Abstract
Erosion–corrosion of 6061 aluminum alloy was attenuated with a green inhibitor Boswellia serrata (BWS) under submerged jet impingement condition by using artificial seawater slurry. Erosion–corrosion rates in the absence and in the presence of inhibitor were achieved by potentiodynamic polarization techniques (PDP). Experiments were performed under the varying concentration of inhibitor at different flow rates and temperatures. Conditions were optimized to obtain maximum inhibition efficiency. Mechanistic aspects of the corrosion and inhibition process were studied in detail by the electrochemical impedance spectroscopy (EIS) technique by correlating the data with appropriate equivalent circuit models. Adsorption of inhibitor was confirmed by surface morphology studies using scanning electron microscopy technique (SEM). Suitable mechanism was proposed for corrosion inhibition process. The inhibition efficiency increased with an increase in its concentration and it decreased with an increase in the flow rate and temperature. The inhibition efficiency of 70% was obtained for 1000 ppm of inhibitor at 303 K at the flow rate of 4 L min−1. It was proved that the mechanism of corrosion inhibition under this tribological condition is charge transfer controlled. The effect of hydrodynamics on the inhibitor efficiency of Boswellia serrata extract was remarkable. Boswellia serrata emerged as an efficient green inhibitor of erosion–corrosion control of 6061 aluminum alloy under submerged jet impingement conditions.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Erosion–corrosion has drawn key attention due to the material loss caused in many engineering applications and flow handling devices such as valves, slurry pumps, and turbines [1, 2]. During erosion, particles entrained in the process fluid strike the wall of the surface leading to a material loss [3]. During corrosion, electrochemical reactions destroy the surface of the material [4]. Erosion–corrosion is one among four types of flow-induced corrosion [5].
In various medium, aluminum alloys are known for erosion–corrosion (E–C). Therefore, the hydrodynamic properties of a fluid medium affect corrosion and its inhibition [6].
The exclusive properties provided by aluminum and aluminum alloys place it among the most versatile, attractive, and economical materials for a variety of applications. In contrast to iron rust, the aluminum oxide layer does not chip off to expose the surface to further reaction. If the protective oxide layer is damaged, it will rapidly reseal itself. Properly alloyed aluminum has the property to resist corrosion by salt, water, and other factors [7,8,9].
The most accepted way to mitigate internal corrosion is by the use of chemical inhibitors [10,11,12,13,14,15]. Chemical inhibitors are organic heterocyclic compounds. The heteroatoms present in the molecule act as electron-rich centers and form a covalent bond with the material. Thus, there will be barrier film formation, which will prevent further dissolution of metals. However, chemical inhibitors are not only expensive, but some are poisonous also. They can have disastrous consequences on the environment. Therefore, industries need to develop alternative solutions. In this regard, green inhibitors have gained much attention. Plant products, ionic liquids, biopolymers are some classes of green inhibitors. Plant products have received the greatest attention in recent years for protecting various classes of metals and alloys against corrosion. A lot of literature reports are available stating the use of plant products as green inhibitor for corrosion mitigation in aluminum and aluminum alloys under static conditions [16]. However, only a few studies have been reported on applying plant products for corrosion control of aluminum alloys under dynamic conditions [17].
As a part of our continuous effort to introduce a new green inhibitor for corrosion mitigation of materials under static and dynamic conditions [18,19,20,21], present work is an effort to introduce Boswellia serrata (BWS) extract as a possible green inhibitor for corrosion mitigation of 6061 aluminum alloy used in heat exchange application. This research work concentrates on studying the hydrodynamic effects on Boswellia serrata and also investigating its efficacy in mitigating erosion–corrosion.
2 Experimental
2.1 Preparation of artificial seawater
Erosion–corrosion was conducted in simulated seawater slurry containing sea sand. The simulated seawater was prepared as per the ASTM D1141-98 standards [22]. The suspended solids in the slurry were sea sand of size 300 μ. Sand selection was done because the industrial processes involving seawater for cooling are usually contaminated with sand. Throughout the experiment, 0.3% sand concentration was maintained. The pH of the artificial seawater was around 8–8.5.
2.2 Erosion–corrosion test rig
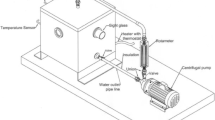
An experimental setup was fabricated locally to carry out the research work. A schematic representation of the experimental rig is shown in Fig. 1. Fluid was circulated through a flow loop with the help of a centrifugal pump. The setup was equipped with a nozzle (8 mm diameter) to obtain the required velocity. The water velocity was varied with the help of a bypass valve. The rig consisted of a sample holder where the specimen was fixed at 90° impingement angle. The material used for the components of the recirculation flow loop was corrosion-resistant 316 L stainless steel. Fluid velocity was measured using a rotameter. Type K thermocouple was used for temperature sensing, whereas a 0.25 hp pump (Massflow Engineers (Chennai)) was used for fluid pumping.
2.3 Preparation of test coupon
The electrode for electrochemical studies was made of 6061 aluminum alloy. The composition of it is given in Table 1.
The specimen preparation included operations like cutting and grinding. The erosion–corrosion samples were 12 mm in diameter and 15 mm in length. The machined alloy was embedded in epoxy resin exposing a working area of 1.1 cm2. The circular surface of the electrode was polished using emery papers of different grades (600–1200). A mirror-polished surface was obtained by treating the metal surface in a polishing machine using levigated alumina as abrasive. The 6061 aluminum alloy specimen was cleaned with acetone, distilled water.
2.4 Test procedure
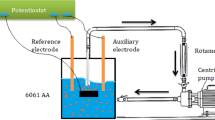
Erosion–corrosion tests were performed in artificial seawater slurry in the absence and presence of Boswellia serrata (BWS) extract. The tank was filled with around 16 L of artificial seawater solution. The 6061 aluminum alloy sample was fixed on the holder inside the test case under an 8 mm nozzle for slurry impingement. Erosion–corrosion experimental runs were carried out at various velocities (4 L min−1, 8 L min−1, and 12 L min−1) and temperatures (303 K, 313 K, and 323 K). The slurry impingement on the target alloy was at a 90° angle. The distance between the sample and the nozzle was 5 mm. The erosion–corrosion test rig was connected to the potentiostat. The auxiliary electrode (Polished platinum), standard reference electrode (saturated calomel electrode), and the working electrode (6061 aluminum alloy) were immersed in the test solution. Erosion–corrosion and inhibition studies were done by electrochemical methods.
2.5 Electrochemical studies
Electrochemical studies were performed by using Electrochemical Workstation (CH600 D-series, U.S. model with beta software). Potentiodynamic polarization (PDP) and electrochemical impedance (EIS) were performed as per the reported literature (Umoren et al. 2010). The working electrode was 6061 aluminum alloy and a platinum electrode was used as the auxiliary electrode. All the studies were carried out with respect to the potential of the saturated calomel electrode.
2.6 Corrosion inhibitor: Boswellia serrata extract
Boswellia serrata extract contains terpenes, amino acids, resin, phenols, β-boswellic acid, and polysaccharides [23]. Boswellic acid present in the extract provides beneficial effects to health. These organic acids include a pentacyclic triterpene, a carboxyl group. An additional hydroxyl group is present in both alpha-boswellic acid and beta-boswellic acid; they vary in their triterpene arrangement. The exudate obtained from Boswellia serrata is an oleo-gum-resin, which chiefly contains an acid resin, essential oil, and water-soluble gum. In the present investigation, the gum portion of the plant Boswellia serrata, was used. It contains various polysaccharides such as arabinose, xylose, galactose, and d-glucuronic acid [24]. Boswellia serrata extract was procured from Ms. Revinto Life sciences Pvt. Ltd., Karnataka.
Figure 2 shows the chemical arrangement of alpha-boswellic acid [25].
2.7 Scanning electron microscope (SEM)
Surface morphology studies were carried out using an analytical scanning electron microscope (JEOL JSM-6380L). SEM images of sand particles were recorded before and after impingement. The surface morphology of 6061 aluminum alloy was studied before and after the addition of the inhibitor.
3 Results and discussion
3.1 Potentiodynamic polarization (PDP) studies
Potentiodynamic polarization measurements were carried out for E–C of 6061 aluminum alloy in artificial seawater slurry in the presence of 500 ppm and 1000 ppm of Boswellia serrata extract (BWS). Potentiodynamic plots are given for 1000 ppm Boswellia serrata extract (BWS) in Fig. 3a–c. at the temperature of 303 K, 313 K, and 323 K.
Various electrochemical parameters like corrosion current density (icorr), corrosion potential (Ecorr), and tafel slopes were obtained from polarization plot.
The corrosion inhibition efficiency is calculated by Eq. (1)
where η = inhibition efficiency, icorr bl = corrosion current density of the solution without inhibitor (µA cm−2), and icorr inh = corrosion current density of the solution with inhibitor (µA cm−2).
If the difference between the Ecorr value of the blank and in presence of the inhibitor varies more than ± 85 mV, the inhibitor is said to be cathodic or anodic [26]. If the shift happens to be less than ± 85 mV then the inhibitor is said to behave like a mixed inhibitor, where the inhibitor controls both the anodic and the cathodic reactions to different levels. The effects of fluid flow on the inhibitor performance vary with flow conditions. With the rise in the slurry flow rate, there was an intensification in the corrosion current density at all three temperatures of 303 K, 313 K, and 323 K. On one hand, hydrodynamic conditions with high turbulence and shear stress will remove the adsorbed inhibitor complex, resulting in a low inhibition efficiency, and on the other hand, the flow of fluid promotes the mass transport of inhibitor molecules, which facilitates inhibitor molecules to reach the electrode surface. The balance between these effects leads to the difference in the inhibition effect of the inhibitor [27]. The increase in corrosion current density indicated that the corrosion resistance of the metal is decreased. This condition may be attributed to the fact that roughness and in-homogeneity increase corrosion rate. As the flow impinges at the target, the sand particles and the liquid jet itself may create scratches, scar and make the surface more rough [28]. The percentage inhibition efficiency decreased when temperature varied from 303 to 323 K. Corrosion rate (CR) increased with increasing temperature while decreasing the inhibitor efficiency. At higher temperature inhibitor molecules rearrange or decompose themselves following desorption further increasing corrosion rate [24]. A lower inhibitor efficiency was a consequence of a decrease in the strength of adsorption on the surface and the desorption of the inhibitor molecules due to the roughening of the alloy surface. A rise in the temperature leads to the weakening of the adsorption forces between the inhibitor and the metal. Stable corrosion product film is formed at higher temperature, whereas the film formed at lower temperature has a higher porosity and is less stable [28]. Figure 4 explains the variation of inhibition efficiency of BWS concerning flow rate and temperature.
It is believed that the corrosion protection ability of the organic inhibitor is achieved via adsorption of its molecules on the aluminum surface through some adsorptive centers present in the inhibitor structure such as heteroatoms (i.e., oxygen from the heterocyclic moiety) and/or functional groups (i.e., hydroxyl functional group) [29]. However, its reduction is less pronounced than that observed at lower temperatures. As stated above, the inhibition of the cathodic reaction may be due to the formation of hydrogen bonds between the inhibitor and the metal surface. It is well known that the hydrogen bond is weaker than the chemical bond and that it generally weakens with increasing temperature due to larger thermal motion. Therefore, the increase in temperature will enhance the metal surface kinetic energy, which has an adverse effect on the adsorption process and encourages desorption processes [29].
Tables 2 and 3 display the results obtained from potentiodynamic polarization measurements for E–C of 6061 aluminum alloy in artificial seawater slurry with 500 ppm and 1000 ppm of inhibitor Boswellia serrata, respectively. Higher Inhibition efficiencies were obtained when the concentration of the inhibitor was raised to 1000 ppm. The protection offered by the inhibitor for the metal corrosion reduced as the flow rate increased. This trend remained the same when aluminum alloy was tested for erosion–corrosion at two different concentrations of the inhibitor.
3.2 Electrochemical impedance spectroscopy (EIS) studies
Two capacitive loops and a diffusive tail characterize the Nyquist plots in Fig. 5. The diffusive tail was observed in the low-frequency region. The diameter of the capacitive loop increased with a drop in the flow rate decreased, following the same trend as the inhibition efficiency. Similar results were obtained by other researchers [30]. It is a known fact that at higher flow rates the mass transfer coefficient is higher. At higher fluid flow rates, corrosive species could reach the metal surface faster resulting in a lower charge transfer resistance [31].
3.3 Equivalent circuit models
The χ2 value reveals the quality of fitting used in the equivalent circuit. The obtained value of χ2 indicates that the proposed circuit is fitted properly [24]. In the current study, χ2 value ranged between 10–3 and 10–4.
The nested circuits indicate that pores in the film can be a source of corrosion by providing zones where the electrolyte has direct contact with the metal surface. When pores are present within the inhibitor film, the active species in the electrolyte can move through the pores to approach the surface of the metal. The electrochemical processes in the presence of inhibitors is charge transfer controlled.
Equivalent circuits retaining constant phase elements (CPEs) are frequently used to fit Nyquist spectra because the related distribution of time constants offers an improved fit. Though equivalent circuits with three-time constants were employed in fitting the EIS spectra, only two semi-circles can be observed in the Nyquist spectra. It may be a consequence of overlapping the peaks due to the intricacy of the reactions associated with the effect of flow on the metal surface. The broadening of the peak angles for the filmed samples toward the high-frequency region also indicates the presence of a duplex corrosion product film [32, 33].
In the Equivalent circuit depicted in Fig. 6b and d, Rs indicates the solution resistance, R1—the porous film resistance, R2—the barrier layer resistance, and R3—the charge transfer resistance. The barrier layer resistance is normally high as compared to the porous layer resistance because of its compactness and non-porous nature [34, 35].
The Warburg element is not found in this circuit (Fig. 6b) indicating the blocking of the pores due to the inhibitor molecules or corrosion products.
3.4 Bode phase plots
Bode phase plots are an additional feature of EIS data. A characteristic of the Bode phase plot is its ability to identify the predominant electrical behavior of the system in a given frequency range [35].
The increased values of phase angle for an inhibited specimen are shown in Fig. 7. Increased values of phase angle indicate the predominance of the capacitive contribution in the overall impedance at this frequency range. The broadening of the single maximum peak further supports the protective film formation by inhibitor molecules [36].
3.5 Surface morphology studies
SEM micrographs of the sand before and after the impingement are shown in Figs. 8 and 9. There was not much change observed in the surface roughness and sharpness of the sand particles. Hence, it can be concluded that the sand was not degraded during the experiment.
The formation of pits and indentations was observed. This may be due to the fact that under erosion–corrosion conditions for 6061 aluminum alloy, electrochemical corrosion mechanism dominates mechanical erosion process. Corrosion attacks the weakened layer at the eroded surface thus exposing the indented area. Hutchings found that erosion of ductile metals at normal incidence occurs by delamination. According to literature, at high impact angles brittle materials fracture, whereas heavy plastic deformation occurs on the surface of ductile materials [36]. As shown in Fig. 10, after the addition of inhibitor, the surface becomes remarkably smooth due to the adsorption of the inhibitor.
4 Conclusions
-
The inhibition efficiency of the inhibitor decreased with an increase in flow rate and temperature of artificial seawater slurry
-
The inhibition efficiency increased with an increase in the inhibitor concentration
-
The maximum inhibition efficiency of 70% was obtained for the addition of 1000 ppm of inhibitor at 303 K at the flow rate of 4 L min−1
-
Mechanism of inhibition was found to be charge transfer controlled.
-
Boswellia serrata emerged as an efficient green inhibitor of erosion–corrosion control of 6061 aluminum alloy under submerged jet impingement conditions.
-
There is an effect of hydrodynamics on the inhibitor efficiency of Boswellia serrata extract.
References
Zheng ZB, Zheng YG (2016) Erosion-enhanced corrosion of stainless steel and carbon steel measured electrochemically under liquid and slurry impingement. Corros Sci 102:259–268
Yi JZ, Hu HX, Wang ZB, Zheng YG (2018) Comparison of critical flow velocity for erosion–corrosion of six stainless steels in 3.5 wt% NaCl solution containing 2 wt% silica sand particles. Wear 416:62–71
Nguyen VB, Liu ZG, Wan S, Lim CYH, Zhang YW (2014) A combined numerical–experimental study on the effect of surface evolution on the water–sand multiphase flow characteristics and the material erosion behavior. Wear 319(1–2):96–109
Mahdi E, Rauf A, Eltai EO (2014) Effect of temperature and erosion on pitting corrosion of X100 steel in aqueous silica slurries containing bicarbonate and chloride content. Corros Sci 83:48–58
Heitz E (1991) Chemo-mechanical effects of flow on corrosion. Corrosion 47(2):135–145
Ashassi-Sorkhabi H, Asghari E (2010) Electrochemical corrosion behavior of Al7075 rotating disc electrode in neutral solution containing l-glutamine as a green inhibitor. J Appl Electrochem 40(3):631–637
Wang Y, Hao E, An Y, Hou G, Zhao X, Zhou H (2020) The interaction mechanism of cavitation erosion and corrosion on HVOF sprayed NiCrWMoCuCBFe coating in artificial seawater. Appl Surf Sci 525:146499
Hussain E, Robinson AM (2007) Erosion–corrosion of 2205 duplex stainless steel in flowing seawater containing sand particles. Corros Sci 49(4):1737–1754
Bjordal M, Bardal E, Rogne T, Eggen TG (1995) Erosion and corrosion properties of WC coatings and duplex stainless steel in sand-containing synthetic sea water. Wear 186:508–514
Shrestha S, Hodgkiess T, Neville A (2005) Erosion–corrosion behavior of high-velocity oxy-fuel Ni–Cr–Mo–Si–B coatings under high-velocity seawater jet impingement. Wear 259(1–6):208–218
Zheng Q, Zhang L, Jie X, Huang X, Luo S (2017) Effect of rotating speed and hydrostatic pressure on erosion–corrosion behaviour of X65 pipeline steel. Int J Electrochem Sci 12:2593–2605
Ituen E, Mkpenie V, Yuanhua L, Singh A (2020) Inhibition of erosion corrosion of pipework steel in descaling solution using 5-hydroxytryptamine-based additives: empirical and computational studies. J Mol Struct 1204:127562
Deyab MA (2018) The effect of disodium cocoamphodiacetate on corrosion–erosion resistance of steel in saline water. J Mol Liq 217:240–245
Enatore EV, Taleb W, Owen HY, Gomes JP, Barker NA (2018) Evaluation of high shear inhibitor performance in CO2-containing flow-induced corrosion and erosion–corrosion environments in the presence and absence of iron carbonate films. Wear 404:143–152
Ige OO, Barker R, Hu X, Umoru LE, Neville A (2013) Assessing the influence of shear stress and particle impingement on inhibitor efficiency through the application of in-situ electrochemistry in a CO2-saturated environment. Wear 304(2):49–59
Umoren SA, Obot IB, Madhankumar A, Gasem ZM (2015) Performance evaluation of pectin as ecofriendly corrosion inhibitor for X60 pipeline steel in acid medium: experimental and theoretical approaches. Carbohydr polym 124:280–291
Yahya S, Othma NK, Ismail MC (2019) Corrosion inhibition of steel in multiple flow loop under 3.5% NaCl in the presence of rice straw extracts, lignin and ethylene glycol. Eng Fail Anal 100:365–380
Lavanya M, Murthy VR, Rao P (2019) Performance evaluation of a potent green inhibitor on 6061 aluminum alloy under liquid/solid jet impingement. J Bio-and Tribo-Corrosion 5(4):1–10
Charitha BP, Rao P (2018) Pullulan as a potent green inhibitor for corrosion mitigation of aluminum composite: electrochemical and surface studies. Int J Biol Macromol 112:461–472
Charitha BP, Rao P (2017) An ecofriendly approach for corrosion control of 6061 Al-15%(v) SiC (P) composite and its base alloy. Chin J Chem Eng 25(3):363–372
Mulky L, Murthy VR, Rao P (2021) An insight into inhibitory performance of Commiphora Mukul on corrosion of aluminum alloy under tribological conditions. J Iran Chem Soc 18:1–11
Standard (2013) A.S.T.M. D1141-98: Standard Practice for the Preparation of Substitute Ocean Water. ASTM International, West Conshohocken
Gulati K, Rai N, Chaudhary S, Ray A (2016) Nutraceuticals in respiratory disorders. In: Gupta RC (ed) Nutraceuticals. Academic Press, New York
Mobin M, Basik M, Aslam J (2018) Boswellia serrata gum as highly efficient and sustainable corrosion inhibitor for low carbon steel in 1 M HCl solution: experimental and DFT studies. J Mol Liq 263:174–186
Gupta RC (2016) Nutraceuticals in arthritis. Academic Press, Boca Raton
Luo Q, Zhang QZ, Wu Z, Shen B, Liu L, Hu W (2018) The synergistic effect of cavitation erosion and corrosion of nickel-aluminum copper surface layer on nickel-aluminum bronze alloy. J Alloys Compd 747:861–868
Zeng L, Zhang GA, Guo XP, Chai CW (2015) Inhibition effect of thioureidoimidazoline inhibitor for the flow accelerated corrosion of an elbow. Corros Sci 90:202–215
Zhao Y, Zhou F, Yao J, Dong S, Li N (2015) Erosion–corrosion behavior and corrosion resistance of AISI 316 stainless steel in flow jet impingement. Wear 328:464–474
Palumbo G, Berent K, Proniewicz E, Banaś J (2019) Guar gum as an eco-friendly corrosion inhibitor for pure aluminium in 1-M HCl solution. Materials 12(16):2620
Jiang X, Zheng YG, Ke W (2005) Effect of flow velocity and entrained sand on inhibition performances of two inhibitors for CO2 corrosion of N80 steel in 3% NaCl solution. Corro Sci 47(11):2636–2658
Chen Y, Jepson WP (1999) EIS measurement for corrosion monitoring under multiphase flow conditions. Electrochem Acta 44(24):4453–4464
Ekerenam OO, Ma AL, Zheng YG, He SY, Okafor PC (2018) Evolution of the corrosion product film and its effect on the erosion–corrosion behavior of two commercial 90Cu–10Ni tubes in seawater. Acta Metallurgica Sinica (English Letters) 31(11):1148–1170
Xu J, Xie Q, Peng S, Li Z, Jiang S (2020) Investigation of slurry erosion–corrosion behavior of Ta (Si1−xAlx)2 nanocrystalline coatings. Mater Res Express 7(2):026408
Dias V, Maciel H, Fraga M, Lobo AO, Pessoa R, Marciano FR (2019) Atomic layer deposited TiO2 and Al2O3 thin films as coatings for aluminum food packaging application. Materials 12(4):682
Verma C, Quraishi MA, Kluza K, Makowska-Janusik M, Olasunkanmi LO, Ebenso EE (2017) Corrosion inhibition of mild steel in 1M HCl by d-glucose derivatives of dihydropyrido [2, 3-d: 6, 5-d′] dipyrimidine-2, 4, 6, 8 (1H, 3H, 5H, 7H)-tetraone. Sci Rep 7:44432
Ogunbadejo AS, Oladele OE, Olajide JL, Obolo OSJ, Olubambi PA, Aribo S (2018) Flow-accelerated corrosion inhibition of steel in hydrochloric acid by hexamethylenetetramine: gravimetric, density functional theory and multiphysical studies. J Bio Tribo Corros 4(4):70–81
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lavanya, M., Murthy, V.R. & Rao, P. Protection of material applied in heat exchanger under submerged jet impingement condition with Boswellia serrata: electrochemical approach. J Appl Electrochem 52, 953–962 (2022). https://doi.org/10.1007/s10800-022-01686-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-022-01686-x