Abstract
Introduction
Current recommendations for the management of rheumatoid arthritis (RA) focus on a treat-to-target approach with the objective of maximizing long-term health-related quality-of-life in patients with RA. Published studies from randomized clinical trials have reported limited data regarding the long-term efficacy and safety of adalimumab in patients with RA. This study aims to evaluate the long-term (10+ years) persistency and effectiveness of adalimumab in patients with RA in a real-world setting.
Methods
Included in this study were biologic-naïve adults with RA initiating adalimumab during follow-up enrolled in the Corrona RA registry. More than 10 years of data on persistency of adalimumab and rheumatologist-supplied reasons for discontinuation were examined. Among patients who persisted on adalimumab over the years, clinical [e.g., clinical disease activity index scores (CDAI), physician global assessment, tender joint count, and swollen joint count] and patient-reported outcomes (PRO), such as physical function, pain, fatigue, and morning stiffness, were examined.
Results
Of 1791 biologic-naive patients treated with adalimumab who had ≥1 follow-up registry visit, 64.1% were still on therapy at 1 year and 10.2% were still on therapy by the end of year 12. Among patients who persisted on adalimumab for at least 1 year (77.1% female, mean age 53.9 years), 67.0% were in low disease activity (LDA)/remission (CDAI ≤10) and had clinically meaningful improvements from baseline in all clinical assessments and PROs. Initial improvements in LDA/remission and in clinical and PRO assessments observed at year 1 were sustained in those patients who remained on adalimumab over 10 years of follow-up. Among patients who discontinued adalimumab, 61.6% were not in LDA/remission and 41.9% switched to another biologic within 12 months after discontinuing adalimumab.
Conclusions
Real-world data demonstrate a sustained effectiveness of adalimumab in the treatment of RA for patients who remained on therapy for 10 years.
Funding
Corrona, LLC and AbbVie.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a chronic, progressive inflammatory disease that can lead to irreversible joint damage [1], diminished function, and disability [2]. The introduction of biologic agents, such as tumor necrosis factor (TNF) inhibitors, in the treatment of RA has significantly reduced disability among patients with RA [3] by improving clinical disease activity, physical function, radiographic outcomes, and rates of joint surgery [4,5,6,7,8,9]. A primary goal for RA treatment is to maximize the long-term health-related quality of life in patients [10]. Because RA is a chronic and progressive disease, most patients require long-term therapy to slow or prevent joint damage. The average patient with RA frequently needs multiple changes in therapy regimens over time to achieve and maintain control of the disease [10, 11]. Reasons to discontinue TNF inhibitors often include intolerance, adverse safety events, and primary or secondary loss of efficacy [12,13,14]. A number of clinical trials have provided data on the effectiveness of TNF, but for only short periods of time [15,16,17,18,19,20]. Long-term assessments are needed to provide a complete understanding of the real-world benefit-risk profile of TNF-inhibitor therapy.
Adalimumab is a TNF inhibitor that has been proven to be efficacious in patients with RA in a number of clinical trials [17, 20,21,22,23,24]. Recently, data from long-term, open-label clinical studies have shown that the benefits of adalimumab therapy persist for up to 10 years [25, 26]; however, there is little information on the effect of adalimumab therapy on long-term outcomes in real-world settings. This study examined the long-term (i.e., more than 10 years), real-world persistency and effectiveness of adalimumab using data from a large, observational RA registry (Corrona) that was prospectively and regularly collected in the clinic from both rheumatologists and patients.
Methods
Study Setting
Corrona (NCT01402661) is an ongoing, prospective, observational disease-based (all treatment modalities included) registry designed to collect information on utilization patterns, effectiveness, and safety of therapeutic agents used in the management of RA [27]. The Corrona registry was founded by academic rheumatologists to collect data at the time of routine clinic visits. As of January 2017, the Corrona RA registry has recruited patients with RA from approximately 170 private and academic practice sites across 40 states in the USA, with approximately 670 participating rheumatologists. The registry has data on approximately 44,500 patients with RA, 337,500 patient visits, and approximately 152,200 patient-years (PY) of follow-up observation time, with mean time of patient follow-up of 4.2 years (median 3.5 years). Data collection, which began in 2001, is performed at clinical visits with follow-ups as frequent as every 3–6 months [28, 29]. The current analysis is a descriptive study of long-term adalimumab effectiveness.
All procedures followed are in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Approvals for practice-level data collection and analyses are obtained from local institutional review boards of participating academic sites and central institutional review boards (Western and New England Institutional Review Boards) for private practice sites. All patients provide written informed consent, and all data are de-identified to protect patient confidentiality.
Study Population
The persistency assessment included all adults with RA enrolled in the Corrona registry who were naïve to targeted immunomodulators [i.e., biologics and targeted synthetic disease-modifying anti-rheumatic drugs (DMARDs)] at the time of initiation and had at least one follow-up visit. Among this cohort, descriptive analyses were performed for patients who had at least 12 months (±3 months visit window) of follow-up while being treated with adalimumab. If adalimumab was initiated in between registry visits, then the visit immediately before the adalimumab initiation visit was used to retrieve baseline data (such as baseline disease activity) as long as the immediately prior visit occurred within 4 months of adalimumab initiation. If neither of the above requirements was fulfilled, the patient was excluded from the analysis.
Outcomes and Assessments
Patient demographics (age, sex, race, employment and insurance status) and clinical characteristics (disease activity, history of comorbidities, and treatment history) were assessed at the time of initiation of adalimumab.
Persistency was estimated for the overall study population (including those with follow-up <12 months) and was also stratified by year of adalimumab initiation.
Reasons for discontinuing or switching from adalimumab were reported by the physician. Physicians could report up to three reasons (related to efficacy, safety, and other) for discontinuing the drug. Reasons for discontinuing because of efficacy included inadequate initial response, failure to maintain response, and lack of efficacy—the latter was used for cases where not even a partial response was noted. Safety reasons for discontinuing therapy included serious and minor side effects. Other reasons for discontinuing therapy included fear of future side effects, patient preference, cost/insurance, patient doing well, frequency of administration, to improve tolerability, route of administration, need for alternative mechanism of action, and temporary interruption.
Disease activity was assessed using the Clinical Disease Activity Index (CDAI) [30]. Percentage of time patients spent in each disease category as defined by CDAI [remission (CDAI ≤2.8), low (CDAI >2.8 to ≤10.0), moderate (CDAI >10.0 to ≤22.0), high (CDAI >22.0)] was estimated by linear interpolation of CDAI between visits. An exploratory definition of flare (defined as an increase in CDAI of 8 points; an increase in the frequency of adalimumab use that occurred >6 months after initiation of adalimumab; initiation of methotrexate, leflunomide, or sulfasalazine or increase in dose frequency of these DMARDs that occurred >6 months after initiation of the DMARD; an increase in prednisone dose of ≥5 mg; or any combination of these changes) was used to determine when patients were experiencing recurring, acute episodes of pain and inflammation. Rate of flares (defined as the number of flares/PY of follow-up) was calculated. The components of the CDAI include swollen joint counts (SJC 0–28 scale), tender joint counts (TJC 0–28 scale), Physician Global Assessment on a visual analog scale (VAS 0–100 scale), and Patient Global Assessment on a visual analog scale (VAS 0–100).
PROs included functionality as assessed by the modified Health Assessment Questionnaire (mHAQ, on a scale of 0–3), Patient Global Assessment on a VAS (0–100 scale), pain (VAS 0–100 scale), fatigue (VAS 0–100 scale), and morning stiffness in hours categorized by duration (0 to <1 and ≥1 h).
Analysis of Data
Patient characteristics, clinical outcomes, PROs, and reasons for discontinuing therapy were summarized using descriptive statistics and stratified by the number of years of follow-up during extended treatment with adalimumab. Follow-up categories were defined as follows: patients who had 1 year (0 to ≤1 year), 2 years (>1 to ≤2 years), 3 years (>2 to ≤3 years), 4 years (>3 to ≤4 years), 5 years (>4 to ≤5 years), 6 years (>5 to ≤6 years), 7 years (>6 to ≤7 years), and 8 years (>7 to ≤8 years) of therapy with adalimumab. Categories beyond 8 years were not included because the number of patients was too small to provide meaningful summary statistics.
Persistency of adalimumab therapy was estimated using product limit estimator (Kaplan-Meier analysis), where follow-up time was calculated as time in months from initiation of adalimumab until discontinuation/switch of adalimumab and last follow-up visit in Corrona; remaining on adalimumab was considered a censored time point. Persistency was estimated for the overall population and also stratified by patients who initiated adalimumab before or during 2009 and those who initiated adalimumab after 2009. The year 2009 was chosen as the cutoff point because most of the currently approved biologics used to treat RA were on the market. In addition, using this cutoff allowed sufficient follow-up time to assess persistency of adalimumab therapy in the two cohorts.
Results
Study Population
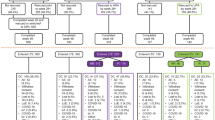
As of August 2016, there were 1912 targeted immunomodulator-naïve patients who initiated adalimumab in the Corrona registry. Of these, 1791 patients had at least one follow-up registry visit and were included in the persistency analysis (Fig. 1). Among these patients, 872 had ≥9 months of follow-up and 746 patients had a registry visit within 4 months of initiating adalimumab. Thus, data from 746 patients who remained on adalimumab therapy for at least 1 year were available for analysis of the long-term effectiveness of adalimumab.
Baseline demographic and clinical characteristics of the study population are provided in Table 1. The average age [standard deviation (SD)] of patients at initiation was 53.9 years (11.8 years). Patients were predominantly female (77.1%) and white (90.3%). The average disease duration at the time of adalimumab initiation was 7 years (8.4 years). The average CDAI at initiation was 19.9 (13.6), with the majority (73.5%) of patients in moderate (38.1%) or severe (35.4%) disease activity. Mean mHAQ at initiation was 0.5 (0.5) with 42.7% of patients having an mHAQ ≥0.5. Most patients (82.5%) reported experiencing morning stiffness with 61.8% reporting a duration of at least 1 h.
Persistency
The persistency of adalimumab therapy is presented as a Kaplan-Meier curve (Fig. 2). Kaplan-Meier estimates of adalimumab persistency were derived using all patients who initiated adalimumab therapy and had at least one follow-up visit after initiation (N = 1791). Over 10 years of follow-up, the number of patients still on adalimumab gradually diminished from 1791 patients at initiation to 26 patients at year 10. Based on the Kaplan-Meier analysis, the percentages of patients who remained on adalimumab therapy were 64.1%, 48.0%, 26.7%, and 13.3% at 1, 2, 5, and 10 years, respectively.
The persistency of adalimumab therapy before and after 2009 was examined in an attempt to investigate whether the availability of more biologics after 2009 had an impact on persistency of adalimumab (Fig. 3). Kaplan-Meier estimates of adalimumab persistency were derived for 1075 patients who initiated adalimumab during or before 2009 and had at least one registry visit after initiating adalimumab and 837 patients who initiated adalimumab after 2009, irrespective of whether they had a registry visit after initiation. Overall, persistency was significantly (p = 0.001) greater among patients who initiated therapy before or during 2009 compared with those who initiated after 2009. For patients who initiated adalimumab during or before 2009, 67.5%, 52.2%, and 20.7% remained on therapy at 1, 2, and 5 years, respectively, after initiation. For those who initiated therapy after 2009, 61.1%, 43.9%, and 22.6% remained on therapy at 1, 2, and 5 years, respectively, after initiation.
Effectiveness of Adalimumab Over Time
Only the first 8 years of follow-up were used for this part of the analysis to maintain statistical power and allow meaningful conclusions (<50 patients were available for analysis afterwards). Over 8 years of follow-up, a significant number of patients treated with adalimumab achieved LDA and remission. As shown in Fig. 4, LDA and remission were noted in the majority of patients during the first year. Specifically, 481 (67.0%), 107 (78.7%), and 52 (89.7%) patients were in LDA/remission at years 1, 5, and 8, respectively. Over time, the number of patients on adalimumab decreased and the proportion of patients in LDA/remission increased over the years.
Table 2 summarizes the clinical characteristics and PROs of patients remaining on adalimumab at each year of follow-up through year 8. More patients with well-controlled disease tended to stay on adalimumab, whereas those without controlled disease discontinued the medication, making the mean disease activity lower each year. Consistent with the reduction in disease activity seen at year 1, significant decreases of 3.6, 4.0, and 13.5 points in mean SJC, TJC, and pain scores, respectively, were observed in the first year. Similar improvements in the mean values were also seen in the Patient and Physician Global Assessments. As shown in Table 2, these improvements in clinical characteristics and PROs remained stable over the 8-year follow-up period for patients who remained on adalimumab therapy. The flare rate was 0.43 per person at year 1 and did not increase over the follow-up period for those patients who remained on adalimumab therapy (Table 3). During year 1, the percentage of time that patients spent in remission/LDA, moderate disease activity, and high disease activity was 54.3%, 30.5%, and 15.2%, respectively. The low levels of moderate and high disease activity were maintained over long-term follow-up in patients who continued adalimumab therapy.
Discontinuation of Therapy
Of the 105 patients who discontinued adalimumab therapy at year 1, only 26 (24.8%) switched to another biologic at the registry visit when discontinuation was reported and 44 (41.9%) had switched to another biologic by the first year after discontinuation. A total of 33 (38.4%) patients were in LDA/remission when they discontinued adalimumab therapy (Table 4). Overall, 36 (34.3%) patients had a reason reported for discontinuing therapy. Of these, 21 discontinued because of efficacy, 6 discontinued because of side effects, and 9 discontinued for other reasons not related to safety or efficacy. Available reasons for discontinuation were not 100% because as opposed to how the registry has operated in the last few years—reporting reasons for discontinuation of biologics are now mandatory—in the first years of the registry operations the reporting of reasons for discontinuation was not mandatory.
Discussion
Long-term, real-world effectiveness data on adalimumab in patients with RA are limited. Observational registry data can shed light on the long-term effectiveness of adalimumab in a real-world setting. In this study, we followed biologic-naïve patients with RA who initiated adalimumab and had up to 12 years of follow-up. Approximately 67% of all patients who initially started adalimumab and had 12 months of follow-up were in LDA/remission and those who remained on adalimumab continued to do well for long periods of time. Over 5 years of follow-up, 38–53% of patients who had a recent visit (within 4 months) before discontinuing therapy and had a CDAI score at that visit were in LDA/remission when they discontinued adalimumab and only 12–25% switched to another biologic at the time the discontinuation was reported. Thirty-eight to 44 percent of patients started another biologic or targeted synthetic DMARD within the 1 year after discontinuation and 58–72% any time after discontinuation. Approximately half (48%) of the study population was still on adalimumab at 2 years with satisfactory control of their disease, and a small proportion (10%) of patients continued to be treated for up to 12 years. Persistency was significantly greater among patients who initiated adalimumab therapy before or during 2009 than among those who initiated adalimumab after 2009. One explanation for the lower persistency after 2009 is the increased availability of other biologics including agents with different mechanisms of action. Another possibility is that the follow-up period for patients initiating adalimumab before 2009 is longer than for those initiating therapy after 2009.
Patient demographics in Corrona are consistent with national Medicare administrative claims data, with a higher representation of females and older patients [31], suggesting that the results obtained in our study are applicable to the general population of patients with RA. Current recommendations stress a treat-to-target approach, with achievement of remission or LDA as a primary goal, and emphasis on improving patient quality-of-life and functionality [10, 11, 32]. Our findings show that, for the majority of patients, adalimumab offers good disease control in a real-world setting, and for a proportion of adalimumab-treated patients, satisfactory disease control may persist for a long period of time along with consistent improvement in PROs.
Recently, two clinical trials have reported 10-year efficacy data that serve as useful comparisons to this real-world analysis. The PREMIER [25] and DE019 [26] trials both evaluated the efficacy and safety of adalimumab treatment in combination with MTX. Both trials involved a study population with a higher representation of females (around 74%) and an average age of 53 years. The PREMIER and DE019 trials demonstrated that long-term treatment with adalimumab and MTX offered good disease control over 10 years of follow-up. Comparable to the results of the Corrona analysis, the improvement of clinical characteristics and PROs in the trials peaked within the first year of treatment initiation, with a subsequent plateau. Although our study did not follow patients to determine outcomes after discontinuation of adalimumab therapy, other studies have shown that patients who discontinue treatment with TNF inhibitors have an increase in the number of flares and are less likely to maintain low disease activity [33, 34]. It should be noted that patients enrolled in randomized clinical trials are selected because they have fewer significant comorbidities and fulfill very strict inclusion criteria, which may not reflect the real-world population. In contrast, disease-based registries, such as Corrona, include patients with various comorbidities no matter how severe and concomitant medications can be more readily adjusted as needed in registry patients. Thus, patients in Corrona are more representative of the general population than patients participating in randomized, controlled clinical trials, making it more likely that the persistent benefits seen with long-term adalimumab treatment will also be observed in the general population. Similar observational results focusing on long-term effectiveness have been reported in the literature reporting persistence between 22% and 41% by 3 years, but these studies did not observe patients for the length of time ours did [35, 36]. A multitude of other observational and registry derived data are available which either followed patients for fewer years [37] or had followed considerably fewer patients [38].
A key strength of this study is the large number of patients enrolled in Corrona and the ability to capture medication use (including initiation and discontinuation), clinical outcomes, and PROs over time. Another strength of using registry data is that all patients treated with adalimumab were included regardless of the presence of comorbidities or minimal disease activity measures typical of randomized controlled trials or phase 4 follow-up of these same patients. Limitations of the use of registry data must be kept in mind when interpreting the results of this study. Specifically, the registry is limited in terms of data collection at times of office visits and by the frequency of reporting (3–6 months intervals between visits). There is also an obvious channeling bias as patients who respond well without toxicity are more likely to remain on treatment. Thus, there may be unidentified confounding factors associated with this analysis. In the initial versions of the Corrona questionnaires, reasons for discontinuation of therapy were not always collected. Having all of the reasons for discontinuation available would give a more complete picture of why some patients discontinue adalimumab while having relatively good control of their disease and whether insurance concerns or patient preference, decreased adherence, or safety concerns played a role in the decision process. Future studies need to focus on evaluating factors associated with longer persistency and better outcomes in a real-life population and identifying patient and disease characteristics that increase the persistency and effectiveness of adalimumab in the long-term.
Conclusions
Results of this real-world analysis show that adalimumab offers satisfactory disease control in the majority of patients and that long-term disease control is achieved for a proportion of patients. These findings support the role of adalimumab in achieving treat-to-target goals and maintaining long-term disease control for patients with RA and are consistent with long-term results observed in clinical trials.
References
Lindqvist E, Jonsson K, Saxne T, Eberhardt K. Course of radiographic damage over 10 years in a cohort with early rheumatoid arthritis. Ann Rheum Dis. 2003;62(7):611–6.
Aletaha D, Smolen J, Ward MM. Measuring function in rheumatoid arthritis: identifying reversible and irreversible components. Arthritis Rheum. 2006;54(9):2784–92.
Krishnan E, Lingala B, Bruce B, Fries JF. Disability in rheumatoid arthritis in the era of biological treatments. Ann Rheum Dis. 2012;71(2):213–8.
Ziegler S, Huscher D, Karberg K, Krause A, Wassenberg S, Zink A. Trends in treatment and outcomes of rheumatoid arthritis in Germany 1997–2007: results from the National Database of the German Collaborative Arthritis Centres. Ann Rheum Dis. 2010;69(10):1803–8.
Sokka T, Kautiainen H, Hakkinen A, Hannonen P. Radiographic progression is getting milder in patients with early rheumatoid arthritis. Results of 3 cohorts over 5 years. J Rheumatol. 2004;31(6):1073–82.
Shourt CA, Crowson CS, Gabriel SE, Matteson EL. Orthopedic surgery among patients with rheumatoid arthritis 1980–2007: a population-based study focused on surgery rates, sex, and mortality. J Rheumatol. 2012;39(3):481–5.
Harty L, O’Toole G, FitzGerald O. Profound reduction in hospital admissions and musculoskeletal surgical procedures for rheumatoid arthritis with concurrent changes in clinical practice (1995–2010). Rheumatology (Oxford). 2015;54(4):666–71.
Kievit W, Fransen J, de Waal Malefijt MC, den Broeder AA, van Riel PL. Treatment changes and improved outcomes in RA: an overview of a large inception cohort from 1989 to 2009. Rheumatology (Oxford). 2013;52(8):1500–8.
Strand V, Rentz AM, Cifaldi MA, Chen N, Roy S, Revicki D. Health-related quality of life outcomes of adalimumab for patients with early rheumatoid arthritis: results from a randomized multicenter study. J Rheumatol. 2012;39(1):63–72.
Smolen JS, Aletaha D, Bijlsma JW, Breedveld FC, Boumpas D, Burmester G, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69(4):631–7.
Smolen JS, Landewe R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73(3):492–509.
Du Pan SM, Dehler S, Ciurea A, Ziswiler HR, Gabay C, Finckh A. Comparison of drug retention rates and causes of drug discontinuation between anti-tumor necrosis factor agents in rheumatoid arthritis. Arthritis Rheum. 2009;61(5):560–8.
Marchesoni A, Zaccara E, Gorla R, Bazzani C, Sarzi-Puttini P, Atzeni F, et al. TNF-alpha antagonist survival rate in a cohort of rheumatoid arthritis patients observed under conditions of standard clinical practice. Ann N Y Acad Sci. 2009;1173:837–46.
Neovius M, Arkema EV, Olsson H, Eriksson JK, Kristensen LE, Simard JF, et al. Drug survival on TNF inhibitors in patients with rheumatoid arthritis comparison of adalimumab, etanercept and infliximab. Ann Rheum Dis. 2015;74(2):354–60.
van der Heijde D, Klareskog L, Rodriguez-Valverde V, Codreanu C, Bolosiu H, Melo-Gomes J, et al. Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis: two-year clinical and radiographic results from the TEMPO study, a double-blind, randomized trial. Arthritis Rheum. 2006;54(4):1063–74.
Maini RN, Breedveld FC, Kalden JR, Smolen JS, Furst D, Weisman MH, et al. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum. 2004;50(4):1051–65.
Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48(1):35–45.
Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343(22):1594–602.
Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000;343(22):1586–93.
Keystone EC, Kavanaugh AF, Sharp JT, Tannenbaum H, Hua Y, Teoh LS, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50(5):1400–11.
Curtis JR, Singh JA. Use of biologics in rheumatoid arthritis: current and emerging paradigms of care. Clin Ther. 2011;33(6):679–707.
van de Putte LB, Atkins C, Malaise M, Sany J, Russell AS, van Riel PL, et al. Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann Rheum Dis. 2004;63(5):508–16.
Furst DE, Schiff MH, Fleischmann RM, Strand V, Birbara CA, Compagnone D, et al. Adalimumab, a fully human anti tumor necrosis factor-alpha monoclonal antibody, and concomitant standard antirheumatic therapy for the treatment of rheumatoid arthritis: results of STAR (Safety Trial of Adalimumab in Rheumatoid Arthritis). J Rheumatol. 2003;30(12):2563–71.
Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54(1):26–37.
Keystone EC, Breedveld FC, van der Heijde D, Landewe R, Florentinus S, Arulmani U, et al. Longterm effect of delaying combination therapy with tumor necrosis factor inhibitor in patients with aggressive early rheumatoid arthritis: 10-year efficacy and safety of adalimumab from the randomized controlled PREMIER trial with open-label extension. J Rheumatol. 2014;41(1):5–14.
Keystone EC, van der Heijde D, Kavanaugh A, Kupper H, Liu S, Guerette B, et al. Clinical, functional, and radiographic benefits of longterm adalimumab plus methotrexate: final 10-year data in longstanding rheumatoid arthritis. J Rheumatol. 2013;40(9):1487–97.
Corrona Registries: Rheumatoid Arthritis. http://www.corrona.org/registries/rheumatoid-arthritis. Accessed 24 Oct 2016.
Kremer JM. The CORRONA database. Autoimmun Rev. 2006;5(1):46–54.
Kremer JM. The Corrona US registry of rheumatic and autoimmune diseases. Clin Exp Rheumatol. 2016;34(5 Suppl 101):96–9.
Aletaha D, Smolen J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol. 2005;23(5 Suppl 39):S100–8.
Curtis JR, Chen L, Bharat A, Delzell E, Greenberg JD, Harrold L, et al. Linkage of a de-identified United States rheumatoid arthritis registry with administrative data to facilitate comparative effectiveness research. Arthritis Care Res (Hoboken). 2014;66(12):1790–8.
Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64(5):625–39.
Ghiti Moghadam M, Vonkeman HE, Ten Klooster PM, Tekstra J, van Schaardenburg D, Starmans-Kool M, et al. Stopping tumor necrosis factor inhibitor treatment in patients with established rheumatoid arthritis in remission or with stable low disease activity: a pragmatic multicenter, open-label randomized controlled trial. Arthritis Rheumatol. 2016;68(8):1810–7.
van Herwaarden N, den Broeder AA, Jacobs W, van der Maas A, Bijlsma JW, van Vollenhoven RF, et al. Down-titration and discontinuation strategies of tumor necrosis factor-blocking agents for rheumatoid arthritis in patients with low disease activity. Cochrane Database Syst Rev. 2014;9:Cd010455.
Iannone F, Sinigaglia L, Favalli EG, Sarzi-Puttini P, Atzeni F, Caporali R, et al. Drug survival of adalimumab in patients with rheumatoid arthritis over 10 years in the real-world settings: high rate remission together with normal function ability. Clin Rheumatol. 2016;35(11):2649–56.
Neubauer S, Cifaldi M, Mittendorf T, Ganguli A, Wolff M, Zeidler J. Biologic TNF inhibiting agents for treatment of rheumatoid arthritis: persistence and dosing patterns in Germany. Health Econ Rev. 2014;4(1):32.
Markenson JA, Gibofsky A, Palmer WR, Keystone EC, Schiff MH, Feng J, et al. Persistence with anti-tumor necrosis factor therapies in patients with rheumatoid arthritis: observations from the RADIUS registry. J Rheumatol. 2011;38(7):1273–81.
Degli Esposti L, Favalli EG, Sangiorgi D, Di Turi R, Farina G, Gambera M, et al. Persistence, switch rates, drug consumption and costs of biological treatment of rheumatoid arthritis: an observational study in Italy. Clinicoecon Outcomes Res. 2017;9:9–17.
Acknowledgements
This study is sponsored by Corrona, LLC. The Corrona LLC has been supported through contracted subscriptions in the last 2 years by AbbVie, Amgen, BMS, Crescendo, Eli Lilly and Company, Genentech, GSK, Horizon Pharma USA, Janssen, Momenta Pharmaceuticals, Novartis, Pfizer, Roche, and UCB. The design, study conduct, and financial support for the study were provided by AbbVie. AbbVie participated in developing the study design, writing, review, and approval of the manuscript, and the decision to submit the manuscript for publication. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. Medical writing assistance in the preparation of this manuscript was provided by Arthur Holland, Ph.D., and Joann Hettasch, Ph.D., of Fishawack Communications, Conshohocken, PA. Support for this assistance was funded by AbbVie.
Disclosures
Dimitrios A. Pappas is an employee of Corrona, LLC, and a Novartis instructor. Joel M. Kremer is an employee of Corrona, LLC; holds stock in Corrona, LLC; is a consultant to AbbVie, Amgen, BMS, Genentech, GSK, Lilly, Medimmune, Pfizer, and Sanofi; and received research grants from AbbVie, Genentech, Lilly, Novartis, Pfizer. Jenny Griffith is an employee of AbbVie and may hold stocks or stock options in AbbVie. Vishvas Garg is an employee of AbbVie and may hold stocks or stock options in AbbVie. George Reed is an employee Corrona, LLC; holds stock in Corrona, LLC and holds an appointment at UMASS. Bob Salim is an employee of Axio Research, LLC. Chitra Karki is an employee of Corrona, LLC.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Approvals for practice-level data collection and analyses were obtained from local institutional review boards of participating academic sites and a central institutional review board for private practice sites. Informed consent was obtained from all patients before being included in the study.
Data Availability
The data sets analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/D7FBF0601CCA76AD.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Pappas, D.A., Kremer, J.M., Griffith, J. et al. Long-Term Effectiveness of Adalimumab in Patients with Rheumatoid Arthritis: An Observational Analysis from the Corrona Rheumatoid Arthritis Registry. Rheumatol Ther 4, 375–389 (2017). https://doi.org/10.1007/s40744-017-0077-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-017-0077-z