Abstract
Introduction
Etanercept (ETN) has been shown to slow radiographic progression of rheumatoid arthritis (RA) and psoriatic arthritis (PsA) in clinical trials. This real-world, non-interventional study assessed radiographic progression in patients with RA or PsA treated with ETN for ≤ 36 months in outpatient care in Germany (NCT01623752).
Methods
Patients with RA or PsA attended ≤ 10 visits across two study phases (phase 1: seven visits, baseline to month 18; phase 2: three visits until month 36). Radiographs were taken at baseline (Rx1), months 12–18 (Rx2), and/or months 30–36 (Rx3). Historic radiographs (Rx0) taken 12–48 months pre-baseline were also evaluated (if available). The primary endpoint was the change in modified total Sharp score (mTSS). The erosion score (ES) and joint space narrowing score (JSN) were also evaluated.
Results
Overall, 1821 patients were enrolled (RA: n = 1378; PsA: n = 440). In patients with Rx1 and Rx2 (RA: n = 511; PsA: n = 167), the mean mTSS remained stable for both disease groups, and the annualized median change in mTSS was 0. In patients with Rx0, Rx1, and Rx2 (RA: n = 180; PsA: n = 47), annualized radiographic progression in mTSS, ES, and JSN was larger in the pre-ETN treatment phase than during ETN treatment in both disease groups. The percentage of patients with radiographic non-progression was higher during ETN treatment versus pre-ETN. Improvement in clinical disease activity and patient-reported outcomes was also observed.
Conclusions
This was the first real-world, non-interventional study to report systematically collected radiographic data in a large cohort of patients with RA or PsA under treatment with a biologic. In patients with available radiographic data, mean radiographic progression was lower and the proportion of patients without progression was greater during ETN treatment than in the pre-ETN period.
Plain Language Summary
Rheumatoid arthritis (RA) and psoriatic arthritis (PsA) are diseases in which inflammation can lead to damage in the joints. X-ray images can show whether the disease gets worse; this is called radiographic progression. Etanercept is a drug that acts on the body’s immune system and can reduce inflammation in the joints. In clinical studies, radiographic progression was slower in people with RA or PsA who received etanercept compared with people who received another drug called methotrexate.
In this study, we wanted to know how radiographic progression changes in people in Germany who receive etanercept as part of their routine treatment. A total of 1378 people with RA and 440 people with PsA received etanercept for up to 36 months. We observed little to no radiographic progression for most people during the study. Radiographic progression was worse before people started taking etanercept. More people had no radiographic progression while taking etanercept compared with before they started treatment. The proportion of people who responded to treatment with etanercept as measured by the number of painful joints increased throughout the study. Overall, people felt that their health improved after they started taking etanercept.
This was the first large study in which we investigated how radiographic progression changes when people with RA or PsA start taking etanercept as part of their routine treatment. We observed a slowing or halting of radiographic progression in most people and an improvement in their overall health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Reducing radiographic progression is an important goal in the treatment of rheumatoid arthritis (RA) and psoriatic arthritis (PsA) |
Etanercept (ETN) has been shown to slow radiographic progression in patients with RA and PsA in randomized prospective clinical trials |
PRERA was a real-world, non-interventional study reporting radiographic data collected over 36 months from a large cohort of patients with RA or PsA treated with ETN in routine clinical practice |
Radiographic progression slowed or halted in the majority of patients with RA and PsA during ETN treatment compared with the pre-ETN period |
Introduction
Rheumatoid arthritis (RA) is associated with rapid functional loss [1] and reduced life expectancy [2]. Disease remission and prevention of structural damage as documented by radiographic non-progression are major goals of RA treatment [3]. In several randomized, double-blind clinical trials (COMET [4], TEMPO [5, 6]), the tumor necrosis factor alpha (TNF-α) inhibitor etanercept (ETN) in combination with methotrexate (MTX) has been shown to reduce disease activity, slow radiographic progression, and improve function. It is unclear, however, what proportion of patients with RA achieve remission and radiographic non-progression under the conditions of routine rheumatologic care.
Psoriatic arthritis (PsA) is another chronic inflammatory disorder that causes joint pain and disability and leads to joint destruction, which can be measured and quantified radiologically [7]. Several studies have demonstrated the efficacy of ETN in reducing the signs and symptoms of PsA and psoriasis, as well as in inhibiting radiological progression [7, 8].
There is evidence to suggest that radiographic progression may continue in patients receiving conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), even when clinical remission is achieved—the so-called silent progressors [9,10,11]. In contrast, radiographic progression was found to be stopped in patients being treated with TNF blocking agents, even if they were not in clinical remission [12]. The question of whether radiographic progression can be halted in patients treated with TNF blockers and its correlation to clinical response in routine clinical practice are therefore of particular relevance.
Although clinical trials are paramount for investigating the efficacy and safety of treatments in a controlled manner, real-world studies offer insight into outcomes of patients treated in daily practice who would not be selected for clinical trials due to strict inclusion and exclusion criteria [13, 14]. This non-interventional study assessed radiographic progression and disease activity in patients with RA or PsA treated with ETN for up to 36 months as part of routine outpatient care in German hospitals and private practices.
Methods
Study Design
The PRospective Evaluation of The RAdiographic Efficacy of Etanercept in Patients With Rheumatoid Arthritis or Psoriatic Arthritis (PRERA) trial was a non-interventional, prospective, multicenter study (NCT01623752). Up to 10 visits took place during the two phases of the study: phase 1 comprised visit 1 at baseline and visits 2–7 every 3 months until month 18. Enrollment in phase 2 was optional and comprised visits 8–10 every 6 months until month 36. Mandatory radiographs of hands and feet were taken at baseline (± 3 months with respect to the start of ETN treatment), and optional radiographs were taken at months 12–18 (towards the end of phase 1) and/or at months 30–36 (towards the end of phase 2); if available, historic radiographs taken 12–48 months prior to study start were also collected at baseline and assessed (Fig. 1).
Inclusion and Exclusion Criteria
Adult patients with a diagnosis of RA or PsA (confirmed by their rheumatologists) who were naïve to treatment with ETN, eligible for treatment with ETN according to the summary of product characteristics (SmPC), and for whom there were plain radiographs of hands and feet within 3 months prior to or after the initiation of treatment with ETN were included in the study. Patients who previously received ETN or any investigational drug within 3 months of study inclusion, or who were ineligible for ETN treatment according to the SmPC, were excluded from the study.
Effectiveness and Safety Endpoints
The primary effectiveness endpoint was the change (absolute and annualized) in van der Heijde modified total Sharp score for RA patients (mTSSRA) [15] or van der Heijde modified total Sharp score adapted for PsA patients (mTSSPsA) [16] as assessed by two blinded assessors (SW, RR). Radiographic non-progression was defined as a change in mTSSRA/PsA of < 0.5.
Secondary efficacy parameters included the change in disease activity score in 28 joints (DAS28) [17]. The DAS28 included information from the Patient’s Global Assessment, the number of swollen and tender joints as determined by the physician, and the laboratory values for erythrocyte sedimentation rate and/or C-reactive protein.
Additional secondary efficacy endpoints included the erosion score (ES) and joint space narrowing (JSN) score, separately. Patient-reported outcomes included the pain visual analog scale (VAS), the Hannover Functional Ability Questionnaire (FFbH) [18], comprising 18 questions on functional ability in activities of daily living, and the Euro Quality of Life–5 Dimensions (EQ-5D) [19], comprising health-related quality of life questions in which patients were asked if they have “extreme problems,” “some problems,” or “no problems” within five sub-categories: “mobility,” “self-care,” “usual activities,” “pain/discomfort,” and “anxiety/depression.”
Safety endpoints included incidence of adverse events (AE) and serious adverse events (SAE). All AEs reported during the course of the study were collected and coded using version 17 of the Medical Dictionary for Regulatory Activities (MedDRA).
Statistical Analyses
Data were reported descriptively and presented as sample size, mean, or median values for continuous variables as appropriate, and as frequencies for categorical variables. A paired t-test was performed for the comparison of normalized radiographic progression. Missing data were generally not imputed; however, for mTSS calculations, missing scores for individual joints were imputed using the mean scores of the remaining joints for that patient, but only X-ray assessments that were performed were included in the analysis.
The relationship of risk factors and baseline disease characteristics (disease duration, concomitant or previous medication, including csDMARD and biologic DMARD [bDMARD] use, anti-citrullinated protein antibodies, rheumatoid factor, baseline DAS28, and radiographic progression) were evaluated using either a linear regression model or an analysis of (co)variance model.
Ethical Conduct of the Study
The study was conducted in accordance with the Declaration of Helsinki and local legal and regulatory requirements. Written informed consent was obtained prior to patients entering the study (before the initiation of study-protocol-specified procedures) by the treating physician. The final observational plan, any amendments, and informed consent documentation were reviewed and approved by the Ethics Committee of the Berlin Chamber of Physicians, Germany.
Patient and Public Involvement Statement
The patients and the public were not involved in the design, conduct, reporting, or dissemination plans of the data obtained from the PRERA study.
Results
Patient Disposition and Baseline Characteristics
Mean age among patients with RA and PsA was 58 and 52 years, respectively (Table 1), and the proportion of patients ≥ 65 years of age was 29% and 11%, respectively. Mean disease duration was 8.0 years in the RA group and 6.4 years in the PsA group. The vast majority of patients received systemic therapy for RA or PsA prior to starting ETN treatment (RA: 97%; PsA: 93%), with MTX being the most common treatment used in each group (RA: 88%; PsA: 87%).
Around half of the patients completed phase 1, and 20.5% and 25.9% of patients with RA and PsA, respectively, entered the optional phase 2. Supplementary Fig. 1 shows the number of patients with data recorded at each visit.
Overall, 36% of patients with RA and 39% of patients with PsA discontinued treatment with ETN before the end of the study. The main reasons for treatment discontinuation were lack of efficacy (RA: 20%; PsA: 22%) and AEs (RA: 10%; PsA: 10%) (Fig. 2). Patients who discontinued ETN treatment had similar baseline characteristics compared with those who continued ETN treatment (Supplementary Table 1).
Effectiveness
Mean mTSS remained stable across all subpopulations in both RA and PsA, and was consistently highest in patients who completed both phase 1 and 2 (Fig. 3A, B). Median change in mTSS was 0 during both phase 1 and phase 2 in both disease groups.
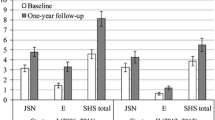
In both disease groups, the times between the baseline X-ray and the historic, first, and second follow-up X-rays varied considerably between individual patients. Therefore, annualized radiographic progression was calculated to facilitate comparison. Annualized radiographic progression (mTSS, ES, and JSN) for patients with RA and PsA is shown in Fig. 4A, B, respectively. In general, mean annualized progression in mTSS, ES, and JSN before the start of ETN treatment was significantly higher than that during ETN treatment in phase 1 in patients with historic, baseline, and first follow-up X-rays in both disease groups. Cumulative annualized progression (mTSS) for patients with RA and PsA with historic, baseline, and first follow-up X-rays was lower during ETN treatment in phase 1 than before the start of ETN treatment (Fig. 4C, D). No statistical difference in annualized progression was seen between patients with RA receiving ETN monotherapy and those receiving ETN + MTX combination therapy (data on file).
Mean annualized radiographic progression in mTSS, ES, and JSN before and during ETN treatment in patients with A RA and B PsA with historic, baseline, and first follow-up X-rays, and cumulative probability plots of progression in patients with C RA and D PsA with historic, baseline, and first follow-up X-rays
In patients with historic, baseline, and first follow-up X-rays, the percentage of patients with radiographic non-progression was higher during ETN treatment in phase 1 compared with pre-ETN treatment for both the RA and PsA groups (Fig. 5A, B).
DAS28 Response
Mean DAS28 decreased from baseline to the end of phase 2 in all patients and in all subpopulations for both RA and PsA groups (Fig. 6A, B). The proportion of patients in DAS28 remission (DAS28 < 2.6) increased steadily from baseline in both the RA (6–62%) and the PsA (10–65%) groups (Fig. 6C, D).
Patient-Reported Outcomes
Mean FFbH scores were 65% for patients with RA and 69% for patients with PsA at visit 1 (baseline) and increased to 73% and 75%, respectively, at month 18 (visit 7, end of phase 1) (Fig. 7A, B). Scores remained stable during phase 2. In the RA group, 27% of patients reported functional remission (FFbH scores of > 83%) at baseline (data on file), which increased to 45% by the end of phase 1. This proportion remained largely stable until the end of phase 2 (42%). In the PsA group, 30% of patients reported functional remission at baseline, which increased to 48% by the end of phase 1. This proportion also remained stable until the end of phase 2 (48%).
In general, the proportion of patients reporting “no problems” increased across all EQ-5D dimensions until the end of phase 1 in both disease groups. This remained stable until the end of phase 2 (Fig. 7C, D). The number of patients with “extreme problems” in “pain/discomfort” declined from baseline until visit 7/month 18 (end of phase 1) in both disease groups (RA: 30–8%; PsA: 30–9%). Proportions remained largely stable until visit 10/month 36 (end of phase 2; RA: 7%; PsA: 11%). The greatest change was seen in the domain of “usual activities:” at visit 1, 26% of patients with RA and 28% of patients with PsA reported “no problems” with “usual activities,” but, by visit 2, 39% of patients with RA and 41% of patients with PsA had “no problems” with “usual activities.” Frequencies further increased until visit 7 (end of phase 1; RA: 51%; PsA: 54%) and remained stable until visit 10 (end of phase 2; RA: 53%; PsA: 58%).
Mean pain VAS at baseline was 56 mm and 58 mm for patients with RA and PsA, respectively, and decreased until the end of phase 1 to 32 mm in both disease groups (Fig. 7E, F). Pain VAS scores remained stable during phase 2.
Safety
A total of 48% and 43% of patients experienced AEs in the RA and PsA groups, respectively (Supplementary Table 2). General disorders and administration-site conditions (RA: 16%; PsA: 16%) and infections and infestations (RA: 16%; PsA 14%) were most common. Twelve percent of patients in the RA and PsA groups, respectively, experienced SAEs (Supplementary Table 3). Overall, 25.0% and 22.7% of patients, respectively, experienced treatment-related AEs, and 2.6% and 2.5%, respectively, experienced serious treatment-related AEs, none of which resulted in death. Of the six deaths that occurred during the study, five were considered unrelated to the study drug by the investigator (causes of death: endocarditis, sepsis, malignant lung neoplasm, bronchial carcinoma, and myocardial infarction). For one death (cause of death unknown), the relationship to ETN was not determined.
Discussion
This was the first real-world, prospective, non-interventional study evaluating radiographic progression before and during treatment with ETN in adult patients with RA and PsA. Treatment of patients with RA or PsA with ETN in routine clinical practice in Germany resulted in an increasing proportion of patients with radiographic non-progression and with disease remission over a period of up to 36 months. In patients who had a historic X-ray available, annualized radiographic progression during the first 18 months (phase 1) of the study was significantly lower than during the pre-ETN treatment period in both RA and PsA. P-values for non-progression as measured by ES were highly significant (p < 0.005), indicating a slowing down in joint erosion during ETN treatment.
These findings are consistent with those from other observational studies which included historic X-rays. While one study from a Swiss longitudinal cohort from the Swiss Clinical Quality Management in Rheumatoid Arthritis (SCQM-RA) registry of patients with RA demonstrated a decrease in radiographic progression once treatment was started, treatments included both chemical as well as biologic DMARDs [20]. However, in another study of patients with RA from the Danish Biologics Registry (DANBIO) who were treated with TNF-α inhibitors, significantly reduced radiographic progression was observed during treatment compared with the pre-treatment period: the median radiographic progression rate decreased from 0.7 total Sharp score units/year to 0 units/year (p < 0.0001) [21]. A British study of patients with PsA treated with TNF-α inhibitors showed that the median modified Sharp/van der Heijde score decreased from 8.5 to 2.1 per year [22].
In addition, several observational studies have shown that radiographic progression can slow down or halt during treatment with ETN and other bDMARDs compared with baseline scores. An earlier study of patients from the SCQM-RA registry showed that radiographic progression halted during treatment with rituximab (an anti-CD20 bDMARD) as well as anti-TNF-α inhibitors [23], as measured using the Ratingen erosion score [24]. In a Dutch study of patients with RA treated with the TNF-α inhibitor adalimumab, 53% and 42% of patients had no radiographic progression (as measured by the original Sharp/van der Heijde score[25]) after 1 and 2 years, respectively [26], compared with 61% after 18 months in this study.
Differences in the methodology used to rate radiographic progression, patient populations, and treatment regimens can make it difficult to compare results from different observational trials. We would welcome the publishing of more data from observational studies, particularly of patients with PsA.
While data from observational trials cannot be compared directly to results from randomized clinical trials, data from the latter can inform real-life treatment practices. Overall, results from this non-interventional trial were similar to those observed in randomized clinical trials with TNF-α inhibitors and other bDMARDs and targeted synthetic DMARDs for the treatment of both RA and PsA [10, 27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49]. In patients with RA, treatment with ETN, infliximab, adalimumab, golimumab or certolizumab, abatacept, tocilizumab [40,41,42], sarilumab, rituximab, tofacitinib, baricitinib, upadacitinib, or filgotinib reduced radiographic progression in addition to improving clinical outcomes compared to MTX. Similarly, ETN, infliximab, adalimumab, certolizumab, golimumab, and the JAK inhibitors tofacitinib and upadacitinib were proven to be superior to MTX or other csDMARDs in terms of radiographic progression and clinical outcomes in controlled trials in patients with PsA.
There is some evidence that combined treatment with ETN and MTX may be clinically more efficacious than treatment for RA with ETN alone [5, 6, 50]. In a Canadian open-label study of patients with RA, progression in joint space narrowing and ES was consistently higher after 12 and 24 months for patients receiving ETN monotherapy compared with combination therapy [51]. In contrast, an analysis of factors that influenced radiographic progression in patients included in the DANBIO registry did not identify concomitant MTX therapy as being related to less progression [52]. In this study, we did not observe a statistically significant difference in annualized progression between patients with RA receiving ETN monotherapy and those receiving ETN + MTX combination therapy. This was consistent across subgroups and indicates that combination therapy does not necessarily provide better outcomes than monotherapy. As this was a non-interventional study, MTX therapy was prescribed at the discretion of the investigator, who may have opted for combination therapy in patients with a more complicated or refractory course of disease.
We also observed a steady increase in the percentage of patients with DAS28 remission. In addition, we observed improvements in patient-reported outcomes, including increased quality of life (QoL) and functional status and reduced pain. Reducing radiographic progression and improving functional status can improve patients’ QoL as well as their ability to participate in everyday activities. Both should be considered when reviewing treatment outcomes.
Limitations of this study included the relatively low number of patients completing both phase 1 and 2; however, this was addressed by analyzing subpopulations within each disease population. The PRERA study initially comprised only phase 1; however, the enrollment period was extended to meet recruitment targets and phase 2 was added later. Study centers had to sign a separate contract and patients needed to sign an additional consent form to enter phase 2, which might explain the low number of patients entering phase 2. Long-term follow-up of patients in a non-investigational study (NIS) setting can be difficult, as demonstrated by the relatively low number of patients completing both phases. A further limitation of this NIS was that only baseline X-ray images were mandatory (and a requirement for study participation) while all other X-rays were voluntary, thereby explaining the lower number of follow-up X-rays. Conclusions regarding radiographic progression are therefore only valid for a smaller subgroup with available data. Missing data may have led to bias, with overestimation of results. Selection criteria for this study were relatively unconstricted, reflecting the variability of the “real world.” However, standardized measurements were taken to ensure the quality and integrity of the data. Regarding the inclusion criteria, the RA or PsA diagnosis was confirmed by the patients’ rheumatologists; however, almost 10% of the patients with PsA were RF positive, even though classification criteria require patients with PsA to be RF negative. As the frequency of RF-positive individuals in the general population increases with age reaching a proportion of around 10% at the age of 60, this finding may not indicate diagnostic mistakes by physicians; it may hint that our data better represent the whole spectrum of patients with PsA in the real world than interventional trials that impose strict classification criteria.
Conclusions
Overall, this study suggests that patients with RA and PsA treated with ETN in a real-world setting experience a slowing of radiographic progression during treatment compared with the pre-treatment period. In patients with available radiographic data, a large proportion of the patients were treated with ETN for up to 3 years without radiographic progression. The treatment with ETN was well tolerated and no new safety signals were reported. Furthermore, disease activity was reduced during ETN treatment, and increased functional remission and improvements in patients’ QoL were observed.
Change history
20 September 2023
A Correction to this paper has been published: https://doi.org/10.1007/s40744-023-00579-5
29 November 2023
A Correction to this paper has been published: https://doi.org/10.1007/s40744-023-00616-3
References
Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6:15.
Listing J, Kekow J, Manger B, et al. Mortality in rheumatoid arthritis: the impact of disease activity, treatment with glucocorticoids, TNFα inhibitors and rituximab. Ann Rheum Dis. 2015;74:415–21.
Reginster JY. The prevalence and burden of arthritis. Rheumatology (Oxford). 2002;41(Supp 1):3–6.
Emery P, Breedveld FC, Hall S, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet. 2008;372:375–82.
Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675–81.
van der Heijde D, Klareskog L, Rodriguez-Valverde V, et al. Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis: two-year clinical and radiographic results from the TEMPO study, a double-blind, randomized trial. Arthritis Rheum. 2006;54:1063–74.
Mease PJ, Kivitz AJ, Burch FX, et al. Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum. 2004;50:2264–72.
Mease PJ, Goffe BS, Metz J, VanderStoep A, Finck B, Burge DJ. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet. 2000;356:385–90.
Joint Damage Progression in Patients with Rheumatoid Arthritis in Clinical Remission. Do Biologics Perform Better Than Synthetic Antirheumatic Drugs? Ciubotariu E, Gabay C, Finckh A. J Rheumatol 2014;41:1576–1582.
Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum. 2005;52:3381–90.
Brown AK, Conaghan PG, Karim Z, et al. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum. 2008;58:2958–67.
Juhasz P, Mester A, Biro AJ, Hejj G, Poor G. Clinical and radiological dissociation of anti-TNF plus methotrexate treatment in early rheumatoid arthritis in routine care: results from the ABRAB study. BMC Musculoskelet Disord. 2014;15:251.
Zink A, Strangfeld A, Schneider M, et al. Effectiveness of tumor necrosis factor inhibitors in rheumatoid arthritis in an observational cohort study: comparison of patients according to their eligibility for major randomized clinical trials. Arthritis Rheum. 2006;54:3399–407.
Sokka T, Pincus T. Eligibility of patients in routine care for major clinical trials of anti-tumor necrosis factor alpha agents in rheumatoid arthritis. Arthritis Rheum. 2003;48:313–8.
van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol. 2000;27:261–3.
van der Heijde D, Sharp J, Wassenberg S, Gladman DD. Psoriatic arthritis imaging: a review of scoring methods. Ann Rheum Dis. 2005;64(Suppl 2):ii61–4.
Prevoo ML, van ’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8.
Raspe HH, Kindel P, Vesterling K. Kohlmann T. Change in functional capacity and pain intensity of 81 cP patients treated with Azulfidine RA or aurothioglucose. Preliminary statistical assessment of the German multicenter study of the treatment of chronic polyarthritis with Azulfidine RA. Z Rheumatol. 1987;46:71–5.
Kind P. The EuroQoL instrument: an index of health—related quality of life. In: Spilker B, editor. Quality of life and pharmacoeconomics in clinical trials. 2nd ed. Philadelphia (PA): Lippincott–Raven Publishers; 1996. p. 191–201.
Mueller RB, Kaegi T, Finckh A, et al. Is radiographic progression of late-onset rheumatoid arthritis different from young-onset rheumatoid arthritis? Results from the Swiss prospective observational cohort. Rheumatology (Oxford). 2014;53:671–7.
Ornbjerg LM, Ostergaard M, Boyesen P, et al. Impact of tumour necrosis factor inhibitor treatment on radiographic progression in rheumatoid arthritis patients in clinical practice: results from the nationwide Danish DANBIO registry. Ann Rheum Dis. 2013;72:57–63.
Allard A, Antony A, Shaddick G, et al. Trajectory of radiographic change over a decade: the effect of transition from conventional synthetic disease-modifying antirheumatic drugs to anti-tumour necrosis factor in patients with psoriatic arthritis. Rheumatology (Oxford). 2019;58:269–73.
Finckh A, Moller B, Dudler J, et al. Evolution of radiographic joint damage in rituximab-treated versus TNF-treated rheumatoid arthritis cases with inadequate response to TNF antagonists. Ann Rheum Dis. 2012;71:1680–5.
Rau R, Wassenberg S, Herborn G, Stucki G, Gebler A. A new method of scoring radiographic change in rheumatoid arthritis. J Rheumatol. 1998;25:2094–107.
van der Heijde DM, van Riel PL, Nuver Zwart IH, Gribnau FW, van de Putte LB. Effects of hydroxychloroquine and sulphasalazine on progression of joint damage in rheumatoid arthritis. Lancet. 1989;15:1036–8.
den Broeder AA, Joosten LA, Saxne T, et al. Long term anti-tumour necrosis factor alpha monotherapy in rheumatoid arthritis: effect on radiological course and prognostic value of markers of cartilage turnover and endothelial activation. Ann Rheum Dis. 2002;61:311–8.
Mease PJ, Ory P, Sharp JT, et al. Adalimumab for long-term treatment of psoriatic arthritis: 2-year data from the Adalimumab Effectiveness in Psoriatic Arthritis Trial (ADEPT). Ann Rheum Dis. 2009;68:702–9.
McInnes IB, Kato K, Magrey M, et al. Upadacitinib in patients with psoriatic arthritis and an inadequate response to non-biological therapy: 56-week data from the phase 3 SELECT-PsA 1 study. RMD Open. 2021;7.
Kavanaugh A, Antoni CE, Gladman D, et al. The Infliximab Multinational Psoriatic Arthritis Controlled Trial (IMPACT): results of radiographic analyses after 1 year. Ann Rheum Dis. 2006;65:1038–43.
van der Heijde D, Kavanaugh A, Gladman DD, et al. Infliximab inhibits progression of radiographic damage in patients with active psoriatic arthritis through one year of treatment: results from the Induction and Maintenance Psoriatic Arthritis Clinical Trial 2. Arthritis Rheum. 2007;56:2698–707.
Mease PJ, Kivitz AJ, Burch FX, et al. Continued inhibition of radiographic progression in patients with psoriatic arthritis following 2 years of treatment with etanercept. J Rheumatol. 2006;33:712–21.
Kavanaugh A, McInnes IB, Mease P, et al. Clinical efficacy, radiographic and safety findings through 5 years of subcutaneous golimumab treatment in patients with active psoriatic arthritis: results from a long-term extension of a randomised, placebo-controlled trial (the GO-REVEAL study). Ann Rheum Dis. 2014;73:1689–94.
Mease P, Deodhar A, Fleischmann R, et al. Effect of certolizumab pegol over 96 weeks in patients with psoriatic arthritis with and without prior antitumour necrosis factor exposure. RMD Open. 2015;1: e000119.
van der Heijde D, Gladman DD, Kavanaugh A, Mease PJ. Assessing structural damage progression in psoriatic arthritis and its role as an outcome in research. Arthritis Res Ther. 2020;22:18.
Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37.
Markusse IM, Akdemir G, Dirven L, et al. Long-term outcomes of patients with recent-onset rheumatoid arthritis after 10 years of tight controlled treatment: a randomized trial. Ann Intern Med. 2016;164:523–31.
Smolen J, Landewe RB, Mease P, et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann Rheum Dis. 2009; 68:797–804.
Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50:1400–11.
Weinblatt ME, Schiff M, Valente R, et al. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: findings of a phase IIIb, multinational, prospective, randomized study. Arthritis Rheum. 2013;65:28–38.
Dougados M, Kissel K, Sheeran T, et al. Adding tocilizumab or switching to tocilizumab monotherapy in methotrexate inadequate responders: 24-week symptomatic and structural results of a 2-year randomised controlled strategy trial in rheumatoid arthritis (ACT-RAY). Ann Rheum Dis. 2013;72:43–50.
Kremer JM, Blanco R, Brzosko M, et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum. 2011;63:609–21.
Nishimoto N, Hashimoto J, Miyasaka N, et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis. 2007;66:1162–7.
Genovese MC, Fleischmann R, Kivitz AJ, et al. Sarilumab plus methotrexate in patients with active rheumatoid arthritis and inadequate response to methotrexate: results of a phase III study. Arthritis Rheumatol. 2015;67:1424–37.
van der Heijde D, Tanaka Y, Fleischmann R, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 2013;65:559–70.
Dougados M, van der Heijde D, Chen YC, et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA-BUILD study. Ann Rheum Dis. 2017;76:88–95.
Taylor PC, Keystone EC, van der Heijde D, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med. 2017;376:652–62.
Fleischmann R, Pangan AL, Song IH, et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind, randomized controlled trial. Arthritis Rheumatol. 2019;71:1788–800.
Combe B, Kivitz A, Tanaka Y, et al. Filgotinib versus placebo or adalimumab in patients with rheumatoid arthritis and inadequate response to methotrexate: a phase III randomised clinical trial. Ann Rheum Dis. 2021;80:848–58.
Mease P, Hall S, FitzGerald O, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med. 2017;377:1537–50.
van Vollenhoven RF, Ernestam S, Harju A, Bratt J, Klareskog L. Etanercept versus etanercept plus methotrexate: a registry-based study suggesting that the combination is clinically more efficacious. Arthritis Res Ther. 2003;5:R347–51.
Keystone EC, Pope JE, Thorne JC, et al. Two-year radiographic and clinical outcomes from the Canadian Methotrexate and Etanercept Outcome study in patients with rheumatoid arthritis. Rheumatology (Oxford). 2016;55:327–34.
Ørnbjerg LM, Østergaard M, Bøyesen P, et al. Which factors influence radiographic progression during treatment with tumor necrosis factor inhibitors in clinical practice? Results from 930 patients with rheumatoid arthritis in the nationwide Danish DANBIO registry. J Rheumatol. 2014;41:2352–60.
Keystone E, Heijde D, Mason D Jr, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 2008;58:3319–29.
Keystone E, Genovese MC, Klareskog L, et al. Golimumab in patients with active rheumatoid arthritis despite methotrexate therapy: 52-week results of the GO-FORWARD study. Ann Rheum Dis. 2010;69:1129–35.
Declarations
The authors would like to thank Dr Thomas Fisher from Winicker Norimed GmbH for his advice on statistical methods.
Funding
The PRERA study, medical writing assistance, and the Rapid Service Fee were funded by Pfizer.
Medical Writing Assistance
Medical writing support was provided by Andrea Schauenburg, PhD, of Engage Scientific Solutions and was funded by Pfizer.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors' Contributions
All authors were involved in the writing and reviewing process and have read and approved this manuscript. Additional contributions to this body of work were as follows: Siegfried Wassenberg and Rolf Rau: conception/design of the study, acquisition and interpretation of data. Thilo Klopsch: acquisition of data. Anja Plenkse, Jürgen Jobst, Thomas Meng, and Peter-Andreas Löschmann: conception/design of the study and interpretation of data. Pascal Klaus: interpretation of data.
List of Investigators
Please see full list of investigators in Supplementary Table 4.
Prior Presentation
Data included in this manuscript have partially been presented at congresses:
-
Wassenberg S, Rau R, Klopsch T, Plenske A, Jobst J, Klaus P, Meng T, Löschmann P-A. Effektivität von Etanercept auf die radiologische Progression bei erwachsenen Patienten mit Rheumatoider Arthritis oder Psoriasis-Arthritis (PRERA). Poster presented at the Congress of the German Society for Rheumatology (DGRh) 2019
-
Wassenberg S, Rau R, Klopsch T, Plenske A, Jobst J, Klaus P, Meng T, Löschmann P-A. Effectiveness of etanercept on radiographic progression in adult patients with rheumatoid arthritis or psoriatic arthritis: final results from a German non-interventional, prospective, multi-center study (PRERA). Poster presented at the Annual Meeting of the American College of Rheumatology 2019.
Disclosures
SW: Pfizer Pharma GmbH (speaker/honoraria), Pfizer Pharma GmbH, AbbVie, Amgen, BMS, Gilead, Lilly, Sanofi, UCB (consulting fees), Gilead, Mylan (support for meeting attendance/travel), Nichi-Iko (data safety monitoring or advisory board); TK: Amgen, Johnson & Johnson, Morphosys, Evotec, Novo Nordisk, GlaxoSmithKline, Fresenius; AP: Pfizer Pharma GmbH (employee); JJ: Pfizer Pharma GmbH (employee); PK: Pfizer Pharma GmbH (employee); TM: Pfizer Pharma GmbH (employee); P-AL: Pfizer Pharma GmbH (employee at the time that the study was conducted and during the initial part of manuscript development), gesundheitspolitik.de (chief correspondent and head at the time of submission; RR: Pfizer Pharma GmbH (grant/research support).
Compliance with Ethics Guidelines
The final protocol and subject information and informed consent documentation were reviewed and approved by the Ethics Committee of the Berlin Chamber of Physicians, Germany. This study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients by the treating physician or a designated person prior to patients entering the study.
Data Availability
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Author information
Authors and Affiliations
Corresponding author
Additional information
The affiliation shown for Peter-Andreas Löschmann was his affiliation when the study was conducted.
The original online version of this article was revised: To correct the keywords.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wassenberg, S., Rau, R., Klopsch, T. et al. Etanercept is Effective and Halts Radiographic Progression in Rheumatoid Arthritis and Psoriatic Arthritis: Final Results from a German Non-interventional Study (PRERA). Rheumatol Ther 10, 117–133 (2023). https://doi.org/10.1007/s40744-022-00491-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-022-00491-4