates of adjusted mean changes are from a repeated measures mixed model that includes treatment group, time, time-by-treatment interaction, baseline value, and time-by-baseline value interaction. Patients receiving ABA EOW + MTX were switched to ABA PBO + MTX at DE week 24 in a blinded manner. Number of patients with measurement at DE weeks 0, 12, 24, 40, and 48, respectively: ABA QW + MTX: 50, 45, 42, 41, and 40; ABA EOW + MTX/ABA PBO + MTX: 50, 45, 43, 41, and 37; ABA + MTX PBO: 47, 39, 34, 33, and 31. ABA abatacept, DE de-escalation, EOW every other week, MTX methotrexate, PBO placebo, QW once weekly, SDAI Simplified Disease Activity Index. Figure reprinted from ACR/ARHP Annual Scientific Meeting held 8–13 November 2019. The American College of Rheumatology does not guarantee, warrant, or endorse any commercial products or services. Reprinted by Springer Nature
Sustained inhibition of structural damage was seen in all arms at DE period week 48 (Table 2). Most patients (84–87%) were radiographic non-progressors (change from DE weeks −4 to 48, mTSS ≤ 0.5).
PROs
PROs improved in all groups during the IP (Supplementary Material Tables S4 and S5). The proportion of patients achieving a HAQ-DI ≥ 0.3 decrease was maintained through DE period week 48 (84.0% of those continuing abatacept and 64.0% and 74.5% in the DE/withdrawal and abatacept monotherapy arms, respectively) (Supplementary Material Fig. S4). At DE period week 48, the adjusted mean change in HAQ-DI declined slightly in the abatacept combination arm but increased to some extent in the DE/withdrawal and abatacept monotherapy arms (Fig. 4a). Similar trends were seen for SF-36 PFS and FACIT-F (Supplementary Material Table S5). FACIT-F scores improved during the DE period in all arms (Fig. 4b), while WPAI-RA scores remained stable in the abatacept + MTX continuation (adjusted mean change: 3.34) and monotherapy arms (adjusted mean change: 2.53) but worsened in the DE and withdrawal arm (adjusted mean change: 13.08) (Fig. 4c).
Adjusted mean change in a HAQ-DI, b FACIT-F, and c WPAI-RA activity impairment in the DE period among randomized patients. HAQ-DI and WPAI-RA activity impairment: decrease in adjusted mean change denotes improvement; FACIT-F: increase in adjusted mean change denotes improvement. ABA abatacept, CI confidence interval, DE de-escalation, EOW every other week, FACIT-F Functional Assessment of Chronic Illness Therapy–Fatigue, HAQ-DI Health Assessment Questionnaire-Disability Index, PBO placebo, QW once weekly, WPAI-RA Work Productivity and Activity Impairment–Rheumatoid Arthritis. Figure adapted from Emery P, et al. EULAR Congress 2020; 6 June 2020; poster SAT0104 (with permission of the authors)
Safety
Safety profiles were similar across treatment arms during the study period with no unexpected safety signals noted. During the IP, 38 patients (30 [6.7% abatacept + MTX] and 8 [2.7% abatacept monotherapy]) reported SAEs, leading to 5 and 3 discontinuations, respectively (Supplementary Material Table S6).
Discussion
Although the primary endpoint of the proportion of patients achieving SDAI remission was not met in the AVERT-2 study, clinically meaningful benefits of abatacept + MTX therapy compared with abatacept placebo + MTX were observed in ACPA-positive, MTX-naive patients with early RA with SDAI and Boolean remission, DAS28 (CRP), HAQ-DI improvement, and change at week 52 in mTSS. To our knowledge, this study is the first to evaluate bDMARD efficacy using the strict metric of SDAI remission as a primary endpoint. Not meeting the primary endpoint may have been due to the stringency of achieving SDAI remission by week 24 rather than a later time point, in patients with high disease activity who were treatment naive. If a less restrictive endpoint had been used, such as DAS28 (CRP) at week 24, which has been reported in numerous other trials [20, 21], this study would have met the primary endpoint (the difference in DAS28 [CRP] < 2.6 of 38.7% for abatacept + MTX and 25.3% for abatacept placebo + MTX monotherapy was nominally significant [p = 0.0112] at week 24). We elected to use SDAI, one of the two ACR/EULAR-approved metrics for sustained remission, as we felt this would be clinically meaningful and more likely to result in limiting radiographic progression and improving patient function and health-related quality of life over time compared with DAS28 (CRP). It is well recognized that patients with DAS28 (CRP) < 2.6 can still have significant disease activity [22]. Consistent with this hypothesis are the clinically meaningful benefits of abatacept + MTX therapy we observed in SDAI and Boolean remission, improvement in PROs, and slowing radiographic progression at week 52.
Importantly, in a pre-planned analysis, we investigated dose DE over 48 weeks. The results suggest that in patients with sustained SDAI remission during the IP, the continuation of combination therapy (abatacept QW + MTX) was more effective for maintenance of SDAI remission than abatacept monotherapy or DE and withdrawal of abatacept. Of note, the DE of abatacept to EOW + MTX preserved SDAI remission as well as the PRO response in a large proportion of patients, suggesting that this may be a viable alternative in the real world. Abatacept withdrawal was associated with the greatest loss of patients in remission (although changes in mean SDAI score were minor) as well as worsening of PROs. Of interest, radiographic non-progression was maintained in all three abatacept arms including the arm with eventual abatacept withdrawal. One possible explanation for this is that radiographic improvements might be more persistent and slower to worsen than clinical outcomes, and that progression would not be expected in patients whose disease is under reasonable control, although it is unclear if radiographic progression would increase over a longer timeframe than 3 months in the abatacept withdrawal arm. Safety was similar across treatments with no unexpected events reported.
Dose reduction data are available for many DMARDs; notably, unlike the AVERT-2 study, these data are not from randomized controlled studies and do not include radiographic outcomes [2, 23–32]. Most reports conclude that discontinuation of bDMARDs is often associated with eventual worsening of disease [25– 30]. Findings from trials assessing dose reduction/withdrawal of other bDMARDs have some similarities with the current study. The PRESERVE trial showed that conventional or reduced doses of etanercept + MTX in patients with active RA were more effective in maintaining low disease activity than MTX alone [31]. The PRIZE study of etanercept tapering in MTX- and bDMARD-naive patients with early RA also noted that reduced doses of etanercept + MTX were more effective in maintaining remission/low disease activity than MTX alone or treatment discontinuation [33]. In addition, PRIZE data showed that continuing MTX with or without etanercept, compared with switching to placebo, did not affect radiographic progression [33]. A trial of certolizumab pegol showed that it cannot be withdrawn in most patients with low-to-moderate active RA achieving Clinical Disease Activity Index ≤ 2.8, as most patients were unable to maintain remission [25]. These findings, combined with data from this study, may help guide clinician decision making once a patient is in sustained remission. Guidelines suggest that treatment dose adjustments following achievement of sustained remission should focus on tapering by dose reduction or interval increase rather than by discontinuation [2, 3]. The AVERT-2 data shown here provide practice-informing evidence to aid in defining a treatment tapering/withdrawal strategy for patients with RA treated with abatacept.
There are some limitations to this study. The generalizability of the data may be limited, as this study included a select group of patients with very early ACPA-positive RA, many of whom were from South America. The choice of SDAI remission by 6 months as primary endpoint in patients with very active RA may have been overly optimistic; 12 months may have been a more reasonable time frame. The DE/withdrawal part of this study was not powered to show whether the results were statistically significant or not. Furthermore, the choice of primary endpoint and/or its timing may not be appropriate for head-to-head clinical trials that have MTX as a comparator in patients with early RA who are MTX naive and are highly sensitive to the effects of MTX early in disease.
Conclusions
In summary, in this study of ACPA-positive, MTX-naive patients with early RA and high disease activity, a numerically but not statistically greater proportion of patients receiving abatacept + MTX achieved SDAI remission at week 24 than those receiving abatacept placebo + MTX, using the stringent primary endpoint of SDAI remission. However, abatacept in combination with MTX led to meaningful improvements in many other clinical assessments and PROs, consistent with previous trials in MTX-naive patients with early RA [34]. In addition, during the DE period, among patients with sustained SDAI remission following treatment with abatacept + MTX, the continuation of abatacept + MTX combination therapy was more effective at maintaining SDAI remission than abatacept monotherapy or DE followed by subsequent withdrawal. Sustained inhibition of structural damage was maintained at DE period week 48 even after abatacept withdrawal.
The data suggest that some patients may tolerate abatacept DE regimens, which may be a viable option in clinical practice for patients with early RA in sustained remission according to stringent SDAI criteria without risking joint damage progression.
References
Martin-Mola E, Balsa A, Garcia-Vicuna R, et al. Anti-citrullinated peptide antibodies and their value for predicting responses to biologic agents: a review. Rheumatol Int. 2016;36:1043–63.
Smolen JS, Landewe RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79:685–99.
Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology Guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2021;73:1108–23.
Felson DT, Smolen JS, Wells G, et al. American College of Rheumatology/European League against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum Dis. 2011;70:404–13.
Harrold LR, Litman HJ, Connolly SE, et al. A window of opportunity for abatacept in RA: is disease duration an independent predictor of low disease activity/remission in clinical practice? Clin Rheumatol. 2017;36:1215–20.
Raza K, Filer A. The therapeutic window of opportunity in rheumatoid arthritis: does it ever close? Ann Rheum Dis. 2015;74:793–4.
Barnabe C, Sun Y, Boire G, et al. Heterogeneous disease trajectories explain variable radiographic, function and quality of life outcomes in the Canadian early arthritis cohort (CATCH). PLoS One. 2015;10: e0135327.
Ajeganova S, Huizinga T. Sustained remission in rheumatoid arthritis: latest evidence and clinical considerations. Ther Adv Musculoskelet Dis. 2017;9:249–62.
Heimans L, Akdemir G, Boer KV, et al. Two-year results of disease activity score (DAS)-remission-steered treatment strategies aiming at drug-free remission in early arthritis patients (the IMPROVED-study). Arthritis Res Ther. 2016;18:23.
Tanaka Y. Stopping tumour necrosis factor-targeted biological DMARDs in rheumatoid arthritis. Rheumatology (Oxford). 2016;55:ii15–22.
Malmstrom V, Catrina AI, Klareskog L. The immunopathogenesis of seropositive rheumatoid arthritis: from triggering to targeting. Nat Rev Immunol. 2017;17:60–75.
Genovese MC, Becker JC, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353:1114–23.
Genovese MC, Covarrubias A, Leon G, et al. Subcutaneous abatacept versus intravenous abatacept: a phase IIIb noninferiority study in patients with an inadequate response to methotrexate. Arthritis Rheumatol. 2011;63:2854–64.
Sokolove J, Schiff M, Fleischmann R, et al. Impact of baseline anti-cyclic citrullinated peptide 2 antibody titre on efficacy outcomes following treatment with subcutaneous abatacept or adalimumab: 2-year results from the AMPLE trial. Ann Rheum Dis. 2015;74:983–4.
Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81.
Greenwood MC, Doyle DV, Ensor M. Does the Stanford Health Assessment Questionnaire have potential as a monitoring tool for subjects with rheumatoid arthritis? Ann Rheum Dis. 2001;60:344–8.
Schiff M, Weinblatt ME, Valente R, et al. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: two-year efficacy and safety findings from AMPLE trial. Ann Rheum Dis. 2014;73:86–94.
Kremer JM, Blanco R, Brzosko M, et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum. 2011;63:609–21.
Wells GA, Tugwell P, Kraag GR, Baker PR, Groh J, Redelmeier DA. Minimum important difference between patients with rheumatoid arthritis: the patient’s perspective. J Rheumatol. 1993;20:557–60.
van Vollenhoven R, Takeuchi T, Pangan AL, et al. Efficacy and safety of upadacitinib monotherapy in methotrexate-naive patients with moderately-to-severely active rheumatoid arthritis (SELECT-EARLY): a multicenter, multi-country, randomized, double-blind, active comparator-controlled trial. Arthritis Rheumatol. 2020;72:1607–20.
Genovese MC, Kalunian K, Gottenberg JE, et al. Effect of filgotinib vs placebo on clinical response in patients with moderate to severe rheumatoid arthritis refractory to disease-modifying antirheumatic drug therapy: the FINCH 2 randomized clinical trial. JAMA. 2019;322:315–25.
Sheehy C, Evans V, Hasthorpe H, Mukhtyar C. Revising DAS28 scores for remission in rheumatoid arthritis. Clin Rheumatol. 2014;33:269–72.
Emery P, Burmester GR, Bykerk VP, et al. Evaluating drug-free remission with abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann Rheum Dis. 2015;74:19–26.
Liu D, Yuan N, Yu G, Song G, Chen Y. Can rheumatoid arthritis ever cease to exist: a review of various therapeutic modalities to maintain drug-free remission? Am J Transl Res. 2017;9:3758–75.
Smolen JS, Emery P, Ferraccioli GF, et al. Certolizumab pegol in rheumatoid arthritis patients with low to moderate activity: the CERTAIN double-blind, randomised, placebo-controlled trial. Ann Rheum Dis. 2015;74:843–50.
Emery P, Burmester GR, Bykerk VP, et al. Re-treatment with abatacept plus methotrexate for disease flare after complete treatment withdrawal in patients with early rheumatoid arthritis: 2-year results from the AVERT study. RMD Open. 2019;5: e000840.
Takeuchi T, Genovese MC, Haraoui B, et al. Dose reduction of baricitinib in patients with rheumatoid arthritis achieving sustained disease control: results of a prospective study. Ann Rheum Dis. 2019;78:171–8.
Tanaka Y, Smolen JS, Jones H, Szumski A, Marshall L, Emery P. The effect of deep or sustained remission on maintenance of remission after dose reduction or withdrawal of etanercept in patients with rheumatoid arthritis. Arthritis Res Ther. 2019;21:164.
Schett G, Emery P, Tanaka Y, et al. Tapering biologic and conventional DMARD therapy in rheumatoid arthritis: current evidence and future directions. Ann Rheum Dis. 2016;75:1428–37.
Tanaka Y. Next stage of RA treatment: is TNF inhibitor-free remission a possible treatment goal? Ann Rheum Dis. 2013;72(Suppl 2):ii124–7.
Smolen JS, Szumski A, Koenig AS, Jones TV, Marshall L. Predictors of remission with etanercept-methotrexate induction therapy and loss of remission with etanercept maintenance, reduction, or withdrawal in moderately active rheumatoid arthritis: results of the PRESERVE trial. Arthritis Res Ther. 2018;20:8.
Zhang W, Bansback N, Sun H, Pedersen R, Kotak S, Anis AH. Impact of etanercept tapering on work productivity in patients with early rheumatoid arthritis: results from the PRIZE study. RMD Open. 2016;2: e000222.
Emery P, Hammoudeh M, FitzGerald O, et al. Sustained remission with etanercept tapering in early rheumatoid arthritis. N Engl J Med. 2014;371:1781–92.
Westhovens R, Robles M, Ximenes AC, et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis. 2009;68:1870–7.
Acknowledgements
We thank the patients who participated in the study.
Funding
Sponsorship for this study and Rapid Service Fee were funded by Bristol Myers Squibb.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Study conception and design: PE, YT, VPB, TWJH, COB, SB, BPS, MN, RW, K-HGH, RF; acquisition of data: PE, YT, GC, BPS, K-HGH, RF; analysis and interpretation of data: PE, YT, VPB, TWJH, GC, COB, SB, BPS, MN, SEC, KLL, JZ, RW, K-HGH, RF.
Medical Writing, Editorial, and Other Assistance
We thank all the investigators who participated in the study. The authors acknowledge the support of Robert Cohen and Sandra Overfield as protocol managers. Professional medical writing and editorial assistance was provided by Fiona Boswell, PhD, at Caudex, and was funded by Bristol Myers Squibb. Prof. Emery is Versus Arthritis Professor of Rheumatology and Director of Leeds Biomedical Research Centre (BRC). This paper presents independent research supported by the National Institute for Health Research (NIHR) Leeds BRC. The views expressed are those of the authors and not necessarily those of the NIHR or the UK Department of Health and Social Care.
Disclosures
PE has received honoraria from Bristol Myers Squibb, Celltrion, GlaxoSmithKline, Lilly, Novartis, and Samsung (all ≤ $10,000); research grants from Bristol Myers Squibb, Lilly, Novartis, and Samsung (> $10,000); consulting fees from Boehringer Ingelheim, Bristol Myers Squibb, Lilly, and Novartis; and received support for meetings/travel from Novartis. YT has received honoraria from AbbVie, Boehringer Ingelheim, Chugai, Eli Lilly, Gilead, Mitsubishi Tanabe (all > $10,000), AstraZeneca, Bristol Myers Squibb, Eisai (all ≤ $10,000), Daiichi-Sankyo, GlaxoSmithKline, and Pfizer; and research grants from AbbVie, Asahi Kasei, Chugai, Daiichi Sankyo, Eisai, Takeda (all > $10,000), Boehringer Ingelheim (≤ $10,000), and Corrona. VPB has received consulting fees from Amgen, Bristol Myers Squibb, Genzyme, Gilead, Janssen, Sanofi, and UCB (all ≤ $10,000); is supported by NIH/fNIH (Accelerated Medicines Program; funds to institution; grants 1UH2AR067691-01 and GRANT11652401) and Cedar Hill; has participated on a Data Safety Monitoring Board for KAI; and acted as Project Advisor for Pfizer. Additionally, VPB’s spouse is an employee of and has ownership interest in Brainstorm Therapeutics. TWJH (with the Department of Rheumatology of Leiden University Medical Center) has received research support/lecture fees/consultancy fees from Bristol Myers Squibb, Eli Lilly, Galapagos, Janssen, and Pfizer (all ≤ $10,000). The institution of GC has received grant/research support from Pfizer; and GC has received consulting fees and speaking fees and/or honoraria from AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Gema Biotech, Janssen, Pfizer, and Roche, and support for meeting attendance and/or travel from AbbVie and Pfizer, and participated on a Data Safety Monitoring Board or Advisory Board for AbbVie, Eli Lilly, Janssen, and Pfizer (all ≤ $10,000). COB has received grant/research support from Bristol Myers Squibb (> $10,000); has received royalties from Up-to-Date; has received consulting fees from AbbVie, Bristol Myers Squibb, Janssen, Pfizer, Regeneron, and Sanofi; has participated on a Data Safety Monitoring Board for Moderna (≤ $10,000); and is on the OMERACT executive committee (unpaid). SB, SEC, KLL, and JZ are employees of and shareholders in Bristol Myers Squibb. BPS was an employee of and shareholder in Bristol Myers Squibb at the time of analysis, and is currently an employee of and shareholder in Novo Nordisk. MN was an employee of and shareholder in Bristol Myers Squibb at the time of analysis. RW was an employee of Bristol Myers Squibb at the time of analysis and has stock options in Bristol Myers Squibb. KHGH was an employee of and shareholder in Bristol Myers Squibb at the time of analysis, and is currently an employee of and shareholder in Janssen R&D US. RF has received consulting fees from AbbVie, Bristol Myers Squibb, Pfizer (all > $10,000), Amgen, GlaxoSmithKline, Novartis (all ≤ $10,000), Cambrian, Teijin, and Vyne; has received honoraria from AbbVie and Pfizer; has participated on Data Safety Monitoring Boards for AbbVie, GlaxoSmithKline, and Pfizer; and is Editor-in-Chief of the journal, Rheumatology and Therapy.
Compliance with Ethics Guidelines
The study was conducted in accordance with the Declaration of Helsinki of 1964 and its later amendments, and the International Conference on Harmonization Good Clinical Practice guidelines. The protocol and patient-informed consent received institutional review board/independent ethics committee approval prior to study initiation. The study was governed by both a central institutional review board (IRB, the New England IRB) as well as local and university-based IRBs if required at individual sites. IRB approval numbers per site were not provided and are not available. All patients provided written informed consent prior to enrollment.
Data Availability
Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html. The datasets generated during and/or analyzed during the current study are available from Bristol Myers Squibb, as detailed in the data sharing policy, on reasonable request.
Prior Presentation
These data were previously presented at the 2018 ACR/ARHP Annual Scientific Meeting, Chicago (Emery P, et al., 19–24 October 2018, poster 563); 2019 ACR/ARHP Annual Scientific Meeting, Atlanta (Emery P, et al., 8–13 November 2019, poster L11); and EULAR 2020 eCongress, virtual (Emery P, et al., 3–6 June 2020, poster SAT0104).
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Emery, P., Tanaka, Y., Bykerk, V.P. et al. Sustained Remission and Outcomes with Abatacept plus Methotrexate Following Stepwise Dose De-escalation in Patients with Early Rheumatoid Arthritis. Rheumatol Ther 10, 707–727 (2023). https://doi.org/10.1007/s40744-022-00519-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-022-00519-9