Abstract
Background
For rheumatoid arthritis (RA), the treat-to-target concept suggests attaining remission or at least low disease activity (LDA) after 12 weeks.
Objectives
This German, prospective, multicenter, non-interventional study aimed to determine the proportion of patients with RA who achieved their treat-to-target aim after 12 and 24 weeks of etanercept (ETN) treatment in a real-life setting, as opposed to patients achieving their therapeutic target at a later timepoint (week 36 or 52).
Methods
A total of 824 adults with a confirmed diagnosis of RA without prior ETN treatment were included. Remission and LDA were defined as DAS28 < 2.6 and DAS28 ≤ 3.2, respectively.
Results
The proportion of patients achieving remission was 24% at week 12 and 31% at week 24. The proportion of patients achieving LDA was 39% at week 12 and 45% at week 24. The proportion of patients achieving remission or LDA further increased beyond week 24 up to week 52. Improvement in pain and reduction in concomitant glucocorticoid treatment were observed. Improvements in patient-reported outcomes were also seen in patients who did not reach remission or LDA. No new safety signals were detected.
Conclusions
A considerable proportion of patients with RA attained the target of remission or LDA after 12 weeks of ETN treatment. Even beyond that timepoint, the proportion of patients achieving treatment targets continued to increase up to week 52.
Trial Registration
ClinicalTrials.gov Identifier: NCT02486302.
Plain Language Summary
Physicians measure response to treatment of rheumatoid arthritis using a disease activity score (DAS28). People with a DAS28 of less than 2.6 have very few to no symptoms (also called remission). People with a DAS28 of 3.2 or less, called low disease activity, may experience mild symptoms. When people do not respond to treatment after 12 weeks, it is usually recommended to prescribe a different treatment. Researchers do not know how many people who do not respond after 12 weeks would respond if treatment were continued. A total of 824 German people with rheumatoid arthritis who received a drug called etanercept for up to 52 weeks took part in this study. Researchers wanted to know how many people had remission or low disease activity after 12 weeks and 24 weeks of treatment.
After 12 weeks, 24 in 100 people had remission; this increased to 31 in 100 people after 24 weeks. Thirty-nine in 100 people had low disease activity after 12 weeks; this increased to 45 in 100 people after 24 weeks. The number of people with remission or low disease activity increased with longer treatment (up to 52 weeks). People needed less additional treatment with a type of drug called glucocorticoids. The people in this study experienced side effects that were similar to those reported by people who took etanercept in previous studies.
The researchers concluded that a considerable proportion of people responded to treatment with etanercept after 12 weeks. This proportion increased when treatment was continued for longer than 12 weeks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The treat-to-target concept for rheumatoid arthritis (RA) requires patients to attain remission or at least low disease activity (LDA) after 12 weeks of treatment. |
What did this study ask? |
The objective of this study was to evaluate the proportion of patients who benefit from treatment with the tumor necrosis factor alpha inhibitor etanercept (ETN) beyond 12 weeks. |
What were the study outcomes/conclusions? |
In this non-interventional study, 24% and 39% of patients with RA treated with ETN as part of routine care in Germany achieved remission and LDA, respectively, at 12 weeks; improvements in patient-reported outcomes and reductions in concomitant glucocorticoid treatment were also observed. |
What has been learned from the study? |
Rates of remission and LDA increased up to week 52 of treatment, suggesting that some patients may benefit from extending treatment with ETN beyond 12 weeks. |
Introduction
For patients with rheumatoid arthritis (RA), the treat-to-target concept suggests a goal of remission within the first 3 months of treatment, after which time treatment should be adjusted if the target is not reached [1,2,3]. In patients who are unlikely to achieve remission, e.g., those with long-standing disease who failed previous therapies, low disease activity (LDA) can be an acceptable alternative target to remission. In patients showing some improvement in disease activity but who have not yet achieved their target, treatment may be continued with a goal of reaching the target after 6 months [1, 3, 4]. Pain and concomitant glucocorticoid use can also affect patients’ quality of life and should be considered in setting treatment targets [3, 5].
In the COMET study, patients with early RA receiving the tumor necrosis factor alpha (TNF-α) inhibitor etanercept (ETN) were more likely to attain clinical remission compared with patients receiving methotrexate (MTX) alone. After 12 weeks of treatment, 36% of patients treated with ETN attained disease activity score in 28 joints (DAS28) remission, while 50% and 57% attained this target at week 24 and week 52, respectively [6]. These findings suggest that continuing ETN treatment beyond 12 weeks may be worthwhile, even if remission or LDA criteria have not yet been reached.
While clinical trials like COMET are paramount in investigating the efficacy and safety of treatments in a controlled manner, real-world studies and registry data analyses (e.g., RABBIT [7]) offer insight into patient outcomes in routine practice [8, 9]. The non-interventional ADEQUATE study evaluated the effectiveness of ETN over a period of 12 months in the routine treatment of patients with rheumatic diseases and plaque psoriasis, with particular attention to patients who attained their desired treatment goal after more than 12 weeks of treatment. To this end, the aim of this study was to evaluate the proportion of patients who benefited from continued ETN treatment beyond 12 weeks, even among those who did not formally attain their desired treatment target at week 12. We describe results from the cohort of patients with RA from ADEQUATE.
Methods
Study Design
ADEQUATE was a prospective, non-interventional study in patients with RA, axial spondyloarthritis, psoriatic arthritis, or plaque psoriasis enrolled at multiple centers in Germany. We report data from the cohort of patients with RA only. ADEQUATE was designed to evaluate the effectiveness of ETN after 12, 24, 36, and 52 weeks of routine treatment (Fig. 1a). Initial treatment decisions were made prior to study inclusion. The study physicians were encouraged to include all eligible patients consecutively.
a Study design and b patient disposition. aPossible reasons: consent withdrawn; center excluded; deviation between therapy start and baseline visit; never took study medication; excluded due to lack of post-baseline values. bAll treated patients with ≥ 1 post-baseline value including documentation of an AE. cTS patients who adhered to the protocol without any major protocol deviation. dNon-completer with treatment discontinuation, multiple reasons possible. eNon-completer without treatment discontinuation. fCompleted week 52 and treatment not discontinued. AE adverse event, ETN etanercept, PPS per-protocol set, PRO patient-reported outcome, RA rheumatoid arthritis, SmPC summary of product characteristics for ETN, TS treated set
Data were collected during five regular study visits (baseline and every 3 months thereafter) over the course of the 12-month observation period (Fig. 1a and Supplementary Table S1). Written informed consent was obtained by the treating physician or designated study site personnel before study entry. The final protocol and subject information and informed consent documentation were reviewed and approved by the Ethics Committee of the Faculty of Medicine of the Goethe University of Frankfurt am Main, Germany. This study was conducted in accordance with the Declaration of Helsinki.
This study was registered with the Federal Institute for Drugs and Medical Devices, as well as the Federal Association of Statutory Health Insurance Physicians, and the Head Association of Health Insurers, and on ClinicalTrials.gov (NCT02486302).
Inclusion and Exclusion Criteria
Patients with RA were diagnosed by the treating physician and treated according to the summary of product characteristics (SmPC) for ETN [10]. Accordingly, patients aged ≥ 18 years with moderate-to-severe active RA and inadequate response to disease-modifying antirheumatic drugs (DMARDs) including MTX, as well as patients with severe, active, and progressive RA not previously treated with MTX were eligible for the study. No prior treatment with ETN was permitted (prior treatment with other biologics was permitted). Exclusion criteria were the contraindications, special warnings, and precautions according to the SmPC for ETN, including hypersensitivity to the active substance or any of the excipients in ETN, sepsis or risk of sepsis, or active infection, including chronic or localized infections [10]. Following evaluation for active and latent tuberculosis, ETN was not given to patients with active tuberculosis, and patients with latent tuberculosis were treated with anti-tuberculosis therapy before ETN treatment in accordance with local recommendations. Based on monitoring infections before, during, and after treatment with ETN, ETN was discontinued in patients with serious infections. Per the SmPC, special precautions were taken in patients with history of hepatitis B or hepatitis C infection, blood dyscrasias, demyelinating disease, congestive heart failure, or moderate-to-severe alcoholic hepatitis, and elderly patients. Concomitant administration of ETN with anakinra or abatacept was not recommended. Additional documentation of a patient in another post-marketing study of ETN was not permitted.
Primary and Secondary Endpoints
The primary endpoints for this study were the proportions of patients achieving the treatment targets of remission or LDA at week 12 and week 24 of treatment. Remission and LDA were defined as DAS28 < 2.6 and DAS28 ≤ 3.2, respectively, according to the S1 Guidelines of the German Society for Rheumatology (DGRh) [1].
Secondary endpoints included the proportion of patients continuing treatment with ETN despite not achieving remission at week 12 and the overall incidence of adverse events (AEs). Patient-reported outcomes (PROs) included the patient global assessment, depression, evaluated using the Patient Health Questionnaire-2 (PHQ-2), and fatigue and pain, both measured by the visual analog scale (VAS).
Data Collection and Statistical Analyses
Treating physicians were responsible for collecting data at each visit using a case report form. Categorical data were summarized as frequencies (absolute and adjusted), and numerical data were summarized as means (± standard deviation [SD]) or medians (range, 25–75% quartiles). Missing values were not imputed unless otherwise noted. Analysis sets were defined as “all documented” (AD; all documented patients), “treated set” (TS; all patients with ≥ 1 post-baseline value including documentation of an AE), and “per-protocol set” (PPS; all patients without major protocol deviations).
Logistic regression analysis was conducted to identify any parameters associated with attainment of remission or LDA by week 36 or 52 as well as parameters associated with high or very high disease activity at week 36 or 52. Parameters analyzed were disease duration, baseline disease activity, fatigue, pain, patient assessment of disease activity, and depression.
A sensitivity analysis was conducted to estimate the effect of incomplete documentation: effectiveness outcomes from patients for whom data were available for at least four out of five visits, or who had documented treatment discontinuation, were compared with results for the overall study population.
Results
Patient Disposition
A total of 845 patients with documented RA entered the study, of whom 824 (98%) were included in the TS. A total of 472 (57%) patients completed the study while 239 (28%) patients discontinued prematurely and 113 (13%) discontinued treatment without complete documentation and were lost to analysis. The main reasons for discontinuation were insufficient efficacy and AEs (Fig. 1b). The number of patients documented per visit is shown in Supplementary Table S2.
Baseline Characteristics
Patients with RA in the TS were predominantly females (72%), with a mean age of 59 years (Table 1). The median disease duration was 5.5 years, and most patients had received two (47%) or at least three (24%) previous conventional DMARDs (cDMARDs; Table 1). MTX was the most common prior therapy (88%). The most common concomitant medications received at least once during the study treatment period were MTX (46%) and prednisolone (32%) (Supplementary Table S3). The most common concomitant diseases recorded at baseline were hypertension (36%), osteoarthritis (13.1%), and type 2 diabetes mellitus (8.6%) (Supplementary Table S4).
Effectiveness
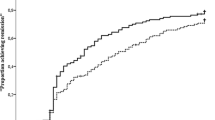
After 12 weeks of ETN treatment, 24% (194/794) of patients with RA achieved remission. This proportion increased steadily over time among patients who remained on study, with 31% of patients achieving remission at both week 24 (203/664) and week 36 (173/561), and 37% (187/502) achieving remission at week 52 of treatment (Fig. 2a).
Percentage of patients achieving a remission (DAS28 < 2.6) and LDA (DAS28 ≤ 3.2) and percentage of patients achieving b remission (DAS28 < 2.6) and LDA (DAS28 ≤ 3.2) at weeks 12 and 24 and maintaining it until week 52. Error bars show CI. Data are based on observed cases. N refers to the number of patients included in the per-protocol set. Sample sizes exclude missing values, based on the number of patient documentations available at the indicated visit. Remission was defined as DAS28 < 2.6 and LDA was defined as DAS28 ≤ 3.2; as such, the category of LDA also includes patients in remission. CI confidence interval, DAS Disease Activity Score, LDA low disease activity
At week 12, 39% (313/794) of patients achieved LDA, and this proportion increased to 45% (300/664) at week 24. Extending treatment duration up to 52 weeks was associated with a further increase in the proportion of patients achieving LDA to 54% (273/502) (Fig. 2a).
An increase in the proportion of patients achieving remission and LDA between week 12 and week 24 was also observed using alternative disease activity scores (Simplified Disease Activity Index [SDAI], Clinical Disease Activity Index [CDAI], American College of Rheumatology/European League Against Rheumatism [ACR/EULAR] Boolean remission criteria). However, remission rates were consistently lower than those observed using the DAS28 score (Supplementary Table S5).
Among patients who did not reach their treatment goal at week 12, 19% (93/481) attained their goal at week 24. Among patients who achieved remission at week 12, 39% (58/147) maintained it up to week 52. This proportion increased to 52% (88/169) among patients who achieved remission at week 24 (Fig. 2b).
Similarly, 45% (107/238) of patients who achieved LDA at week 12 and 59% (146/249) of those who achieved LDA at week 24 maintained it up to week 52 (Fig. 2b).
Patient-Reported Outcomes
A clinically relevant pain reduction was observed over the course of the study (Fig. 3a). Fatigue and mean PHQ-2 score, indicating depression, also decreased steadily throughout the study, with the most pronounced reductions observed during the first 12 weeks of treatment with ETN (Fig. 3b, c). Similarly, the proportion of patients without depression (PHQ-2 score = 0) increased steadily over the course of the study, from 10% (78/807) at baseline to 30% (140/463) at week 52.
a Global assessment of pain, b global assessment of fatigue, c PHQ-2 score for depression, d concomitant glucocorticoid use, and e mean daily glucocorticoid dose. Error bars show SD. Data are based on observed cases. PHQ-2 Patient Health Questionnaire-2, SD standard deviation, VAS visual analog scale
Concomitant Glucocorticoid Treatment in Rheumatologic Indications
The proportion of patients with concomitant glucocorticoid treatment continuously decreased from 86% (621/718) of patients at baseline to 65% (289/443) at week 52 (Fig. 3d). The mean daily concomitant glucocorticoid dose was during ETN treatment compared with baseline and previously reported doses (Fig. 3e). The most pronounced dose reduction from baseline was observed at week 12, although further reductions were observed until week 52. This apparent trend was not only seen in the overall study population but also in patients who achieved remission or LDA at a later timepoint (week 36 or week 52) or not at all by week 52.
A greater proportion of patients who had previously received biologic DMARD (bDMARD) therapy were using glucocorticoids at baseline (88% [128/145]) compared with the rate among bDMARD-naïve patients (86% [493/577]), and the proportion remained higher throughout the study (73–79% [61/84–112/142] vs. 64–75% [228/359–414/556], respectively). The mean daily dose of glucocorticoids decreased to a lesser extent among prior bDMARD recipients compared with those who had not received bDMARD therapy. No relationship between mean daily glucocorticoid dose and AEs was observed.
Disease Course
Some patients with RA did not reach remission or LDA at week 12 or 24 and remained under treatment with ETN until week 52: 18% (102/568) and 13% (65/508) had DAS28 > 3.2 at week 36 and week 52, respectively, without having reached DAS28 ≤ 3.2 previously. While some patients achieved remission at week 36 or 52, others achieved remission or LDA at week 12 or 24 but subsequently lost response. In addition, some patients were lost to follow-up or discontinued the study (Supplementary Fig. S1).
Predictors of Response
No relevant baseline parameters (disease duration, baseline disease activity, fatigue, pain, patient assessment of disease activity, and depression) predicting achievement of remission or LDA at weeks 36 or 52 were identified in the logistic regression analysis. However, descriptive analysis showed that patients with moderate or high disease activity (DAS28 > 3.2) at weeks 36 or 52 (who had not achieved DAS28 < 3.2 at any previous time point) had clinically relevant improvements in PROs including pain, fatigue, and depression, as well as patient and physician global assessment (Supplementary Tables S6A, S6B). Patients who did not attain their treatment target at any point during the study had a numerically longer disease duration (median [Q1; Q3]: 8.1 years [2.6; 17.5]) compared with the overall study population (median [Q1; Q3] 5.5 years [0.0; 61.6]). Overall, 62% (315/502) of patients received ETN until the end of the study despite not reaching the treatment target.
Subsequent Treatment After Discontinuation of ETN
Out of 239 patients who discontinued ETN, subsequent therapy was specified for 191 patients. While 28 (15%) patients received no further treatment, seven (4%) were treated with an ETN biosimilar, 48 (25%) were treated with another TNF inhibitor, and 64 (34%) were treated with a biologic with a different mode of action.
Sensitivity Analysis
A sensitivity analysis was performed for 739 patients for whom records were available for at least four out of the five visits, or who had documented treatment discontinuation. Effectiveness outcomes for these patients were comparable to those of the overall study population, which included patients with a higher rate of incomplete documentation.
Safety
A total of 402 (49%) patients with RA experienced ≥ 1 treatment-emergent AE during the observational period. The most common classes of AEs were general disorders and administration-site reactions (28%), infections and infestations (12%), musculoskeletal and connective tissue disorders (10%), and skin and subcutaneous disorders (6%) (Table 2). Treatment-emergent serious adverse events (SAEs) were reported in 92 patients (11%), most commonly musculoskeletal and connective tissue disorders (4%) and general disorders and administration-site conditions (3%). One case of tuberculosis was reported as a treatment-emergent SAE. Four patients with RA died during the observational period (two women and two men; all > 70 years of age); none of the deaths was determined to be study drug-related.
Discussion
In this non-interventional study, a considerable proportion of patients with RA treated with ETN under routine daily practice conditions achieved the treatment goal of remission or LDA at 12 weeks and maintained their target up to 52 weeks of treatment. In addition, patients reported marked improvement in PROs including pain and fatigue.
Remission rates from other real-world observations of patients treated with TNF inhibitors or other bDMARDs vary greatly. In the German GO-NICE study, 34% of patients had achieved DAS28 remission after 3 months of treatment with the TNF inhibitor golimumab, increasing to 45% after 3 years [11]. Data from the Rheumatic Diseases Portuguese Register showed that 23% and 40% of patients achieved remission and LDA, respectively, after 6 months of treatment with TNF inhibitors [12], which is generally in line with our findings. However, in a post hoc analysis of an Australian non-interventional cohort study of patients with RA treated with bDMARDs (abatacept, adalimumab, anakinra, certolizumab pegol, etanercept, golimumab, infliximab, rituximab, and tocilizumab), rates of remission were higher than those observed here: nearly 60% of patients achieved remission at 3 months, which increased to 64% after 18 months [13]. In contrast, in an analysis of data from the British Society for Rheumatology Biologics Registry for Rheumatoid Arthritis, only 10% of patients treated with infliximab, etanercept, or adalimumab achieved remission at 6 months [14]. Direct comparisons of real-world data are often difficult due to variations seen in patient populations, treatment settings, and methods. Despite this, general trends such as an increase in the rate of remission over time can be observed, particularly in studies with observation periods of several years. For example, a comparison of infliximab, adalimumab, and ETN in Greek patients with RA showed an increase of DAS28 response beyond 24 weeks and up to 8 years of treatment [9]. In a prospective study in patients with RA attending an outpatient clinic in Ireland and treated with ETN, infliximab, adalimumab, golimumab, or certolizumab, 35% of patients achieved remission after 1 year of treatment, which increased to 61% after 12 years [15].
While the proportion of patients in remission or with LDA increased throughout the course of the study, it must be considered that 16% of patients discontinued treatment due to insufficient response. This observation along with the high proportion of patients subsequently receiving another bDMARD suggests that investigators based their treatment decisions on the treat-to-target concept, as detailed in the relevant guidelines [1, 3, 4, 16]. Nevertheless, a sizable proportion of patients received ETN until the end of the study, despite not achieving the treatment target. These patients experienced improvements in PROs such as fatigue and pain, suggesting that, for some patients, objective assessments of disease activity may not be the only relevant factor for treatment continuation in clinical practice. Some patients with substantial improvements in PROs may benefit from remaining on a given treatment despite not reaching the target. The finding that patients who did not reach treatment target at any point had a longer disease duration compared with the overall study population is in agreement with the benefit of treatment with ETN in early phases of the disease, as previously identified in the ERA and COMET studies [17, 18].
A number of studies have shown the benefits of the treat-to-target principle [19, 20]; however, a change in treatment may not always be possible for all patients due to comorbidities or contraindications. Our results show that extending treatment with ETN beyond 12 weeks increases the proportion of patients achieving the treatment goal or LDA, with some patients achieving treatment goal for the first time at 24 weeks. This result, along with the improvements in PROs seen in patients who reached the treatment goal at a later point in the study, indicates that continuing treatment with ETN, even if treat-to-target goals have not been reached, can be beneficial to patients with RA.
Side effects of glucocorticoid treatment, which range from decreased quality of life to cardiovascular effects, are generally dose-dependent [21]. In this study, we observed a decrease in concomitant glucocorticoid use over time on ETN treatment. However, most patients remained on long-term, low-dose glucocorticoid treatment. These results reflect the situation in daily practice and highlight the future need for improved disease management in accordance with new treatment guidelines [3].
In line with the guidelines for defining remission and LDA at the time of study conception, DAS28 criteria were used to define remission and LDA [1]. ACR/EULAR guidelines now recommend the usage of SDAI and CDAI scores to measure remission and LDA [4, 16]. However, these scores tend to yield lower rates of remission than DAS28, as observed in this study. Despite this, the proportion of patients achieving SDAI and CDAI remission and LDA increased at week 12 and continued to improve during the study. Few patients met the more stringent ACR/EULAR Boolean remission criteria at any timepoint during the study (i.e., 0.1% at baseline to 3.5% at week 52). It is common for patients to not meet the stringent patient global assessment (PGA) component (≤ 1 on a 0–10 scale) for remission despite a good clinical response, and patients achieving remission for joint counts and C-reactive protein but not the PGA criteria are frequently categorized as “near-remission” [22, 23]. While near-remission was not evaluated in this study, the low rate of ACR/EULAR Boolean remission incorporating the strict PGA criteria was not unexpected.
Inherent limitations of non-interventional, observational studies are the risk of selection/ascertainment bias and the inability to attribute causation. To limit potential selection bias, physicians were encouraged to include all eligible patients consecutively. Due to the study duration of 52 weeks, no conclusions on long-term outcomes can be made. In addition, ETN is indicated in combination with MTX or, in cases of intolerance to MTX or when continued treatment with MTX is inappropriate, as monotherapy. Nearly half of the patients received concomitant MTX at least once during this study, which may have influenced response rates. Consistent with the known greater risk of RA in women versus men [24, 25], the majority of patients (72%) in this study were women, potentially limiting generalizability of results to both genders.
A further limitation is the decreasing number of observations over time, as 42% of enrolled patients discontinued prematurely or were missing documentation. Reasons for discontinuation included switching to a different treatment due to AEs or insufficient effectiveness; in addition, some patients were lost to follow-up. This leads to a potential risk of bias due to missing data. Missing values were not imputed in the effectiveness analyses. Therefore, denominators were included where possible to provide context. In addition, sensitivity analyses comparing patients with data from at least four of five visits or documented treatment discontinuation with the overall study population indicated that incomplete documentation had little impact on our observations. Such a decrease in the number of patients over time (due to discontinuation of treatment or lost follow-up) is a common observation in non-interventional studies [9, 26]. Documentation may be incomplete as only routine clinical investigations are included due to the non-interventional nature of the study, which may vary from practice to practice. However, randomized controlled trials (RCTs) usually have much stricter inclusion and exclusion criteria and therefore are not representative of the general population of patients eligible for a certain medication. Data from the German biologics registry RABBIT showed that only 21–33% of patients included in this registry would have been eligible for an RCT [27].
Conclusions
In summary, our results show that decision-making regarding continuation or switching of therapy is complex. Optimal treatment decisions should always take into account patient preferences. While the treat-to-target approach has proven clinical benefits, some patients may benefit from prolonged treatment with ETN beyond 12 weeks before considering switching treatment.
Our findings confirm the effectiveness and safety of ETN in a real-world setting and highlight the potential benefits of continuing treatment with ETN in patients who have not reached their treatment goal after 12 weeks of treatment.
Change history
05 September 2023
A Correction to this paper has been published: https://doi.org/10.1007/s40744-023-00578-6
References
Kruger K, Wollenhaupt J, Albrecht K, et al. German 2012 guidelines for the sequential medical treatment of rheumatoid arthritis. Adapted EULAR recommendations and updated treatment algorithm. Z Rheumatol. 2012;71:592–603.
Smolen JS, Breedveld FC, Burmester GR, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis. 2016;75:3–15.
Smolen JS, Landewe RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79:685–99.
Smolen JS, Aletaha D. Scores for all seasons: SDAI and CDAI. Clin Exp Rheumatol. 2014;32:S-75-9.
Ishida M, Kuroiwa Y, Yoshida E, et al. Residual symptoms and disease burden among patients with rheumatoid arthritis in remission or low disease activity: a systematic literature review. Mod Rheumatol. 2018;28:789–99.
Emery P, Breedveld FC, Hall S, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet. 2008;372:375–82.
Listing J, Strangfeld A, Rau R, et al. Clinical and functional remission: even though biologics are superior to conventional DMARDs overall success rates remain low–results from RABBIT, the German biologics register. Arthritis Res Ther. 2006;8:R66.
Gaubitz M, Gottl KH, Behmer O, Lippe R, Meng T, Loschmann PA. Etanercept is effective as monotherapy or in combination with methotrexate in rheumatoid arthritis: subanalysis of an observational study. Clin Rheumatol. 2017;36:1989–96.
Papadopoulos CG, Gartzonikas IK, Pappa TK, et al. Eight-year survival study of first-line tumour necrosis factor alpha inhibitors in rheumatoid arthritis: real-world data from a university centre registry. Rheumatol Adv Pract. 2019;3:rkz007.
European Medicines Agency. Summary of product characteristics: Enbrel. 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/enbrel#product-information-section. Accessed 24 Aug 2021.
Kruger K, Burmester GR, Wassenberg S, Bohl-Buhler M, Thomas MH. Effectiveness and safety of golimumab in patients with rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis under real-life clinical conditions: non-interventional GO-NICE study in Germany. BMJ Open. 2018;8:e021082.
Ganhao S, Lucas R, Fonseca JE, et al. Remission and low disease activity matrix tools: results in real-world rheumatoid arthritis patients under anti-TNF therapy. Acta Reumatol Port. 2020;45:245–52.
Bird P, Littlejohn G, Butcher B, et al. Real-world evaluation of effectiveness, persistence, and usage patterns of monotherapy and combination therapy tofacitinib in treatment of rheumatoid arthritis in Australia. Clin Rheumatol. 2021. https://doi.org/10.1007/s10067-021-05853-x (Epub ahead of print).
Druce KL, Bhattacharya Y, Jones GT, Macfarlane GJ, Basu N. Most patients who reach disease remission following anti-TNF therapy continue to report fatigue: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology (Oxford). 2016;55:1786–90.
Murray K, Turk M, Alammari Y, et al. Long-term remission and biologic persistence rates: 12-year real-world data. Arthritis Res Ther. 2021;23:25.
Felson DT, Smolen JS, Wells G, et al. American College of Rheumatology/European League against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum Dis. 2011;70:404–13.
Emery P, Kvien TK, Combe B, et al. Combination etanercept and methotrexate provides better disease control in very early (<=4 months) versus early rheumatoid arthritis (>4 months and <2 years): post hoc analyses from the COMET study. Ann Rheum Dis. 2012;71:989–92.
Genovese MC, Bathon JM, Martin RW, et al. Etanercept versus methotrexate in patients with early rheumatoid arthritis: two-year radiographic and clinical outcomes. Arthritis Rheumatol. 2002;46:1443–50.
Solomon DH, Bitton A, Katz JN, Radner H, Brown EM, Fraenkel L. Review: treat to target in rheumatoid arthritis: fact, fiction, or hypothesis? Arthritis Rheumatol. 2014;66:775–82.
Zak A, Corrigan C, Yu Z, et al. Barriers to treatment adjustment within a treat to target strategy in rheumatoid arthritis: a secondary analysis of the TRACTION trial. Rheumatology (Oxford). 2018;57:1933–7.
Yasir M, Jatana G, Sonthalia S. Corticosteroid adverse effects. 2021. https://www.ncbi.nlm.nih.gov/books/NBK531462/. Accessed 24 Aug 2021.
Gossec L, Kirwan JR, de Wit M, et al. Phrasing of the patient global assessment in the rheumatoid arthritis ACR/EULAR remission criteria: an analysis of 967 patients from two databases of early and established rheumatoid arthritis patients. Clin Rheumatol. 2018;37:1503–10.
Vermeer M, Kuper HH, van der Bijl AE, et al. The provisional ACR/EULAR definition of remission in RA: a comment on the patient global assessment criterion. Rheumatology (Oxford). 2012;51:1076–80.
McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–19.
van Vollenhoven RF. Treatment of rheumatoid arthritis: state of the art 2009. Nat Rev Rheumatol. 2009;5:531–41.
Iking-Konert C, von Hinuber U, Richter C, et al. ROUTINE-a prospective, multicentre, non-interventional, observational study to evaluate the safety and effectiveness of intravenous tocilizumab for the treatment of active rheumatoid arthritis in daily practice in Germany. Rheumatology (Oxford). 2016;55:624–35.
Zink A, Strangfeld A, Schneider M, et al. Effectiveness of tumor necrosis factor inhibitors in rheumatoid arthritis in an observational cohort study: comparison of patients according to their eligibility for major randomized clinical trials. Arthritis Rheumatol. 2006;54:3399–407.
Acknowledgements
Funding
The ADEQUATE study was funded by Pfizer Pharma GmbH. The Rapid Service Fee was funded by Pfizer.
Medical Writing Assistance
Medical writing support was provided by Andrea Schauenburg, PhD, of Engage Scientific Solutions and was funded by Pfizer.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors' Contributions
All authors were involved in the writing and reviewing process and have read and approved this manuscript. Additional contributions to this body of work were as follows: Eugen Feist, Xenofon Baraliakos, Frank Behrens, and Diamant Thaçi: conception/design of the study, acquisition and interpretation of data. Thilo Klopsch: acquisition of data. Anja Plenkse, Thomas Meng, and Peter-Andreas Löschmann: conception/design of the study and interpretation of data. Lisa K. Blindzellner and Pascal Klaus: interpretation of data.
List of Investigators
The full list of investigators for the ADEQUATE study is shown in Supplementary Table S7.
Prior Presentation
Data included in this manuscript have partially been presented at the following congresses: Feist E, Baraliakos X, Behrens F, Thaçi D, Klopsch T, Plenske A, Blindzellner LK, Meng T, Löschmann PA. Efficacy of Etanercept over a Period of 12 Months in the Routine Treatment of Patients with RA, AxSpA, PsA or PsO: Final Results of a German Non-Interventional, Prospective, Multicenter Study (ADEQUATE). Poster presented at the European League Against Rheumatism Congress 2019. Baraliakos X, Feist E, Behrens F, Thaçi D, Klopsch T, Plenske A, Blindzellner LK, Meng T, Löschmann PA. Nicht-interventionelle Studie zur Wirksamkeit und Sicherheit von Etanercept in der zielgerichteten Routinebehandlung von Patienten mit Rheumatoider Arthritis, axialer Spondyloarthritis, Psoriasis-Arthritis und Psoriasis (ADEQUATE). Poster presented at the congress of the German Society for Rheumatology (DGRh) 2019.
Disclosures
Eugen Feist received consulting/speaker fees from AbbVie, BMS, Celgene, Lilly, Novartis, Roche, Sanofi, Pfizer, and UCB. Xenofon Baraliakos received honoraria or grants from AbbVie, Amgen, Biocad, BMS, Celltrion, Chugai, Gilead, Janssen, Lilly, Novartis, Pfizer, Roche, Sandoz, and UCB. Frank Behrens received research grants from Bionorica, Celgene, Chugai, Janssen, Pfizer, and Roche; and consulting/speaker fees from AbbVie, Amgen, Boehringer Ingelheim, Celgene, Chugai, Janssen, Genzyme, Gilead, Lilly, MSD, Novartis, Pfizer, Roche, Sandoz, Sanofi, and UCB. Diamant Thaçi was consultant, investigator, speaker; and has participated in advisory boards for AbbVie, Almirall, Amgen, Biogen Idec, Bristol-Myers Squibb, Janssen-Cilag, LEO Pharma, Lilly, Novartis, Pfizer, Regeneron, Sanofi, and UCB; and has received research/educational grants from AbbVie, Novartis, LEO Pharma, and Sanofi. Thilo Klopsch received consulting/speaker fees from AstraZeneca, BMS, Clinuvel Pharmaceuticals, Eli Lilly, Evotec, Johnson & Johnson, Morphosys, Novartis, Novo Nordisk, Pfizer, Roche, and UCB. Anja Plenske, Lisa K. Blindzellner, Pascal Klaus, and Thomas Meng are employees and shareholders of Pfizer. Peter-Andreas Löschmann was a full-time employee of Pfizer at the time the manuscript was developed and a shareholder of Pfizer. At the time of writing, he acts as the chief correspondent and head of gesundheitspolitik.de.
Compliance with Ethics Guidelines
The final protocol and subject information and informed consent documentation were reviewed and approved by the Ethics Committee of the Faculty of Medicine of the Goethe University of Frankfurt am Main, Germany. This study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients by the treating physician or a designated person prior to patients entering the study.
Data Availability
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dr Löschmann’s name to indicate that this was his affiliation at the time of study conduct.
The original online version of this article was revised: To correct the in-text reference of Figure 2.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Feist, E., Baraliakos, X., Behrens, F. et al. Effectiveness of Etanercept in Rheumatoid Arthritis: Real-World Data from the German Non-interventional Study ADEQUATE with Focus on Treat-to-Target and Patient-Reported Outcomes. Rheumatol Ther 9, 621–635 (2022). https://doi.org/10.1007/s40744-021-00418-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-021-00418-5