Abstract
Tribo corrosion control of 6061 aluminum alloy was done by using naturally available Boswellia serrata (BWS) extract as green inhibitor. Studies were done in artificial sea water slurry containing 0.03% sand particles via a submerged jet impingement technique. Electrochemical studies were the majour techniques adopted for corrosion rate measurement in the absence and in the presence of inhibitor. Investigation was done at different flow rates and at different temperatures. Detailed surface study was done for sand particles before and after impingement using scanning electron microscope. Surface morphology of aluminum sample was done before and after the addition of inhibitor. For a given flowrate inhibition efficiency decreased with increase in temperature. This suggested the possibility of physical adsorption. Results were fitted into suitable adsorption isotherm. Mechanism of adsorption was supported and substantiated by quantum chemical calculations using Density Functional Theory (DFT). The best corrosion–erosion resistance was obtained at a concentration of 1000 ppm Boswellia serrata at 303 K under 4 L min−1. DFT studies supported the adsorption mechanism of the inhibitor on the surface of metal under tribological conditions. The outcome of this work will help in research that is focused on development on green inhibitors for corrosion inhibition under dynamic multi flow conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Tribo-corrosion or erosion–corrosion is a combined process involving metal removal through erosion and electrochemical corrosion. It is detrimental to industrial equipment leading to high operating and maintenance costs [1]. The effect of erosion–corrosion is generally higher as compared to individual processes due to their synergistic effects [2]. Aluminum is plentifully available metal on the earth’s crust, and finds its application in the design of new material because of its advantages like low-cost manufacturing, fully known fabrication techniques and compelling design prospect. Nevertheless, pure aluminum is relatively soft limiting its application range. Therefore, its alloys are developed with enhanced mechanical proper-ties [3]. 6061 aluminum alloy is extensively applied in automotive, aircraft, marine, offshore applications, railway, the infrastructure works, and the construction fields because of their excellent strength-to-weight ratio, good corrosion resistance and low thermal expansion coefficient [4,5,6].
Many devices and machine elements comprising 6061 aluminum alloys display lower resistance to wear and erosion. Therefore, the application of 6061 aluminum alloy s is restricted in the areas involving fluid flow [7]. In order to improve the erosion resistance and tribological characteristics and of aluminum alloys, several protective coatings are being used [8].
The application of corrosion inhibitor is a concrete way to protect these metals from corrosion and has gained importance in industrial applications [9]. Chemical inhibitors are known to be efficient in decreasing metal dissolution, nevertheless its use is minimal due to the environmental hazard involved. Several research works on corrosion inhibition by amino acids, plant extracts, polymers, drugs, and other chemical substance have been performed [10,11,12,13]. Therefore, the current studies have been dedicated to using cost effective, toxin free, and biodegradable corrosion inhibitors [14]. Plant extracts are excellent inhibitors, however, as known, low-to-medium protection efficiency at increased concentrations of inhibitor is one of the limiting factors ascribed to its engagement as corrosion inhibitors [15].
The latest development of Density Functional Theory (DFT) in the advancement of quantum electrochemistry is substantial, and could be associated with the accomplishments in the late 1980s where hybrid functional and gradient-corrected techniques were familiarized. Hohenberg–Kohn was bestowed with the Nobel Prize in physics for his theorem on DFT in 1964. Among quantum chemical techniques for assessment of corrosion inhibitors, DFT comes with few advantages and appears to be appropriate for acquiring the necessary facts causing the inhibitory action [16]. DFT focusses upon electron density r(r), itself as the carter of the complete information in molecule ground state. Overall, the Hohenberg–Kohn theorem launches the foundation of an electronic system. Previous research by our group involved studying the effect of hydrodynamic on corrosion inhibition by plant extracts [17,18,19].
Detailed adsorption study for erosion corrosion and quantum chemical calculation for the molecules used under tribological condition is highly limited. Through this work, we are reporting adsorption behavior of the inhibitor under tribological condition and support it by evaluation of quantum chemical descriptors.
2 Methodology
2.1 Experimental Arrangement
An experimental set up was fabricated to perform the experiments. Schematic is represented in Fig. 1. Fluid was circulated in the flow loop with the help of a centrifugal pump. The water velocity can be varied with the help of the bypass valve and the flowrate is measured with the help of a rotameter. The material used for the components of the recirculation flow loop was corrosion resistant 316 L stainless steel. Type K thermocouple was used for temperature sensing, whereas 0.25 hp pump (Massflow Engineers (Chennai)) was used for fluid pumping.
2.2 Preparation of Test Coupon
The specimen for electrochemical studies was developed using 6061 aluminum alloy. Its composition is mentioned in ST1 .
The erosion corrosion samples size was 12 mm x 15 mm. The alloy was implanted in epoxy resin excluding a working extent of 1.1 cm2. The electrode surface was cleaned with emery papers of 600 grade and finally polished with disc polisher. Finally, they were cleaned with acetone, distilled water.
2.3 Test Procedure
Erosion corrosion tests were performed in synthetic seawater slurry in blank and in the presence of Boswellia Serrata (BWS). The tank was filled with around 16 L of the test solution. The 6061 aluminum alloy sample was fixed on the holder inside the test case such that the slurry impinged directly on the sample ensuring an impact angle of 90°. Nozzle with 8 mm diameter was chosen for slurry impingement. The distance between the sample and the nozzle was 5 mm. The erosion corrosion test rig was connected to the electrochemical work station. Erosion corrosion and inhibition studies were undertaken by electrochemical methods.
2.4 Electrochemical Studies
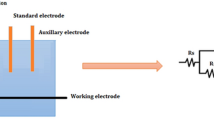
Electrochemical studies were conducted with Electrochemical Workstation (CH600 D-series, U.S. model-beta software). Three electrode test cell consisted of 6061 aluminum alloy—working electrode, platinum electrode-auxiliary electrode, and saturated calomel electrode-standard electrode.
2.4.1 Potentiodynamic Polarization (PDP) Studies
Initially open-circuit corrosion potential was determined by allowing the test specimen to be in contact with the test solution for 1500 s. 6061 aluminum alloy was then polarized by applying -500 mV cathodically and + 500 mV anodically pertaining to OCP with a scan rate of 1 mV/ sec.
2.4.2 Electrochemical Impedance Spectroscopy (EIS) Studies
EIS studies have been performed at a sinusoidal potential input of ± 10 mV amplitude. The frequency spectrum selected for the study ranged between 10 kHz and 10 mHz. The impedance data were analyzed with the Nyquist spectra.
Potentiodynamic polarization studies were conducted post electrochemical impedance measurement bereft additional surface treatment. Each of the experiments was repeated for at least 3–4 trials and the mean of most approving value was reported. Studies were conducted both with and without the presence of Boswellia serrata under differing experimental conditions.
2.5 Corrosion Inhibitor: Boswellia Serrata (BWS)
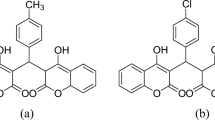
Boswellia serrata is a large sized tree belonging to family Burseraceae and grows in hilly regions of India, Middle East and Northern Africa [20]. The genus Boswellia consists of around 25 species dispersed in India, the Northeastern coast of Africa, and Arabia [21]. The oleo gum-resin comprises of 30–60% resin, 5–10% essential oils, and the remainder with polysaccharides. The resinous part of Boswellia serrata includes monoterpenes, diterpenes and triterpenes, tetracyclic triterpenic acids and the 4 important pentacyclic triterpenic acids which include β-boswellic acid. The extract (Boswellia serrata) was acquired from, Ms Revinto Life sciences Pvt. Ltd. Karnataka, India. Molecular structure of Boswellic acid is given in Fig. 2.
3 Results and Discussion
3.1 Potentiodynamic Polarization Studies (PDP)
PDP studies were performed at various flow rates of artificial slurry 4 L min−1, 8 L min−1, and 12 L min.−1, at various temperatures 303 K, 313 K, 323 K using varying concentration of inhibitors in the range of 500 to 1000 ppm. PDP plots in presence of 1000 ppm BSW extract, at different temperature and at different flow rate of slurry is given in Fig. 3. Inhibition efficiency was calculated using Eq. (1)
Observations indicated increase in corrosion potential and corrosion current density (icorr) with increase in the flow rate of slurry. The higher flow rate and increased particle kinetic energy results in the damage and removal of the naturally formed shielding layer on the metal surface. This is a consequence of de-passivation caused by the particles of sand. De-passivation is predominant phenomena in the absence of inhibitor. The sand particles disrupt the layer and expose fresh metal to the corrosive environment. When the inhibitor was present in the slurry the corrosion current density decreased.
The experimental temperature and the flow rate are major determining factors in assessing tribological behavior of the material. Liquid impingement at elevated flowrates, with or without sand particle resulted in decrease in the inhibition efficiency. Same was the observation when flow rate of the slurry increased at a given temperature. In accordance with studies of Aribo et al. [22], it is as a consequence of impact of flow rate and higher temperature on the inhibitor film formation. At elevated temperature, ductile mechanisms like cutting and plastic deformation are supported and the corrosion activities are accelerated even in the absence of optimum concentration of inhibitor [23].
Shift in the corrosion potential toward the positive side indicated that added inhibitor was more anodic in nature. Results of PDP measurements are consolidated in Tables 1 and 2.
3.2 Electrochemical Impedance (EIS) Studies
Electrochemical Impedance Spectroscopy is used for estimating the electrochemical reactions in the medium and the metal interface. The Nyquist spectrum is the general representation of the process.
The impedance is a complex number with an imaginary component, Zimaginary and a real component, Zreal [24]. As perceived from Fig. 4, Nyquist plot indicates depressed capacitive semicircles at higher frequency domain, because of the charge shift alongside the corrosion product layers. It is worth noting that the semicircle diameter expands with a dropping flowrate, indicating the much larger impedance at lower flowrates after the addition of the inhibitor [25]. The variation on the flowrate does not affect the shape of the loops, signifying that no change in the corrosion mechanism of 6061 AA has occurred.
The circuit displayed in the Fig. 5 [R(C(R(Q(R(CR)))))] -involves 7 components. After the multiphase flow grooves and holes are generated on the sample and hence EC model assumes that the surface is not a homogeneous layer but defective [26]. Therefore, the presence of BSW offers a reliable circuit signifying that the film of inhibitor developed on the metal surface is imperfect [27]. Various heterogeneities prevailing over the alloy surface contribute to the depressed semicircle and therefore a BSW is introduced to the equivalent circuit in place of a pure capacitor [28]. R1 represents the resistance of the porous layer since it is continuously exposed to the electrolyte. It may be also to use as solution resistance amidst the pores of the layer which depends on the defective coating formation. R2 denotes the resistances of the inner compact layer of the passive film.
3.3 Evaluation of Kinetic Parameters
The influence of temperature on corrosion phenomena is useful in obtaining kinetic parameters namely energy of activation (Ea), enthalpy of activation (\(\Delta {H}_{a})\), and entropy (\(\Delta {S}_{a})\) of activation. Ea was calculated by Arrhenius and Transition state equation as given by Eq. (2) and (3) respectively
T denotes temperature, R for universal gas constant (8.314 JK−1 mol−1), N is Avogadro's number (6.022 × 1023 mol−1), h is the Planck's constant (6.626 × 10–34 Js) Kinetic parameters are given in S T2.
Energy of activation in case of an inhibited solution is greater as compared to that of the blank solution. Further these values increased at higher inhibitor concentrations. This clearly indicated the barrier formed by the inhibitor molecules for the continuous metal degradation. This could be attributed to the physical adsorption occurring at the initial stage. Higher activation energy depicts the significant decrease in the inhibitor adsorption on the 6061 aluminum alloy with the rise in temperature. Desorption of the inhibitor takes place along with the increase in its adsorption since both are reverse processes at equilibrium. Because of this desorption process, more surface of the alloy gets in contact with the medium producing higher corrosion rates alongside an increase in temperature [29].
3.4 Adsorption Considerations
The obtained results were tried to fit in several adsorption isotherms but the best fit was observed in Langmuir isotherm given by Eq. (4).
It is shown schematically in Fig. 6
The intercept of the isotherms gives an estimate of adsorption equilibrium constant (K). Standard free energy of adsorption (\(\Delta {G^\circ }_{ads})\) value was gained from Eq. (5)
R = universal gas constant (8.314 J mol─1 K─1), T = absolute temperature.
The extent of protection from corrosion is dependent on the surface area covered by the adsorption of Boswellia serrata molecules. The amount of the adsorbed Boswellia serrata molecules grows over the surface with an expanding Boswellia serrata concentration, resulting in enhanced protection. The surface coverage parameter, θ, specifies the fraction of 6061 aluminum alloy covered with the inhibitor molecules. Examining Table 2, a conclusion is drawn that θ (surface coverage) shows an upward trend with the rising Boswellia serrata concentration. Boswellia serrata impedes 6061 aluminum alloy corrosion at each experimental temperature. The physical method of adsorption of Boswellia serrata molecules over the 6061 aluminum alloy layer in artificial sea water slurry is established by the remark which suggests that the protection efficiency decreases with the rising medium temperature. With the decrease in the slurry temperature, desorption of H2O molecules from the alloy layer is more feasible, causing a spike in the adsorption of Boswellia serrata molecules with the accessibility to greater surface area [15, 30,31,32]. The standard enthalpy (\(\Delta H^\circ )\) and standard entropy of adsorption (\(\Delta S^\circ )\) were estimated by plotting \(\Delta G^\circ\) against T using the following equation
The plot of \(\Delta {G^\circ }_{ads}\) vs. T is given in Fig. 7, and the estimated thermodynamic parameters are mentioned in ST3.
The spontaneity associated with the phenomena and the firmness in the adsorbed film layer over the surface is ensured by negative ΔG° values [26, 33, 34]. ΔG° values of − 40 kJ mol−1 or greater include charge transfer or sharing of the inhibitor molecules with the surface forming coordinate bond (chemisorption) and the order − 20 kJ mol−1 or lesser indicates physisorption [35, 36]. The obtained ΔG data is less than − 20 kJ mol−1 which implies that the adsorption mechanism considered for the study of Boswellia Serrata on 6061 aluminum alloy in artificial seawater is common in physisorption. Physical adsorption of the inhibitor is confirmed by an enthalpy of adsorption value less than zero, which is a characteristic of exothermic reaction. Negative value for the parameter - entropy of activation suggests an increase in the orderliness during the process of adsorption.
3.5 Surface Morphology Studies
Surface morphology of sand particles before and after impingement with the material is shown in the Fig. 8 and Fig. 9 under different magnifications.
There was not much change observed in the surface roughness and sharpness of the sand particles. Hence, it can be concluded that the sand was not degraded during the course of the experiment. Even though sand particles have not gone much morphological changes, their kinetic energy was enough to remove the passivated layer on the surface of 6061 aluminum alloy resulting in accelerated corrosion.
F 9 SEM images (a) Fresh surface alloy (b) Corroded surface (c) Surface coated with BWS.
[(a)And (b) Ref: Lavanya et.al https://doi.org/https://doi.org/10.24874/ti.2018.40.04.04].
The formation of pits, deep craters, and indentations were observed. However, platelets and lips formation is minimal. One of the explanations may be that under erosion corrosion conditions for 6061 aluminum alloy, electrochemical corrosion mechanism dominates over mechanical erosion process. Corrosion attacks the weakened layer at the eroded surface thus exposing the indented area. Hutchings found that erosion of ductile metals at normal incidence occurs by delamination. According to literature, at high impact angles brittle materials fracture whereas heavy plastic deformation is experienced by the surface of ductile materials.
The corrosion pits have a large size of about 100 μm. The erosion pits formed by the jet impacts have sizes of a few micrometers. The formation of corrosion products inside and around the pit is a characteristic of the corrosion pit. It has a large size and shallow which is characterized by traces of particle removal. The erosion pit has a small size of about 10 μm or less. The added inhibitor is expected to cover the pits and the craters formed due to impingement. There will be physical barrier formation which prevents further deterioration of the metal. The surface of 6061 aluminum alloy in presence of BWS is smooth compared to other inhibitors. This is in agreement with the results obtained by PDP results.
3.6 Mechanism of adsorption
The adsorption of the inhibitor molecules was associated to the Langmuir isotherm. The monolayer adsorption is shown in Fig. 1. Here, the procedure of adsorption occurs on the alloy surface comprising a specified amount of adsorption sites. All of these sites are engaged by the inhibitor [37]. The inhibitor molecules and metal substrate interact with each other, without any interaction among the inhibitor molecules [38]. A majority of the organic inhibitors getting adsorbed on the surface of the metal can be articulated using this Eq. (7) [39]
where—water molecules superseded by inhibitor molecules upon adsorption over alloy surface, CI(aq) and CI(AA)—inhibitor molecules in the bulk solution and corrosion inhibitor molecules that have been adsorbed on 6061 aluminum alloy respectively. H2O(s) is water molecule adsorbed over the 6061 aluminum alloy. As the processes of adsorption and desorption of corrosion inhibitor reaches equilibrium, there exists a balance among the coverage ratio (θ) and inhibitor concentration. This expression can be articulated with the adsorption isotherm.
3.7 Quantum Chemical Calculations
Quantum chemical techniques have already established their usefulness in defining the molecular structure and explicating the electronic form along with the reactivity which might be useful in developing new and highly efficient corrosion inhibitors [40]. High value of EHOMO denotes the ability to donate electrons. Whereas, low EHOMO values are related to the capacity to receive electrons from a donor [41]. From Koopman’s theorem, the energies of the molecular orbital of the frontier molecular orbital, could be associated to the ionization potential and the electron affinity as per the Eqs. (8) and (9) [42].
The electronegativity and hardness can be computed from the above parameters as per the Eqs. (10) and (11) [43, 44]
where χ denotes the absolute electronegativity, η represents the chemical hardness, and σ depicts the global softness which is the reciprocal of hardness. The electronegativity value is an indicator of the electron transfer that occurs among the alloy and the inhibitor. This value is used to explicate the reactivity in inorganic and organic chemistry. It is also a parameter applied to describe the inhibitory properties [45].
The nucleophilicity (ε), electrophilicity index (ω), can be calculated using Eq. (12) [46]
Calculated values of chemical hardness (η) and electronegativity (χ) permits the assessment of fraction of electron transferred [47]. The electronic properties of the Boswellic acid were calculated by density function theory (DFT). The details of HOMO and LUMO investigation for Boswellic acid molecules were displayed in Fig. 10.
The calculated quantum chemical parameters were displayed in ST4.
The capacity of Boswellic acid molecule toward adsorption is explained by the difference between EHOMO and ELUMO. EHOMO of molecule represent electron donating capacity and ELUMO represent electron accepting capacity. The difference between ELUMO and EHOMO represents the overall binding capacity of the molecule. Binding energy of Boswellic acid has E equal to 1.0885 eV. This demonstrates the greater interaction between the inhibitor molecule and metal surface.
The inhibitor molecule that has a lowest value of electro negativity (χ) shows exceptional inhibition efficiency. Among various quantum chemical descriptors, dipole moment of the inhibitor molecule plays decisive role in the adsorption process. Dipole moment of water is 1.88 Debye. Calculated value of dipole moment of inhibitor molecule is 2.5895 D. This clearly demonstrated the polar nature of molecule which can easily replace water molecule and get itself adsorbed on the metallic surface.
4 Conclusion
-
Increased temperature and flow rate lead to greater erosion corrosion rate.
-
Inhibition efficiency of BWS decreased with flow rate of slurry and with temperature
-
Maximum efficiency of 70% could be achieved for the addition of 1000 ppm of BWS at 303 K for the flow rate of slurry 4 L min.−1
-
EIS studies proved the mechanism of erosion corrosion which is charge transfer controlled as well as diffusion controlled
-
BWS obeyed Langmuir adsorption isotherm and followed physical adsorption
-
Surface morphology studies supported the adsorption of inhibitor onto the metal surface.
-
Quantum chemical calculations supported the mechanism of adsorption.
Data availability
Data will be available upon request to the corresponding author.
References
Dong H, Qi P-Y, Li XY, Llewellyn RJ (2006) Improving the erosion–corrosion resistance of AISI 316 austenitic stainless steel by low-temperature plasma surface alloying with N and C. Mater Sci Eng A 431:137–145.
Zhang GA, Xu LY, Cheng YF (2009) Investigation of erosion–corrosion of 3003 aluminum alloy in ethylene glycol–water solution by impingement jet system. Corros Sci 51:283–290
Liu P, Zhang QH, Ji-Ming HU, Cao FH (2021) Interesting phenomena for Al-Zn-Mg aluminum alloy after two years of storage: a comparative study on microstructure, mechanical properties and corrosion behavior of aluminum alloy with different aging treatments. Const Build Mater 276:122210
Zhang T, Wang J, Zhang G, Liu H (2020) The corrosion promoting mechanism of Aspergillus niger on 5083 aluminum alloy and inhibition performance of miconazole nitrate. Corros Sci 176:108930
Wang D, Miao Wu, Ming J, Shi J (2021) Inhibitive effect of sodium molybdate on corrosion behaviour of AA6061 aluminium alloy in simulated concrete pore solutions. Const Build Mater 270:121463
Quebbou Z, Chafi M, El Hachemi L, Omari, (2021) Corrosion resistance of 5005 aluminum alloy by anodizing treatment in a mixture of phosphoric and boric acids. Mater Today Proc 37:3854–3859
Szkodo M, Stanisławska A, Komarov A, Bolewski Ł (2021) Effect of MAO coatings on cavitation erosion and tribological properties of 5056 and 7075 aluminum alloys. Wear 474:203709
Hou G, An Y, Zhao X, Zhou H, Chen J, Li S, Liu X, Deng W (2017) Improving interfacial, mechanical and tribological properties of alumina coatings on Al alloy by plasma arc heat-treatment of substrate. Appl Surf Sci 411:53–66
Dakeshwar Kumar V (2018) Density functional theory (DFT) as a powerful tool for designing corrosion inhibitors in aqueous phase. Advanced engineering testing. IntechOpen, London, p 89
Ogwo KD, Osuwa JC, Udoinyang IE, Nnanna LA (2017) Corrosion inhibition of mild steel and aluminium in 1 M hydrochloric acid by leaves extracts of Ficus sycomorus. Phys Sci Int 2017:1–10
Verma DK, Khan F (2016) Green approach to corrosion inhibition of mild steel in hydrochloric acid medium using extract of spirogyra algae. Green Chem Lett Rev 9:52–60
El-Awady AA, Abd-El-Nabey BA, Aziz SG (1992) Kinetic-thermodynamic and adsorption isotherms analyses for the inhibition of the acid corrosion of steel by cyclic and open-chain amines. J. Electrochem Soci 139:2149
Verma C, Quraishi MA, Kluza K, Makowska-Janusik M, Olasunkanmi LO, Ebenso EE (2017) Corrosion inhibition of mild steel in 1M HCl by D-glucose derivatives of dihydropyrido [2, 3-d: 6, 5-d′] dipyrimidine-2, 4, 6, 8 (1H, 3H, 5H, 7H)-tetraone. Sci Rep 7:1–17
Basik M, Mobin M, Shoeb M (2020) Cysteine-silver-gold Nanocomposite as potential stable green corrosion inhibitor for mild steel under acidic condition. Sci Rep 10:1–12
Mobin M, Rizvi M, Olasunkanmi LO, Ebenso EE (2017) Biopolymer from Tragacanth gum as a green corrosion inhibitor for carbon steel in 1 M HCl solution. ACS Omega 2:3997–4008
Lashkari M, Arshadi MR (2004) DFT studies of pyridine corrosion inhibitors in electrical double layer: solvent, substrate, and electric field effects. Chem phys 299:131–137
Lavanya M, Ramachandra Murthy V, Rao P (2020) Erosion corrosion control of 6061 aluminum alloy in multi-phase jet impingement conditions with eco-friendly green inhibitor. Chin J Chem Eng 28:340–347
Lavanya M, Murthy R, Rao P (2019) Performance evaluation of a potent green inhibitor on 6061 aluminum alloy under liquid/solid jet impingement. J Bio Tribo Corros 5:1–10
Lavanya M, Murthy R, Rao P (2021) An insight into inhibitory performance of Commiphora Mukul on corrosion of aluminum alloy under tribological conditions. J Iran Chem Soc 18:1–11.
Siddiqui MZ (2011) Boswellia serrata, a potential antiinflammatory agent: an overview. Ind J Pharm Sci 73:255
Kishor RN, Parama D, Banik K, Bordoloi D, Devi AK, Thakur KK, Padmavathi G (2019) An update on pharmacological potential of boswellic acids against chronic diseases. Int J Mol Sci 20:4101
Aribo S, Fakorede A, Ige O, Olubambi P (2017) Erosion-corrosion behaviour of aluminum alloy 6063 hybrid composite. Wear 376:608–614
Mesa DH, Toro A, Tschiptschin AP (2003) The effect of testing temperature on corrosion–erosion resistance of martensitic stainless steels. Wear 255:139–145
Huh J-H, Kim SH, Chu JH, Kim SY, Kim JH, Kwon S-Y (2014) Enhancement of seawater corrosion resistance in copper using acetone-derived graphene coating. Nanoscale 6:4379–4386
Li Q, Feng Z, Liu L, Hong Xu, Ge W, Li F, Maozhong, (2015) Deciphering the formation mechanism of a protective corrosion product layer from electrochemical and natural corrosion behaviors of a nanocrystalline zinc coating. RSC Adv 5:32479–32490
Emran KM, Ahmed NM, Torjoman BA, Al-Ahmadi AA, Sheekh SN (2014) Cantaloupe extracts as eco friendly corrosion inhibitors for aluminum in acidic and alkaline solutions. J Mater Environ Sci 5(6):1940–1950
Shetty SK, Shetty AN (2017) Eco-friendly benzimidazolium based ionic liquid as a corrosion inhibitor for aluminum alloy composite in acidic media. J Mol Liq 225:426–438
Wei NY, Jiang Z, Liu Y, Ying X, Guo Y, Wu Y, Yang W (2018) 4-Phenylpyrimidine monolayer protection of a copper surface from salt corrosion. RSC Adv 14:7340–7349
Emran KM (2015) Effects of concentration and temperature on the corrosion properties of the Fe–Ni–Mn alloy in HCl solutions. Res Chem Intermed 41(6):3583–3596
Qiang Y, Li H, Lan X (2020) Self-assembling anchored film basing on two tetrazole derivatives for application to protect copper in sulfuric acid environment. J Mater Sci Tech 52:63–71
Nethaji S, Sivasamy A, Mandal AB (2013) Adsorption isotherms, kinetics and mechanism for the adsorption of cationic and anionic dyes onto carbonaceous particles prepared from Juglans regia shell biomass. Int J Env Sci Tech 10(2):231–242
Radovanović M, Mihajlović MP, Tasić Ž, Simonović A, Antonijević M (2021) Inhibitory effect of L-Threonine and L-Lysine and influence of surfactant on stainless steel corrosion in artificial body solution. J Mol Liq 342:116939
Ishak A, Adams FV, Madu JO, Joseph IV, Olubambi PA (2019) Corrosion inhibition of mild steel in 1M hydrochloric acid using Haematostaphis barteri leaves extract. Procedia Manuf 35:1279–1285
Li H, Zhang S, Qiang Y (2021) Corrosion retardation effect of a green cauliflower extract on copper in H2SO4 solution: Electrochemical and theoretical explorations. J Mol Liq 321:114450
Fakrudeen SP, Murthy C, Bheema R (2012) Corrosion inhibition of AA6061 and AA6063 alloy in hydrochloric acid media by Schiff base compounds. J Chil Chem Soc 57(4):1364–1370
Wang C, Zou C, Cao Y (2021) Electrochemical and isothermal adsorption studies on corrosion inhibition performance of β-cyclodextrin grafted polyacrylamide for X80 steel in oil and gas production. J Mol Struc 1228:129737
Chauhan DS, Madhan Kumar A, Quraishi MA (2019) Hexamethylenediamine functionalized glucose as a new and environmentally benign corrosion inhibitor for copper. Chem Eng Res Des 150:99–115
Udayappan B, Veawab A (2022) Performance analysis of methionine as an environmentally friendly corrosion inhibitor for carbon steel in the amine based carbon capture process. Int J Greenh Gas Control 114:103565
Umoren SA, AlAhmary AA, Gasem ZM, Solomon MM (2018) Evaluation of chitosan and carboxymethyl cellulose as ecofriendly corrosion inhibitors for steel. Int J Bio Macromol 117:1017–1028
Oguike RS, Kolo AM, Shibdawa AM, Gyenna HA (2013) Density functional theory of mild steel corrosion in acidic media using dyes as inhibitor: adsorption onto Fe (110) from gas phase. Int Sch Res Notices 2013:175910
Zinad DS, Hanoon M, Salim RD, Ibrahim SI, Al-Amiery AA, Takriff MS, Kadhum, (2020) A new synthesized coumarin-derived Schiff base as a corrosion inhibitor of mild steel surface in HCl medium: gravimetric and DFT studies. Int J Corros Scale Inhib 9:228–243
Pais M, Rao P (2021) Electrochemical, spectroscopic and theoretical studies for acid corrosion of zinc using glycogen. Chem Pap 75(4):1387–1399
Mubarik A, Rasool N, Hashmi MA, Mansha A (2021) Computational study of structural, molecular orbitals, optical and thermodynamic parameters of thiophene sulfonamide derivatives. Crystals 11:211
Krishtal A, Senet P, Van Alsenoy C (2009) Local softness, softness dipole, and polarizabilities of functional groups: application to the side chains of the 20 amino acids. J Chem Phys 131:044312
Tüzün B, Bhawsar J (2021) Quantum chemical study of thiaozole derivatives as corrosion inhibitors based on density functional theory. Arab J Chem 14:102927
Jordaan MA, Ebenezer O, Mthiyane K, Damoyi N, Shapi M (2021) Amide imidic prototropic tautomerization of efavirenz, NBO analysis, hyperpolarizability, polarizability and HOMO–LUMO calculations using density functional theory. Comput Theor Chem 1201:13273
Sastri VS, Perumareddi JR (1997) Molecular orbital theoretical studies of some organic corrosion inhibitors. Corrosion 53:617–622
Acknowledgements
Dr. Lavanya Mulky acknowledges the laboratory facility provided by Department of Chemistry, MIT Manipal.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. This project is not funded.
Author information
Authors and Affiliations
Contributions
Dr. Lavanya Mulky: Conceptualisation, experimental and manuscript writing. Dr. Padmalatha Rao: Validation and finalisation of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mulky, L., Rao, P. Tribo-Corrosion Control with Molecules of Bio-Origin: Experimental Studies and Theoretical Insights. J Bio Tribo Corros 10, 46 (2024). https://doi.org/10.1007/s40735-024-00846-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-024-00846-0