Abstract
Introduction
Information is limited on the prevalence and clinical characteristics of nonradiographic axial spondyloarthritis (nr-axSpA) among patients with inflammatory back pain (IBP) in African countries. A global study estimated the prevalence of nr-axSpA among patients with IBP from 19 countries in Latin America, Europe, Asia, and Africa. This post hoc subset analysis focused on estimating prevalence of nr-axSpA and clinical characteristics among patients with IBP from Northwest Africa (Morocco and Algeria) and South Africa.
Methods
Patients from Northwest Africa and South Africa diagnosed with nr-axSpA according to protocol completed patient-reported outcome measures to assess disease activity and functional limitations, including Ankylosing Spondylitis Disease Activity Score (ASDAS).
Results
Of the 206 patients with IBP from Africa (n = 168, Northwest Africa and n = 38, South Africa), 33 (16.0%) were diagnosed with nr-axSpA (n = 26, Northwest Africa and n = 7, South Africa), corresponding to prevalence rates of 15.5% and 18.4%, respectively. Disease activity per region, measured as mean ASDAS, was 2.4 ± 1.4 and 2.4 ± 0.9, respectively, based on erythrocyte sedimentation rate and 2.4 ± 1.3 and 2.7 ± 0.7 based on C-reactive protein.
Conclusions
Although the number of patients available for the analysis was low, it appears that the prevalence of nr-axSpA among patients with IBP is similar between Northwest and South Africa, and the disease burden is substantial. Limited access to magnetic resonance imaging may hinder early detection in these areas, thereby affecting the assessment of prevalence.
Funding
Pfizer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spondyloarthritis (SpA) is a group of similar inflammatory diseases that includes psoriatic arthritis, ankylosing spondylitis (AS), reactive arthritis, and enteropathic arthritis [1]. SpA can be categorized into axial SpA (axSpA), wherein symptoms are mainly localized in the spine and sacroiliac joints, and peripheral SpA (including articular or enthesitic involvement), wherein symptoms are localized in the peripheral joints. In axSpA, chronic inflammatory back pain (IBP) is a common feature [2] and has a strong association with expression of human leukocyte antigen (HLA)-B27 [3].
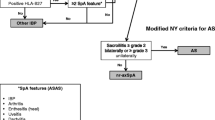
Several sets of criteria have evolved to classify patients with SpA [4]. In the modified New York criteria for assessing AS, conventional radiographs must show the patient to have at least grade II bilateral sacroiliitis or grade III unilateral sacroiliitis and at least one other clinical feature from the following: IBP, limited mobility of the lumbar spine, or limited chest expansion at the fourth intercostal space [5]. According to the 2009 criteria developed and validated by the Assessment of SpondyloArthritis international Society (ASAS) [6, 7], patients with radiographic evidence of sacroiliitis are diagnosed as having AS (referred to as radiographic SpA). Patients without radiographic evidence of sacroiliitis, but with evidence of sacroiliitis by magnetic resonance imaging (MRI) and IBP and/or HLA-B27 positivity, or without MRI evidence but HLA-B27 positive with two or more other clinical features of SpA are classified as having nonradiographic (nr)-axSpA. However, the diagnosis of nr-axSpA remains a challenge and newer and more sensitive methods using MRI for the evaluation of the sacroiliac joint have been developed [8, 9]. This progress in imaging may lead to earlier diagnosis and treatment of these patients, and may delay or prevent disease progression to radiographic axSpA or AS that would otherwise result in increased limitation of function [10].
Findings from a multinational, noninterventional, cross-sectional, epidemiologic study revealed significant variations across the globe in the prevalence of nr-axSpA among patients with IBP, with the highest prevalence reported in Asia (36.5%) and the lowest reported in Africa (16.0%) [11]. However, there is limited information on the prevalence and clinical characteristics of nr-axSpA among patients with IBP in individual African countries [12]. Understanding the prevalence of nr-axSpA in the various regions of Africa and the challenges involved in obtaining this information will provide a foundation for regional rheumatologists and public health agencies to develop programs for early detection and treatment of this disease in their countries. The objective of this post hoc analysis was to provide prevalence estimates of nr-axSpA among patients with IBP across two countries in Northwest Africa (Morocco and Algeria) and in South Africa.
Methods
Study Design and Patients
This report constitutes a post hoc subset analysis of a noninterventional, cross-sectional, epidemiological study that estimated the prevalence of nr-axSpA in patients with IBP from 19 countries in Africa, Asia, Europe, and Latin America [11]. The study was conducted in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Consecutive patients with chronic low back pain (CLBP) from 51 rheumatology outpatient public health clinics who met the criteria for IBP were evaluated for inclusion in the study [11]. Major inclusion criteria were age ≥ 18 years, CLBP ≥ 3 months, and at least four of the following: age of onset was < 40 years, insidious onset, improvement with exercise, no improvement with rest, nighttime pain. The exclusion criteria were: back pain that is non-inflammatory, a condition such as fibromyalgia that mimics IBP, a fever that persists, a neuropathic component, weight loss of more than 10 kg in the past 6 months that cannot be explained, numbness or weakness of the lower extremities bilaterally, urinary retention or incontinence, fecal incontinence or a decrease in anal sphincter tone, saddle anesthesia, or worsening neurologic decline.
Assessments
A medical record review using a case report form was conducted to determine whether patients met the criteria for nr-axSpA, or AS or other types of IBP, according to the ASAS criteria for axial SpA and the modified New York criteria for AS [11]. Patients diagnosed with nr-axSpA were asked to complete a survey to assess patient-reported outcomes. Disease activity was determined using the Ankylosing Spondylitis Disease Activity Score (ASDAS) based on erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP), and the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI).
Statistical Methods
The original epidemiologic study was powered so that the 95% confidence interval around the prevalence estimate for nr-axSpA would be ± 3.00% overall and ± 5.00–10.00% for a region. The actual total sample size was slightly lower than the assumed size (N = 974 vs. N = 981); however, this did not affect the overall statistical power. For this post hoc subset analysis, no sample size estimation was performed.
Data for binary/categorical variables were reported as frequencies, percentages, and 95% confidence intervals, and data for continuous variables were reported as counts, means, and standard deviations.
Results
Prevalence of nr-axSpA in Northwest Africa and South Africa
A total of 206 patients from Africa fulfilled ASAS IBP criteria, of whom 168 were from two countries in Northwest Africa (Morocco and Algeria) and 38 were from South Africa (Fig. 1). Of these patients, 33 (16.0%) were diagnosed with nr-axSpA; 26 patients from Northwest Africa and seven patients from South Africa. This corresponded to prevalence rates of nr-axSpA of 15.5 and 18.4%, respectively.
Demographic and Clinical Characteristics of Patients with nr-axSpA
Patients with nr-axSpA from Northwest Africa were a mean age of 34.2 ± 9.7 years, 42.3% were women, and 80.8% were of Arab ethnicity (Table 1). In South Africa, patients were a mean age of 45.9 ± 10.0 years, 57.1% were women, and 71.4% were white. The mean body mass index was 24.3 ± 3.9 and 26.3 ± 3.9 kg/m2 in Northwest and South Africa, respectively, and the corresponding mean age of IBP onset was 28.0 ± 8.6 and 29.9 ± 9.4 years. Patients experienced CLBP for a mean of 6.9 ± 9.3 years in Northwest Africa and 15.0 ± 12.9 years in South Africa. A total of 16 (69.6%) of 23 patients from Northwest Africa and two (40.0%) of five patients from South Africa had elevated CRP levels. Nine (37.5%) of 24 patients from Northwest Africa, but none from South Africa, had elevated ESR. Nine (64.3%) of 14 patients from Northwest Africa and six (85.8%) of seven patients from South Africa tested positive for HLA-B27 (Table 2).
Delay from IBP to nr-axSpA Diagnosis
There was a mean delay of 2.5 ± 2.4 years between the presentation of IBP and diagnosis of nr-axSpA among patients from Northwest Africa, based on data from six patients, and 14.5 ± 20.5 years for patients from South Africa, based on data from two patients (Table 2).
Patient-Reported Outcomes in nr-axSpA
Disease activity based on mean ASDAS-ESR and ASDAS-CRP for patients from Northwest Africa was 2.4 ± 1.4 and 2.4 ± 1.3, respectively (Fig. 2). The corresponding ASDAS scores in patients from South Africa were 2.4 ± 0.9 and 2.7 ± 0.7. The mean overall BASDAI scores were 3.8 ± 2.5 and 4.8 ± 2.6 in patients from Northwest Africa and South Africa, respectively.
Reported outcomes across Northwest and South African patients with nr-axSpA. ASDAS Ankylosing Spondylitis Disease Activity Score, AS ankylosing spondylitis, BASDAI Bath Ankylosing Spondylitis Disease Activity Index, BASFI Bath Ankylosing Spondylitis Functional Index, BASMI Bath Ankylosing Spondylitis Metrology Index, CRP C-reactive protein, ESR erythrocyte sedimentation rate, nr-axSpA non-radiographic axial spondyloarthritis
Discussion
In this subset analysis, the prevalence of nr-axSpA was similar among patients with IBP from Northwest Africa (Morocco and Algeria) and South Africa, but there was a higher proportion of men among those from Northwest Africa vs. South Africa (57.7 vs. 42.9%). The higher percentage of men vs. women with nr-axSpA in Northwest Africa differs from results of other studies [13, 14]. It is possible that this difference is due to a higher likelihood of men seeking medical help in that region.
There was an apparent difference in the mean number of years between presentation of IBP and diagnosis of nr-axSpA among patients from the two regions (Northwest Africa, 2.5 years; South Africa, 14.5 years), and they both differed from the mean value of 5 years obtained in the global study [11]. However, these data should be interpreted with caution, as they are based on very low numbers of patients with data available (six from Northwest Africa and two from South Africa). Although MRI facilities are widely available in Algerian, Moroccan, and South African hospitals, access to these facilities is limited for some patients and this may have contributed to the low numbers [12].
Similarly, the difference in HLA-B27 test status between Northwest Africa and South Africa, where results were available for 54 and 100% of patients, respectively, (compared with 71% of the entire sample of patients with nr-axSpA) [11] should be interpreted with caution, due to the small number of patients. Even though access to MRI and/or HLA-B27 testing may be challenging in developing countries, education on recognizing and diagnosing nr-axSpA can help increase rates of earlier diagnosis.
Clinical and patient-reported outcomes as assessed by the ASDAS and BASDAI scores, respectively, revealed high levels of disease activity, suggesting suboptimal disease control in both regions. Disease management strategies may vary among African countries. For instance, a study conducted in Morocco found that all patients had used nonsteroidal anti-inflammatory drugs, with phenylbutazone having been used in 15.4% of patients [15]. However, only 52.6% of patients received conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) (sulfasalazine or, less often, methotrexate) and only 2.6% were treated with antitumor necrosis factor (TNF) therapies [15]. Algeria has a public healthcare system in which the majority of the population has access to csDMARDs. However, patient access to biologic therapies is limited, as they are only available for free in public hospitals [16]. Few patients are treated with TNF inhibitors, due to a lack of access and an increased risk of malaria associated with anti-TNF treatment [17].
A limitation of this post hoc subset analysis is that it included a small number of patients. The populations of Algeria and Morocco together represent approximately only one-third of the population of Northern Africa [18]. Although the population of South Africa constitutes almost 90% of the population of Southern Africa, conditions vary widely in the region [18]. Therefore, the extent to which the patients included in this subset analysis are representative of the wider population of patients with nr-axSpA in Africa, or even Northern and Southern Africa, is not clear. Additionally, as this was an observational, cross-sectional study, there was limited longitudinal patient information for further analyses. Although the use of questionnaires and case report forms is a standard procedure for collecting data and patient assessments, this may not provide a comprehensive medical history for each patient.
Another limitation of this analysis is that the ASAS criteria have not been validated in Northern and Southern Africa and may not be the ideal method for diagnosing nr-axSpA in these areas [19]. Differences between these regions and western countries in the genetic background of the patients and in environmental factors such as sanitation and infection may result in a different presentation of the disease [19, 20].
Conclusions
Despite the limitations, this post hoc subset analysis provides insight into the prevalence of nr-axSpA among patients with IBP in Northwest Africa and South Africa. Larger studies evaluating the epidemiology, diagnosis, and treatment of nr-axSpA are needed to have a clear understanding of prevalence rates in these African regions.
References
van Tubergen A, Weber U. Diagnosis and classification in spondyloarthritis: identifying a chameleon. Nat Rev Rheumatol. 2012;8(5):253–61. https://doi.org/10.1038/nrrheum.2012.33.
Rudwaleit M, van der Heijde D, Khan MA, Braun J, Sieper J. How to diagnose axial spondyloarthritis early. Ann Rheum Dis. 2004;63(5):535–43. https://doi.org/10.1136/ard.2003.011247.
Braun J, Bollow M, Remlinger G, et al. Prevalence of spondylarthropathies in HLA-B27-positive and negative blood donors. Arthritis Rheum. 1998;41(1):58–67.
Akgul O, Ozgocmen S. Classification criteria for spondyloarthropathies. World J Orthop. 2011;2(12):107–15. https://doi.org/10.5312/wjo.v2.i12.07.
van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27(4):361–8.
Rudwaleit M, Landewe R, van der Heijde D, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis. 2009;68(6):770–6. https://doi.org/10.1136/ard.2009.108217.
Rudwaleit M, van der Heijde D, Landewe R, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68(6):777–83. https://doi.org/10.1136/ard.2009.108233.
Slobodin G, Eshed I. Non-radiographic axial spondyloarthritis. Isr Med Assoc J. 2015;17(12):770–6.
Braun J, Baraliakos X, Kiltz U, Heldmann F, Sieper J. Classification and diagnosis of axial spondyloarthritis—what is the clinically relevant difference? J Rheumatol. 2015;42(1):31–8. https://doi.org/10.3899/jrheum.130959.
Boonen A, van der Linden SM. The burden of ankylosing spondylitis. J Rheumatol Suppl. 2006;78:4–11.
Burgos-Varga R, Wei JC, Rahman MU, et al. The prevalence and clinical characteristics of nonradiographic axial spondyloarthritis among patients with inflammatory back pain in rheumatology practices: a multinational, multicenter study. Arthritis Res Ther. 2016;18(1):132. https://doi.org/10.1186/s13075-016-1027-9.
Hammoudeh M, Abdulaziz S, Alosaimi H, et al. Challenges of diagnosis and management of axial spondyloarthritis in North Africa and the Middle East: an expert consensus. J Int Med Res. 2016;44(2):216–30. https://doi.org/10.1177/0300060515611536.
Kiltz U, Baraliakos X, Karakostas P, et al. Do patients with non-radiographic axial spondylarthritis differ from patients with ankylosing spondylitis? Arthritis Care Res (Hoboken). 2012;64(9):1415–22. https://doi.org/10.1002/acr.21688.
Wallman JK, Kapetanovic MC, Petersson IF, Geborek P, Kristensen LE. Comparison of non-radiographic axial spondyloarthritis and ankylosing spondylitis patients–baseline characteristics, treatment adherence, and development of clinical variables during three years of anti-TNF therapy in clinical practice. Arthritis Res Ther. 2015;17(1):378. https://doi.org/10.1186/s13075-015-0897-6.
El Mansouri L, Bahiri R, Abourazzak FE, Abouqal R, Hajjaj-Hassouni N. Two distinct patterns of ankylosing spondylitis in Moroccan patients. Rheumatol Int. 2009;29(12):1423–9. https://doi.org/10.1007/s00296-009-0873-z.
Slimani S, Abbas A, Ben Ammar A, et al. Characteristics of rheumatoid arthritis in Algeria: a multicenter study. Rheumatol Int. 2014;34(9):1235–9. https://doi.org/10.1007/s00296-014-2981-7.
Haid S, Teniou I, Magateli A, Abdessemed N, Brahimi N, Ladjouze-Rezig A. Anti-TNF et spondyloarthrite: quel risque infectieux? Revue du Rheumatisme. 2015;82S:A357.
Population Reference Bureau. 2017 World Population Reference Sheet. https://www.prb.org/wp-content/uploads/2017/08/2017_World_Population.pdf. Accessed Apr 2018.
Lebughe P, de Vlam K, Westhovens R, Mbuyi-Muamba J-M, Malemba JJ. Spondyloarthritis in the Democratic Republic of the Congo: a prospective hospital-based study. BMJ Open. 2018;8(5):e020329. https://doi.org/10.1136/bmjopen-2017-020329.
Cao K, Moormann AM, Lyke KE, et al. Differentiation between African populations is evidenced by the diversity of alleles and haplotypes of HLA class I loci. Tissue Antigens. 2004;63(4):293–325. https://doi.org/10.1111/j.0001-2815.2004.00192.x.
Acknowledgements
The authors wish to thank the participants of the study.
Funding
Sponsorship for this study and article processing charges was provided by Pfizer (New York, NY). All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing, Editorial and Other Assistance
Medical writing support was provided by Paul Oakley and Mukund Nori, PhD, MBA, of Engage Scientific Solutions, and was funded by Pfizer.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Khalid Shirazy was an employee of Pfizer at the time the article was written. Constance Hammond is an employee of Pfizer and owns stock or stock options in Pfizer. Heather Jones is an employee of Pfizer and owns stock or stock options in Pfizer. Ron Pedersen is an employee of Pfizer and owns stock or stock options in Pfizer. Bonnie Vlahos is an employee of Pfizer and owns stock or stock options in Pfizer. Najia Hajjaj-Hassouni has received financial support for symposia attendance from Pfizer, AbbVie, Cooper-Pharma, Roche, Janssen, UCB-France and Sothéma. Aicha Ladjouze Rezig has nothing to disclose.
Compliance with Ethics Guidelines
This study was conducted in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Data Availability
Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the US and/or EU or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.6850685.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Shirazy, K., Hajjaj-Hassouni, N., Hammond, C. et al. The Prevalence of Non-radiographic Axial Spondyloarthritis Among Patients with Inflammatory Back Pain from Northwest and South Africa: Data from a Noninterventional, Cross-Sectional Study. Rheumatol Ther 5, 437–445 (2018). https://doi.org/10.1007/s40744-018-0122-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-018-0122-6