Abstract
Introduction
This study aimed to determine the association between extra-articular manifestations (EAMs) and baseline characteristics of patients with ankylosing spondylitis (AS) and identify their potential risk factors in an observational cohort.
Methods
We analyzed the data of consecutive patients with AS obtained between April 2016 and May 2019 from the ongoing Chinese Ankylosing Spondylitis Prospective Imaging Cohort.
Results
Among the 1414 patients with AS, 23.1% had experienced EAMs at baseline. The prevalence rates of acute anterior uveitis (AAU), inflammatory bowel disease, and psoriasis among patients with AS were 16.7, 6.9, and 2.6%, respectively, and the prevalence of AAU increased significantly with the disease duration. Patients with comorbidity of AAU and psoriasis had Ankylosing Spondylitis Disease Activity Score (ASDAS) than patients without EAMs (2.16 ± 0.984 vs. 1.99 ± 0.956 [p = 0.025] and 2.36 ± 1.01 vs. 1.99 ± 0.96 [p = 0.025]). Among the 1087 patients with AS without EAMs at baseline, 98 developed EAMs during follow-up. Long disease duration (> 10 years) and high disease activity at baseline (ASDAS > 2.1) were associated with the risk of new-onset EAMs (hazard ratio [HR] [95% confidence interval, CI], 2.150 [1.229–3.762] and 2.896 [1.509–5.561], respectively) and new-onset AAU (HR [95% CI], 2.197 [1.325–3.642] and 3.717 [1.611–8.574], respectively).
Conclusions
In Chinese patients with AS, patients with comorbidity of AAU and psoriasis had higher disease activity scores than patients without EAMs. Furthermore, the risk of AAU or combined EAMs increases with the duration of AS and appears to be associated with higher cumulative exposure to inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Ankylosing spondylitis (AS) is a chronic inflammatory disease, which often has extra-articular manifestations (EAMs), including acute anterior uveitis (AAU), inflammatory bowel disease (IBD), and psoriasis. |
In China, patients with AS account for 0.3–0.5% of the total population, affecting more than 4 million Chinese individuals. |
EAMs are considered helpful for the diagnosis of axial spondyloarthritis (axSpA)/AS, and they are a part of the axSpA classification standard; currently, information regarding the characteristics and development of EAMs in patients with AS in China is limited. |
This study aimed to determine the characteristics related to the occurrence and development of EAMs in patients with AS and identify differences and potential risk factors for the clinical features at baseline between patients with new-onset AAU, IBD, or psoriasis, and those without EAMs. |
What has been learned from the study? |
Among the 1414 patients with AS, 23.1% had experienced EAMs at baseline; the prevalence rates of acute anterior uveitis (AAU), inflammatory bowel disease, and psoriasis among patients with AS were 16.7, 6.9, and 2.6%, respectively, and the prevalence of AAU increased significantly with disease duration. |
Among 1087 patients with AS without EAMs at baseline, 98 developed EAMs during follow-up, and long disease duration (>10 years) and high disease activity at baseline (ASDAS > 2.1) were associated with the risk of new-onset EAMs (hazard ratio [HR] [95% confidence interval, CI], 2.150 [1.229–3.762] and 2.896 [1.509–5.561], respectively) and new-onset AAU (HR [95% CI], 2.197 [1.325–3.642] and 3.717 [1.611–8.574], respectively). |
In Chinese patients with AS, patients with comorbidity of AAU and psoriasis had higher disease activity scores than patients without EAMs and the risk of AAU or combined EAMs increased with the duration of AS. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14046497.
Introduction
Ankylosing spondylitis (AS) is a chronic inflammatory disease, which can lead to functional damage and quality of life decline. It causes spinal bone fusion and new bone formation due to axial spine injury [1, 2]. Patients with AS often have extra-articular manifestations (EAMs), including acute anterior uveitis (AAU), inflammatory bowel disease (IBD), and psoriasis [3]. The inflammation can also affect the peripheral joints, skin, heart, lungs, eyes, and intestines [1, 2]. The prevalence of AS varies considerably globally [4]. Its prevalence in the Western population is 0.6%, with an estimated annual incidence of 3–7 per ten patients [5,6,7]. In China, patients with AS account for 0.3–0.5% of the total population [8, 9]. The disease affects more than 4 million Chinese individuals, mainly young and middle-aged men.
EAMs are considered helpful for the diagnosis of axial spondyloarthritis (axSpA)/AS [10], and they are a part of the axSpA classification standard [11, 12]. Therefore, it is important to understand the characteristics of EAM occurrence and development. Furthermore, the incidence of EAMs considerably varies with region and race [3]. Currently, information regarding the characteristics and development of EAMs in patients with AS in China is limited. This study aimed to determine the characteristics related to the occurrence and development of EAMs in patients with AS. We also aimed to identify differences and potential risk factors for the clinical features at baseline between patients with new-onset AAU, IBD, or psoriasis, and those without EAMs.
Methods
Patients and Study Design
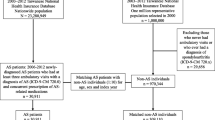
This study was a retrospective review of a prospectively collected database. We analyzed the data of the Chinese Ankylosing Spondylitis Prospective Imaging Cohort (CASPIC) [13], which is a nationwide, prospective, longitudinal, and state-funded cohort comprising both patients with peripheral SpA and those with axial SpA who fulfill the modified New York criteria for AS [14]. There was no other eligibility criterion for our cohort. Past history or current symptoms of AAU, psoriasis, or IBD were collected at baseline. The inclusion criteria were as follows: diagnoses of AS made according to the 1984 modified New York criteria [14]. Exclusion criteria pertained to the selected patients for the following: (1) patients who were unwilling to participate in the study and/or (2) did not sign the consent form. The first visit of patients with AS in the CASPIC was defined as baseline. The data were obtained between April 2016 and May 2019.
The study protocol was authorized by the Ethical Committee of the Chinese PLA General Hospital (S2016-049-02). Written informed consent was obtained from all patients before entry into the study. Our study complies with the Declaration of Helsinki.
EAMs
History or current symptoms of AAU, psoriasis, or IBD were collected at baseline. Patients with newly developed AAU, IBD, or psoriasis during follow-up were considered to have specific EAMs since definitive diagnosis. The diagnosis of IBD, AAU, and/or psoriasis was confirmed by ophthalmologists, gastroenterologists, and/or dermatologists, respectively, unless the patients provided previous medical records with a clear description of the relevant conditions and diagnosis. Moreover, the diagnosis of IBD was further confirmed by endoscopy and pathology.
Collected Variables and Disease Evaluation
The following patients’ details were collected at baseline: sex; age; height; weight; body mass index (BMI); onset time; disease duration; family history of AS, AAU, IBD, and psoriasis; EAMs (AAU, IBD, and psoriatic arthritis); smoking status; human leukocyte antigen B27 (HLA-B27) status with the flow cytometry method (FCM); peripheral arthritis; and enthesitis. Laboratory tests (C-reactive protein [CRP] and erythrocyte sedimentation rate [ESR]) and ASDAS [15, 16] were used for the objective evaluation of disease activity. When the CRP level was lower than the detection limit, a constant value of 2 mg/l was used to calculate the ASDAS [17]. The ASDAS were defined as follows: < 1.3, inactive disease; ≥ 1.3 and ≤ 2.1, low disease activity (LDA); > 2.1 and ≤ 3.5, high disease activity (HDA); and > 3.5, very high disease activity (VHDA) [18]. Blood was collected at the time of inclusion and each visit of patients. The Bath Ankylosing Spondylitis Functional Index (BASFI) (scale 0–10) [19] was used for the functional evaluation of AS, and the Bath Ankylosing Spondylitis Metrology Index (BASMI) [20] was used as a measure of spine and hip mobility. Spinal pain was evaluated using a 10-cm visual analog scale pain score (scale 0–10).
Definition of the Endpoint
The primary endpoint was defined as the occurrence of any new-onset EAMs (AAU, IBD, and psoriasis) during any follow-up visit.
Selection and Recording of the Treatment Strategies
According to disease activity, doctors and patients jointly determined the treatment strategies, without any other study intervention. The use of nonsteroidal anti-inflammatory drugs, conventional synthetic disease-modifying antirheumatic drugs, and tumor necrosis factor inhibitors (TNFi), including biosimilar etanercept (ETN; Yisaipu®; Sunshine Guojian Pharmaceutical Co., Ltd.; Shanghai, China) or adalimumab (ADA; AbbVie®, Ludwigshafen, Germany) was recorded at each visit.
Quality Control
For quality control, an interdisciplinary research team was established, comprising a rheumatologist, an ophthalmologist, a gastroenterologist, and a dermatologist. Before the study, we discussed and formulated a standardized research plan, and per the plan, we trained the members of the project team before patient recruitment, during treatment, and at follow-up of the study participants. Trained specialists educated patients and explained the questionnaires and evaluation contents to ensure the accuracy and comparability of patients’ self-evaluation questionnaires and scores. The inclusion, follow-up, and evaluation of patients with AS were completed by rheumatologists. The disease activity score and function measurement of patients with AS were evaluated by trained and relatively fixed specialists. Regular training sessions were organized to standardize measurement practices. We collected relevant disease information at each treatment and follow-up of patients. After each follow-up, the information was checked by a specially assigned person (JXJ). After completion and correction, the forms were uploaded to the system, and a logic error detection program was set up to detect and check possible logic errors in time.
Statistical Analyses
Continuous variables are presented as mean (standard deviation (SD) [5]), and dichotomous data are presented as frequency. The mean (SD) was used for normally distributed data, and a t test was used for intergroup comparisons of two independent samples. The mean (P25, P75) was used for non-normally distributed data, and the Wilcoxon signed-rank test was used for intergroup comparisons. Counting data are expressed as frequency, and the Chi-square test was used for intergroup comparisons.
The baseline characteristics associated with EAMs, AAU, IBD, and psoriasis were determined using univariate and multivariate logistic regression analyses. The rank-sum test was used to compare the disease activity (ASDAS) at baseline between combined EAMs and without EAMs patients with AS. Patients were excluded from the logical analysis if data about EAMs could not be retrieved. Only features in the univariate analysis with p < 0.20 were input in the (backward) multivariate analysis. In addition, collinearity and interaction were checked. Risk factor for EAMs, odds ratio (OR), hazard ratio (HR), and relative risk (RR) were used in the cross-sectional analysis at baseline and the longitudinal queue.
Survival analysis was used to calculate the incidence of EAMs during follow-up. The Cox regression analysis and Kaplan–Meier curve were used to determine the demographic and clinical characteristics associated with the new-onset of any type of EAMs. Patients were excluded from the Cox regression analysis if EAM data could not be retrieved. First, the baseline features and, subsequently, time-varying features at EAM diagnosis were used. For patients who were not diagnosed with EAMs, the baseline features were used. SPSS 23.0 (IBM Corp., Armonk, NY, USA) software was used for data analysis. Results with p < 0.05 were considered statistically significant.
Results
Baseline Characteristics of Patients
A total of 1414 patients with AS were included in the study. There were 327 patients with AS with EAMs (EAMs group) and 1087 without EAMs (without EAMs group) at baseline. All baseline demographic and clinical characteristics of patients were compared between the EAMs and without EAMs groups (Table 1). The average disease duration was 9.90 ± 6.58 and 7.80 ± 5.84 years in the EAMs and without EAMs groups, respectively. In addition, there were differences between the two groups in terms of age (32.73 ± 8.70 vs. 29.71 ± 8.57 years) and family history of AS (25.7 vs. 15.8%, p < 0.05). The scores of BASFI and ASDAS in the EAMs group were higher than those in the without EAMs group (1.74 ± 1.80 vs. 1.53 ± 1.60 and 2.17 ± 0.97 vs. 1.99 ± 0.96, respectively; p < 0.05). There was no significant difference in age, sex, HLA-B27-positive rate, BMI, peripheral arthritis, enthesitis, acute inflammatory index (ESR and CRP), and BASMI between the two groups (Table 1).
Cross-Sectional Analysis of EAMs in Patients with AS
Overall, 327 (23.1%) patients in the CASPIC had experienced EAMs before inclusion at baseline. At baseline, the prevalence rates of AAU, IBD, and psoriasis in patients with AS were 16.7, 6.9, and 2.6%, respectively. In the EAMs group at baseline, there were 200 patients with only AAU (61.2%), 62 with only IBD (20.0%), and 25 with only psoriasis (7.6%). There were 40 patients (12.2%) with overlapping EAMs. AAU along with IBD (28/40, 70%) was the most common combination in patients with overlapping EAMs. Furthermore, the three EAMs overlapped in 4/40 (10%) patients. At baseline, patients with a history of only AAU and overlap of EAMs were correlated with the duration of disease (p < 0.05), and 46.5% of patients with only AAU and 52.5% of patients with overlapping EAMs had a disease duration of > 10 years (Table 2 and Fig. 1).
Overall, 200 patients had experienced AAU at baseline. In these patients, the prevalence of AAU increased significantly with the disease duration (p < 0.001) (Fig. 2). Furthermore, patients with comorbidity of AAU and psoriasis had higher disease activity scores (ASDAS) at baseline than patients without EAMs (2.16 ± 0.98 vs. 1.99 ± 0.956 [p = 0.025] and 2.36 ± 1.01 vs. 1.99 ± 0.96 [p = 0.025]) (Fig. 3).
First, patients with EAMs were associated with age (OR 1.031; 95% confidence interval [CI], 1.011–1.051), long duration of AS (course of AS, > 10 years) (OR 1.597; 95% CI 1.134–2.249), and a family history of AS (OR 1.768; 95% CI 1.222–2.559) at baseline. Second, patients with a history of only AAU were associated with a long duration of AS (> 10 years) (OR 1.897; 95% CI 1.286–2.796), HLA-B27 positivity (OR 3.175; 95% CI 1.429–7.055), and a family history of AAU (OR 3.029; 95% CI 1.176–7.801) at baseline. Finally, patients with a history of only IBD were associated with age (OR 1.046; 95% CI 1.012–1.082) and a family history of IBD (OR 26.247; 95% CI 6.342–108.621) at baseline. However, unlike AAU, in patients with a history of only IBD, the HLA-B27-positive rate was lower (OR 0.435; 95% CI 0.216–0.875) than that in patients without EAMs at baseline. In the multivariate analysis, sex, BMI, peripheral arthritis symptoms, enthesitis, and functional indicators were not associated with a history of EAMs, only AAU, or only IBD. At baseline, the multivariate analysis was not performed for those with a history of psoriasis due to the low number of patients with psoriasis (n = 25) (Table 3).
Development of New-Onset EAMs
Among patients with AS without EAMs at baseline, excluding 267 patients with no follow-up information, the median follow-up period of 820 patients was 17.0 (1–33) months. Ninety-eight patients developed new-onset EAMs during the follow-up period: 68 (69%) developed AAU, 21 (22%) developed IBD, and nine (9%) developed psoriasis. Among patients with EAMs at baseline, excluding 46 patients with no follow-up information, the median follow-up period of 281 patients was 14.0 (1–32) months. Three patients developed other EAMs based on the history of EAMs at baseline: two patients with AAU developed IBD, and one patient with IBD developed psoriasis.
Baseline Characteristics Associated with New-Onset EAMs During Follow-Up
The new-onset EAMs (including AAU, IBD, and psoriasis) during the follow-up period were correlated with long disease duration (> 10 years) (HR 2.150; 95% CI 1.229–3.762), HLA-B27 positivity (HR 0.542; 95% CI 0.310–0.948), and HDA at baseline (ASDAS > 2.1) (the HRs [95% CI] of HDA and VHDA were 2.896 [1.509–5.561] and 5.536 [2.597–11.802], respectively). New-onset AAU during the follow-up period was correlated with long disease duration (HR 2.197; 95% CI 1.325–3.642) and HDA (the HRs [95% CI] of HDA and VHDA were 3.717 [1.611–8.574] and 6.562 [2.425–17.753], respectively) at baseline (p < 0.05). We did not conduct a multivariate analysis with IBD and psoriasis because there were only a few patients with these two diseases (n = 21 and n = 9, respectively). In addition, in the univariate analysis, TNFi treatment seemed to promote the development of new EAMs (HR 1.511; 95% CI 1.008–2.264), but no statistical significance was observed in the multivariate analysis (Tables 4 and 5).
Furthermore, a Kaplan–Meier curve was developed according to HLA-B27 positivity/negativity based on the outcome index of any new-onset EAMs. The incidence rate of new-onset EAMs in HLA-B27-positive patients was approximately half of that in B27-negative patients (RR 0.542; 95% CI 0.310–0.948; p = 0.032; Fig. 4).
A Kaplan–Meier curve was developed according to baseline disease activity (ASDAS ≤ 2.1/ > 2.1). The incidence rate of new-onset EAMs in patients with unstable disease control at baseline (HAD and VHDA, ASDAS > 2.1) was three times that in stable disease (ID and LDA, ASDAS ≤ 2.1) (RR 3.003; 95% CI 1.977–4.564; p < 0.001) (Fig. 5).
Discussion
Our results showed that in the cohort of patients with a relatively long duration of AS symptoms, a considerable number (23.1%) of patients had ≥ 1 EAMs at baseline. During the follow-up period, the patients often developed new EAMs. We found that the presence of EAMs, AAU alone, and combined EAMs (≥ 2 kinds of EAMs) in patients with AS was significantly correlated with the duration of AS. However, we did not find the same correlation for IBD and psoriasis.
In the baseline cross-sectional analysis, the history of AAU was associated with longer disease duration and older age. Vertically, the new onset of AAU was also related to longer disease duration and higher disease activity index of AS at baseline. Furthermore, the new onset of EAMs was observed more in HLA-B27-negative patients than in HLA-B27-positive patients with AS, and this may be attributed to the effect of patients with IBD in the cohort on the overall new-onset EAMs. This is because in the cross-sectional study at baseline, we found that HLA-B27-negative patients had a relationship with the history of IBD. In patients with psoriasis, no such association was found. This reflects the difference in inflammation accumulation and exposure period, and the degree of inflammation exposure may be relatively higher in patients with AAU and combined EAMs. We also found that the new onset of EAMs and AAU were related to a long duration of AS (> 10 years). Patients with a long duration of AS (> 10 years) have a 1.15-fold higher risk of the new onset of EAMs and a 1.197-fold higher risk of the new onset of AAU than do patients with AS with a disease duration of ≤ 5 years; this is similar to the findings of a clinical study [21]. Furthermore, we found that patients with a relatively HDA (ASDAS > 2.1) at baseline were more likely to develop any new-onset EAMs and AAU.
The risk of new EAMs in HLA-B27-positive patients with AS was small compared to that in HLA-B27-negative patients with AS. These findings were further confirmed using the Kaplan–Meier curve analysis. The risk of any new EAM in HLA-B27-positive patients with AS was 0.542 times higher than that in HLA-B27-negative patients. In patients with AS, HLA-B27 positivity may be a protective factor for new-onset EAMs. The risk of any new EAM in patients with AS with unstable disease control (HAD and VHDA, ASDAS > 2.1) was three times higher than that in patients with a relatively stable disease control (ID and LDA, ASDAS ≤ 2.1). In our study, the history of EAMs was not associated with peripheral arthritis and enthesitis, even in patients with IBD and psoriasis. This finding is contrary to that of Gaelle et al. [22], who reported that in patients without EAMs and those with psoriasis, there was a trend of increased joint swelling. The difference in the findings may be related to the ethnic differences in the subjects studied. A similar finding was also obtained in our previous study: in the CASPIC, the occurrence of peripheral arthritis and enthesitis was not related to the disease duration [13]. Additionally, in the present study, in the univariate analysis, patients with AS with a history of TNFi treatment seemed to be more likely to develop new EAMs, but no such result was obtained in the multivariate analysis. This may be related to the higher proportion of patients with AAU (69.4%) among those with new-onset EAMs. In the CASPIC, tumor necrosis factor receptor fusion protein was the main target component of TNFi [13]. It has been reported that the receptor fusion protein may induce AAU development [23, 24], explaining the result of our study. At the same time, we found that there was also new-onset AAU in patients with AS who used tumor necrosis factor monoclonal antibody (Supplementary Table S1). Our results are similar to a newly published cohort study of 175 patients with AS, which revealed that the receptor fusion protein does not elevate the risk of occurrence of uveitis compared with monoclonal TNF antibodies [25]. Although there was no evidence that tumor necrosis factor monoclonal antibody may promote the occurrence of AAU in patients with AS, it was still worthy of clinical attention and further research.
Patients with AS are more likely to develop AAU, IBD, and psoriasis than the general population. It has been reported that the cumulative incidence rate of AAU in the general population is 0.2–1.0% [3]. Furthermore, the prevalence of IBD is 0.01–0.5% and that of psoriasis is 0.3–2.5% in the general population [26, 27]. A meta-analysis of the results of 156 studies in more than 40,000 patients with AS showed that the prevalence rates of AAU, IBD, and psoriasis were 25.8, 6.8, and 9.3%, respectively [3]. Ivette et al. showed that the prevalence rates of AAU, IBD, and psoriasis at baseline were 18.0, 6.9, and 4.1%, respectively [21]. AAU is the most common EAM in patients with AS [3]. In our study, the prevalence rates of AAU, IBD, and psoriasis at baseline were 16.7, 6.9, and 2.6%, respectively, comparable to those reported by Ivette et al. [21]. In similar clinical studies, the prevalence of different EAMs in patients with AS or nr-axSpA in the DESIR cohort and GESPIC was comparable to that observed in our study [28, 29]. However, the prevalence of psoriasis was slightly lower in our study, which may be due to the difference in the race of the participants. Furthermore, previous studies have shown that the prevalence of AAU increased with disease duration in patients with AS [3, 30]. In a meta-analysis, the prevalence of AAU was related to the disease duration. In patients with AS with an average disease duration of < 10 and > 20 years, the prevalence rates of AAU were 17.4 and 38.5%, respectively [3]. A systematic review of 1989 patients with SpA showed that the prevalence of AAU increased with the disease duration [31]. This is similar to our finding in the CASPIC. At baseline, we found that the history of AAU and IBD was related to the prolongation of AS. However, in the multivariate analysis at baseline and follow-up longitudinal observation study, we found that only the occurrence of AAU was related to a long disease duration, whereas IBD did not show such a relationship. It should be noted that no such association with psoriasis was found in previous studies. This may be because psoriasis and IBD occurred before the onset or diagnosis of AS; however, this has not been proven. This is because EAMs are a part of the current criteria of axSpA and are considered helpful for the diagnosis of axSpA or AS [32, 33]. The relationship between AS and EAMs requires more attention in the future.
HLA-B27 is closely related to the pathogenesis of AS. Strong evidence supports that it is an independent risk gene for the pathogenesis of AS/SpA and that it is the strongest among all known HLAs [34]. There is evidence to show that HLA-B27 and IBD have a strong correlation, but whether there is a difference between the prevalence of HLA-B27-positive and HLA-B27-negative patients is still unclear [35] [36]. In a large-scale study, in 908 HLA-B27-positive and 90 HLA-B27-negative patients with AS, the relationship between HLA-B27 and IBD in patients with AS was analyzed [37]; 9% and 20% of the patients had IBD. This was confirmed in our study; HLA-B27-positive patients with AS had a greater risk of AAU occurrence and lower risk of IBD occurrence than the HLA-B27-negative patients. Our previous study results suggested a similar trend, but there was no statistical difference, which may be related to the small number of patients observed in the previous study (801 patients with AS) [38]. Although there was no statistical difference, the proportion of patients with IBD was different (the incidence rates of IBD in HLA-B27-positive and HLA-B27-negative patients with AS were 2.4 and 3.1%, respectively). Its clinical significance is worthy of attention. We should also pay attention to the possibility of IBD development in HLA-B27-negative patients with AS if they have obvious intestinal symptoms.
In our study, we compared patients with AS with and without EAMs, in whom of EAMs was related to their family history of AS. Patients with AAU or IBD alone showed a relation to their corresponding family history (family history of AAU or IBD). Furthermore, in the longitudinal analysis of new-onset EAMs, we found that any new-onset EAM was related to disease activity (ASDAS) at baseline. Although our findings suggested a link between EAMs and disease activity of AS (ASDAS), it did not necessarily describe causal relationships. The presence of IBD or AAU may also be a manifestation of cumulative exposure to systemic inflammation. Alternatively, patients with IBD or AAU may show more inflammatory activity before its occurrence. Therefore, we should also evaluate the changes in imaging and activity score to determine whether HDA and persistent inflammatory activity are risk factors for EAMs.
This study has several limitations. First, at baseline, we recorded EAMs in patients with AS by asking their medical history and reviewing the medical records of previous related diagnosis and treatment. However, 29 patients could not clearly describe their previous medical history or provide the examination and medical records of diagnosis, which may have led to an underestimation of the prevalence of EAMs. It also highlights the importance of consultation and physical examination of EAMs in daily practice. This can be achieved through some simple inquiries with doctors and then determining whether to recommend the patient to the corresponding specialist for diagnostic examination. Second, because of the relatively long disease duration at baseline, many patients (327/1414, 23.1%) already had EAMs, and we could not include them in the subsequent survival analysis. Among some early cohorts [28, 29] including our study, there was no study in which EAMs or axSpA/AS appeared before the other. This should be further analyzed in collaboration with ophthalmologists, gastroenterologists, and dermatologists. Furthermore, our research was based on real-world data without intervening with patients. Therefore, many patients were lost during the follow-up, the possible reasons for which include a change in long-term residence, transfer of patients to a community hospital for follow-up, and other uncertain factors. We analyzed the baseline characteristics of patients who were lost to follow-up, with no statistical difference; therefore, we believe that there was no significant choice bias (Supplementary Table S2). Finally, we did not discuss the evaluation of imaging, changes in disease activity, and inflammatory markers in this study. This will be supplemented and discussed in our follow-up study.
Herein, we systematically described and analyzed the characteristics and new development of EAMs in Chinese patients with AS. In particular, the occurrence of AAU was positively related to the prolongation of AS duration. Furthermore, HLA-B27-negative patients were more likely to develop IBD. The occurrence of EAMs was related to the increase in disease activity (ASDAS) in patients with AS.
Conclusions
In Chinese patients with AS, patients with comorbidity of AAU and psoriasis had higher disease activity scores than patients without EAMs. Furthermore, the risk of AAU or combined EAMs increased with the duration of AS and appears to be associated with higher cumulative exposure to inflammation. Our results may be a guide for clinical practice. In particular, our study suggests that it is important to closely monitor the disease activity of AS because patients with AS may be prone to EAMs.
References
Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007;369:1379–90. https://doi.org/10.1016/S0140-6736(07)60635-7.
Machado P, Landewé R, Braun J, et al. Both structural damage and inflammation of the spine contribute to impairment of spinal mobility in patients with ankylosing spondylitis. Ann Rheum Dis. 2010;69:1465–70. https://doi.org/10.1136/ard.2009.124206.
Stolwijk C, van Tubergen A, Castillo-Ortiz JD, et al. Prevalence of extra-articular manifestations in patients with ankylosing spondylitis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74:65–73. https://doi.org/10.1136/annrheumdis-2013-203582.
Dean LE, Jones GT, MacDonald AG, et al. Global prevalence of ankylosing spondylitis. Rheumatol (Oxf). 2014;53:650–7. https://doi.org/10.1093/rheumatology/ket387.
Geirsson AJ, Eyjolfsdottir H, Bjornsdottir G, et al. Prevalence and clinical characteristics of ankylosing spondylitis in Iceland—a nationwide study. Clin Exp Rheumatol. 2010;28:333.
Hanova P, Pavelka K, Holcatova I, et al. Incidence and prevalence of psoriatic arthritis, ankylosing spondylitis, and reactive arthritis in the first descriptive population-based study in the Czech Republic. Scand J Rheumatol. 2010;39:310–7. https://doi.org/10.3109/03009740903544212.
Kaipiainen-Seppanen O, Aho K, Heliovaara M. Incidence and prevalence of ankylosing spondylitis in Finland. J Rheumatol. 1997;24:496–9.
Jin J-y, Han S-l, Li K-p, et al. Epidemiological investigation of back pain and spondyloarthritis in Shougang resident communities. Zhonghua Nei Ke Za Zhi. 2010;49:832–5.
Ng SC, Liao Z, Yu DTT, et al. Epidemiology of spondyloarthritis in the People’s Republic of China: review of the literature and commentary. Semin Arthritis Rheum. 2007;37:39–47. https://doi.org/10.1016/j.semarthrit.2007.01.003.
Rudwaleit M, van der Heijde D, Khan MA, et al. How to diagnose axial spondyloarthritis early. Ann Rheum Dis. 2004;63:535–43. https://doi.org/10.1136/ard.2003.011247.
Rudwaleit M, van der Heijde D, Landewé R, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;2009(68):777–83. https://doi.org/10.1136/ard.2009.108233.
Rudwaleit M, van der Heijde D, Landewé R, et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. 2011;70:25–31. https://doi.org/10.1136/ard.2010.133645.
Ji X, Wang Y, Ma Y, et al. Improvement of disease management and cost effectiveness in Chinese patients with ankylosing spondylitis using a smart-phone management system: a prospective cohort study. Biomed Res Int. 2019;2019:2171475. https://doi.org/10.1155/2019/2171475.
van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984; 27:361–8. https://doi.org/10.1002/art.1780270401.
Lukas C, Landewé R, Sieper J, et al. Assessment of SpondyloArthritis international S. Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis 2009; 68:18–24. https://doi.org/10.1136/ard.2008.094870
van der Heijde D, Lie E, Kvien TK, Sieper J, et al. Assessment of SpondyloArthritis international S. ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Ann Rheum Dis 2009; 68:1811–8. https://doi.org/10.1136/ard.2008.100826.
Machado P, Navarro-Compán V, Landewé R, et al. Calculating the ankylosing spondylitis disease activity score if the conventional c-reactive protein level is below the limit of detection or if high-sensitivity c-reactive protein is used: an analysis in the DESIR cohort. Arthritis Rheumatol. 2015;67:408–13. https://doi.org/10.1002/art.38921.
Machado P, van der Heijde D. How to measure disease activity in axial spondyloarthritis? Curr Opin Rheumatol. 2011;23:339–45. https://doi.org/10.1097/BOR.0b013e3283470f23.
Garrett S, Jenkinson T, Kennedy LG, et al. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994;21:2286–91.
Jenkinson TR, Mallorie PA, Whitelock HC, et al. Defining spinal mobility in ankylosing spondylitis (AS). The Bath AS Metrology Index. J Rheumatol. 1994; 21:1694–1698.
Essers I, Ramiro S, Stolwijk C, et al. Characteristics associated with the presence and development of extra-articular manifestations in ankylosing spondylitis: 12-year results from OASIS. Rheumatol (Oxf). 2015;54:633–40. https://doi.org/10.1093/rheumatology/keu388.
Varkas G, Vastesaeger N, Cypers H, et al. Association of inflammatory bowel disease and acute anterior uveitis, but not psoriasis, with disease duration in patients with axial spondyloarthritis: results from two Belgian nationwide axial spondyloarthritis cohorts. Arthritis Rheumatol. 2018;70:1588–96. https://doi.org/10.1002/art.40551.
Nicolela Susanna F, Pavesio C. A review of ocular adverse events of biological anti-TNF drugs. J Ophthalmic Inflamm Infect. 2020;10:11.
Iwahashi C, Ono H, Haruta M, et al. New onset or exacerbation of uveitis with infliximab: paradoxical effects? BMJ Open Ophthalmol. 2019;4:e000250. https://doi.org/10.1136/bmjophth-2018-000250.
Choi EY, Lee M, Lee CS, et al. Uveitis occurrence in AS patients according to the type of anti-TNF-a cohort study of 175 patients. Clin Exp Rheumatol. 2020;38:1132–7.
Plunkett A, Marks R. A review of the epidemiology of psoriasis vulgaris in the community. Australas J Dermatol. 1998;39:225–32. https://doi.org/10.1111/j.1440-0960.1998.tb01478.x.
Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-e30. https://doi.org/10.1053/j.gastro.2011.10.001.
Rudwaleit M, Haibel H, Baraliakos X, et al. The early disease stage in axial spondylarthritis: results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum. 2009;60:717–27. https://doi.org/10.1002/art.24483.
Dougados M, d’Agostino M-A, Benessiano J, et al. The DESIR cohort: a 10-year follow-up of early inflammatory back pain in France: study design and baseline characteristics of the 708 recruited patients. Joint Bone Spine. 2011;78:598–603. https://doi.org/10.1016/j.jbspin.2011.01.013.
Wendling D, Prati C, Demattei C, et al. Impact of uveitis on the phenotype of patients with recent inflammatory back pain: data from a prospective multicenter French cohort. Arthritis Care Res (Hoboken). 2012;64:1089–93. https://doi.org/10.1002/acr.21648.
Zeboulon N, Dougados M, Gossec L. Prevalence and characteristics of uveitis in the spondyloarthropathies: a systematic literature review. Ann Rheum Dis. 2008;67:955–9. https://doi.org/10.1136/ard.2007.075754.
Rudwaleit M, Landewé R, van der Heijde D, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis. 2009;68:770–6. https://doi.org/10.1136/ard.2009.108217.
Rudwaleit M, van der Heijde D, Landewé R, Akkoc N, et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. 2011;70:25–31. https://doi.org/10.1136/ard.2010.133645.
Caffrey MF, James DC. Human lymphocyte antigen association in ankylosing spondylitis. Nature. 1973;242:121. https://doi.org/10.1038/242121a0.
van Lunteren M, Sepriano A, Landewé R, et al. Do ethnicity, degree of family relationship, and the spondyloarthritis subtype in affected relatives influence the association between a positive family history for spondyloarthritis and HLA-B27 carriership? Results from the worldwide ASAS cohort. Arthritis Res Ther. 2018;20:166.
Akkoç N, Yarkan H, Kenar G, et al. Ankylosing spondylitis: HLA-B*27-positive versus HLA-B*27-negative disease. Curr Rheumatol Rep. 2017;19:26. https://doi.org/10.1007/s11926-017-0654-8.
Feldtkeller E, Khan MA, van der Heijde D, et al. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int 2003; 23:61–6. https://doi.org/10.1007/s00296-002-0237-4.
Ji XJ, Sun K, Hu ZY, et al. Comparison of clinical manifestations according to HLA-B(27) genotype in ankylosing spondylitis patients: real-world evidence from smart management system for spondyloarthritis. Zhonghua Nei Ke Za Zhi. 2018;57:179–84. https://doi.org/10.3760/cma.j.issn.0578-1426.2018.03.006.
Acknowledgements
The authors are extremely grateful to the participants for their support. They appreciate the contributions of the present and former members of the CASPIC study group.
Funding
This was a state-funded cohort study supported by the Key Projects in the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period (2014BAI07B05) and the National Key Basic Research Program of China (973 program) (2014CB541806). The financial support was mainly used to develop the database system platform. The journal’s Rapid Service Fee for this paper was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
All authors (Siliang Man, Xiaojian Ji, Lidong Hu, Yiwen Wang, Yingpei Ma, Lei Wang, Jian Zhu and Feng Huang) declare that they have no financial, commercial, or academic conflicts of interest.
Compliance with Ethics Guidelines
Our study complies with the Declaration of Helsinki. Written informed consent was obtained from all patients before entry into the study. The study protocol was authorized by the Ethical Committee of the Chinese PLA General Hospital (S2016-049–02).
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author (F.H.) upon reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xiaojian Ji is the co-first author.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Man, S., Ji, X., Hu, L. et al. Characteristics Associated with the Occurrence and Development of Acute Anterior Uveitis, Inflammatory Bowel Disease, and Psoriasis in Patients with Ankylosing Spondylitis: Data from the Chinese Ankylosing Spondylitis Prospective Imaging Cohort. Rheumatol Ther 8, 555–571 (2021). https://doi.org/10.1007/s40744-021-00293-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-021-00293-0