Abstract
Microbiologically influenced corrosion, also known as microbial or biological corrosion, is produced by particular bacteria adhering to metal in water. It is widely acknowledged to be the direct cause of catastrophic corrosion failures, with associated damage costs accounting to many billions of US$ annually. Certain activities of microbial organisms such as their adherence capabilities are known to lead to the acceleration in corrosion rates of metals. Bacterial adherence is the beginning of the process of colonisation of a surface, known as biofilm development that involves physicochemical and molecular interactions. This process of bacterial adhesion is influenced by a myriad of parameters which are broadly categorised as environment, bacterial, and material characteristics. The following article reviews the mechanisms of bacterial adhesion to biomaterial surfaces, the factors affecting this adhesion, and the techniques used in estimating microbially influenced corrosion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
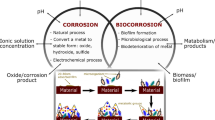
Microbiologically Influenced Corrosion has a lengthy history, with Gaines observing the influence of microorganisms on corrosion initially in 1910 [1]. Microbiologically influenced corrosion (MIC) is known as microbial or biological corrosion. Microbes change the electrochemical reaction at the biofilm/metal interface and either inhibit or accelerate the process of metal corrosion [2]. This concerning process usually involves the stimulation of microorganisms to cathodic or anodic processes or the creation of differential oxygen concentration cells in a confined electrolytic environment by microorganisms. The development of biofilms on metal surfaces and the activities of bacteria beneath the film that induce metal corrosion are both linked to MIC [3]. Microbial corrosion is caused by a mixture of bacteria, medium, and metal [4]. Metal corrosion is caused by a variety of microorganisms. Out of those, the bacteria which are responsible are classified as aerobic or anaerobic. Slime-forming bacteria, sulphate-reducing bacteria (SRB), iron-oxidising bacteria (IOB), and iron-reducing bacteria (IRB) are some of the subgroups that these bacteria may be split into [5].
This localised type of assault by microorganisms typically include the following: pitting, increased erosion corrosion, dealloying, stress corrosion cracking, enhanced galvanic corrosion, and hydrogen embrittlement [6]. Because the extracellular polymeric substances (EPS) formed by bacteria favour cell attachment to the materials, the electrochemical reactions at the metal–biofilm interface are expected to be influenced significantly by the existing macromolecules in the biofilm, such as carbohydrates and proteins [7]. Extracellular polymer substances (EPS) generated by bacteria have been linked to the MIC process in various studies. The presence of organic groups in EPS, such as polysaccharides, proteins, and fatty acids, can influence wettability, surface charge, and free energy [8].
All materials that come into touch with the natural environment, such as wet air, soil, and water, are exposed to MIC in varying degrees [9]. Medical implants, the oil and gas sector, maritime habitats, and water systems are all examples of places where MIC is a major concern [10]. Many sectors are affected by biofilm formation of some kind or another, which can lead to high cleaning and maintenance expenses. Maritime, dairy, food, water systems, oil, paper, cooling systems, power transmissions, opticians, dentists, and hospitals are examples of such sectors [11]. Perhaps the environment where people are exposed to biofilms most frequently is the domestic environment [12].
Corrosion costs were predicted to reach more than 3.4 percent of global GDP or a staggering amount of $2.9 trillion in 2018. MIC is responsible for up to 20% of all corrosion in aquatic systems, according to a conservative estimate. [13, 14]. This estimate represents the significant ambiguity around the precise contribution of MIC, which is primarily due to the difficulties in diagnosing MIC. The primary drawbacks of current corrosion prevention methods are that they are constrained by environmental conditions, they are expensive, are inapplicable in the field, and are occasionally ineffective [15]. MIC has traditionally been researched from a dual standpoint: (1) A biological one where the complex nature of bacteria consortia and biofilm development is taken into account and (2) from a materials corrosion standpoint where the evidence and mitigation of metal pitting under controlled circumstances are considered [16].
Previous reviews have classified MIC into four categories based on organisms.
-
(i)
Nutrient absorption by microbial growths adhering to the metal surface.
-
(ii)
Liberation of corrosive metabolites or end products of fermentative growth.
-
(iii)
Production of sulphuric acid.
-
(iv)
Obligate anaerobe interference in the cathodic process under oxygen-free conditions [17].
2 Bacterial Adhesion
Bacterial adhesion, a dynamic process, is one that occurs between bacterial cells and material surfaces or any substance that has been adsorbed on a surface. For the very first attachment of bacteria, the accessibility of binding sites on the cell surface or perhaps the material surface that bacteria may touch is critical [18]. Traub et al. suggested the division of bacterial adhesion interactions, into three categories: (1) generic physical–chemical interactions, (2) specialised interactions, and (3) surface mechanosensing.
Adhesion is thought to occur in non-specific physical–chemical interactions when appendages and proteins connect with particular chemical fractions on a surface through non-covalent interactions. Specifically, they utilise the usually attractive van der Waals forces, the repulsive electrostatic charges, or acid–base interactions which show dual behaviour. The composition, pressure, pH, oxygen, nutrient availability, and surface conditions of the medium all impact their characteristics. Adhesion processes in specific interactions entail unique appendages that can bind to chemical species on specific surfaces. Coaggregation connections are very specialised, with adhesins in the partner cell recognising receptors in a cell type. An example of this could be—when one particular bacterium’s cell surface adhesion identifies a polysaccharide comprising mannose, glucose, and galactose on the surface of another. The interaction in surface mechanosensing entails a bacterium’s active sensing when it comes into touch with a surface. Adhesion is mediated by sensory organs such as flagella and tension in pili retraction. One of the hallmarks of adhesions is the participation of signal transmission and response from the organism [19].
Van Loosdrecht et al. suggest that bacterial adhesion occur in four phases, based on certain physicochemical characteristics of microorganisms: (1) transport, i.e. the migration of microorganisms in an environment towards the surface of the substrate by either (a) diffusion in static settings, owing to the effects of Brownian motion; (b) convection displacement currents with reference to fluid movement, which essentially provide quicker transport; or (c) active movement, which is the quickest, based on a concentration gradient for specific substances between the two surfaces, (2) reversible initial bonding, with the ability to remove bacteria attached by their own mobility or mild agitation, (3) permanent irreversible bonding with the ability to remove bacteria attaching only under the action of high shaking forces, and (4) colonisation, in which cells get permanently attached to the substrate and begin to grow and proliferate rapidly amongst themselves [20].
Bacterial adhesion to a material surface is a two-phase process with an immediate, initial, and reversible physical phase (phase one) and an irreversible molecular and cellular phase which is time dependent (phase two, initially suggested by Marshall and colleagues) [21]. The influence of physical forces such as gravitational forces, Brownian motion, Van der Waals attraction forces, hydrophobic interactions, and the effect of surface electrostatic charge on the initial attraction of the cells to the surface are all part of phase one of bacterial adhesion [22].
In the beginning, bacterial cells reversibly adhere to the surface at their poles, a process known as reversible attachment. The reversible attachment involves cell appendages, such as pili, flagella, and fimbriae. The bacteria can now either commit to the biofilm lifestyle or depart the surface and return to a planktonic existence. This reversible attachment is then followed by irreversible attachment. Surface proteins and EPS aid attachment between the cell and the surface at this stage. This shift towards irreversible attachment is also implicitly promoted by an internal second messenger and a number of proteins [24].
Physical and chemical interactions govern whether microorganisms adhere to a surface, which can be attractive or repellent based on the intricate interplay of the chemistries of the bacterial and substratum surfaces, as well as the aqueous phase. Figure 1 depicts the interactions between bacterial cell and substratum. Several studies have attempted to investigate if bacterial attachment to surfaces is regulated by the same physicochemical interactions that govern the deposition of non-living colloidal particles in order to better understand the factors that influence adhesion [25].
Various interactions between bacterial cell and substratum depending on distance [23]
The Derjaguin–Landau–Verwey–Overbeek (DLVO) concept and the thermodynamic method are two physicochemical approaches to bacterial adhesion [26].
2.1 DLVO Model Approach (Derjaguin−Landau−Verwey−Overbeek)
Bacteria are the most common species in biofilms and range in size from 0.5 to 2 m, which is close to the size of colloidal particles. As a result, bacteria may be regarded as colloidal particles, and their adherence can be examined using colloid chemistry principles as physicochemical phenomena. Cell surfaces, like most natural surfaces, are typically negatively charged and have varied degrees of hydrophobicity. Bacteria are certainly not inert colloidal particles. Their cell surfaces and properties can vary due to environmental shifts, in ways that are not normally considered in colloid chemistry techniques [26].
What we have to understand is that DLVO makes quite a series of assumptions.
-
1.
The surfaces are smooth and solid at the molecular level.
-
2.
They are inert, unless an electrolyte with bulk liquid characteristics acts as an intervening solvent (water).
-
3.
The van der Waals and electrical double-layer forces are studied separately, and they both offer a source of counterions that leave charged surfaces behind.
-
4.
The double layer’s boundary conditions are either constant charge or constant potential [27].
The DLVO hypothesis defines bacterial adherence to any surface as a direct consequence of the Lifshitz-Van der Waals interactions, electrostatic double-layer interactions, and acid–base binding (in its extended version). DLVO analyses imply that the interfacial Gibbs free energy of adhesion (∆Gadh) is a function of the separation distance between a bacterium and the substratum surface [28].
The interaction energy between two interacting surfaces can be separated as follows:
in which, GEL, GLW, and GTOT denote the electrostatic interaction energy, Lifshitz van der Waals energy, and total energy, respectively. The degradation of these interaction energies with distance, i.e. decay with distance (d) is determined by the geometry of the interacting bodies [28].
2.2 Thermodynamic Approach
The thermodynamic technique is the easiest way to anticipate whether a cell will cling to a surface or not. The surface tension describes the thermodynamics of bacterial cells with liquid or solid surfaces as illustrated in Fig. 2. When a cell attaches to a surface, the system energy transition is from AγBL + AγSL to AγBL because the two interfaces liquid–solid and bacteria–liquid are replaced by one bacteria–solid [29]. The thermodynamic method is based on the interacting surfaces’ surface free energy and does not explicitly account for electrostatic interactions. The surface free energies of the interacting surfaces ∆Gadh can be obtained from a balance of interracial Gibbs energies:
where sm, sl, and ml are the solid–microorganism, solid–liquid, and microorganism–liquid interfacial free energies, respectively [30].
3 Factors Affecting Bacterial Adhesion
As illustrated in Fig. 3, bacterial characteristics, material surface qualities, and climatic variables all influence the adhesion process [30].
3.1 Environmental Factors
Exposure time, bacterial concentration, chemical treatment, and the presence of antibiotics are all factors in the overall environment. By altering physical interactions or changing the surface features of bacteria or materials, all of these variables have the ability to alter bacterial adherence [31].
3.1.1 pH and Temperature
The electrostatic attraction between materials and eco-organisms is hypothesised to be influenced by pH and temperature variations. The effectiveness of chlorination is influenced by pH and temperature. Bacteria are resistant to internal and external pH fluctuations, and they maintain a balance of protein activity and synthesis in biological systems [32]. When we gradually raise the acidity of the cells rather than adding HCL quickly to induce an abrupt drop in pH, the cells have the best chance of surviving. This essentially demonstrates that bacteria have very specialised processes that allow them to adapt to minor pH variations in the environment [33]. The optimum PH range (6.5–7.2) for bacterial development varies greatly depending on the kind of bacteria. At high temperatures (> 35 °C), a bacteria seems to have only one flagellum, but when the temperature drops, the number of flagella rises, increasing the surface area of the bacteria and the chance of bacterial adherence in the first attachment stage [34]. Temperature variations are used to test differences in bacterial strains’ adhesion behaviour. Lower temperatures cause polysaccharide characteristics to be more consistent, which promotes biofilm development. (b) Lower temperatures reduce the amount of cell surface hydrophobicity, resulting in less biofilm development [35].
3.1.2 Hydrodynamics
Bacteria are exposed to flow conditions in natural and man-made habitats. Thus, the relevance of hydrodynamics in their initial adhesion. Whilst the impact of the surrounding liquid is restricted in a static fluid situation to hydrostatic pressure, bacteria in a flowing fluid face hydrodynamic forces that alter their motion (translational and rotational velocity) or deformation (extensional strain and shear strain). As a result, every bacterial attachment investigation done under flow circumstances must take into account the hydrodynamic environment [36]. The surface adherence of bacteria is directly affected by water velocity, compressive strength, and laminar flow. Hydrodynamics has a significant impact on chemical kinetics processes and produces pressures that directly influence biofilm composition, stiffness, and separation [37].
3.2 Bacterial Characteristics
Bacteria promote adhesion through a myriad of characteristics, such as production of extracellular polysaccharides (EPS), metabolic activity, charge, cell viability, cell wall stiffness, and hydrophobicity, adhesin-mediated receptor–ligand binding (adhesins are protein complexes that recognise and bind to protein receptors on the host cell surface) and appendages like pili and curli. The attaching of cells to a substrate is referred to as adhesion, whereas the interaction between cells is referred to as cohesion. Surface charge, hydrophobicity, surface free energy, roughness, topography, specific surface geometry (macro, micro, and nano), and chemistry are all physical–chemical aspects that influence biofilm adherence and early development in addition to the properties of bacteria [38].
3.2.1 Surface Charge
Surface charge and hydrophobicity determine bacterial characteristics. The cellular surface charge is the total net charge carried by a bacterial cell. Because of a plethora of carboxyl and phosphate groups in their cell walls, most bacterial cells are negatively charged [39]. Microorganisms, much like the bulk of substrates they can possibly colonise, have a negative charge and varying degrees of hydrophobicity on their surfaces [40]. Since bacteria has a negative charge, it makes them appealing to positively charged surfaces. Negatively charged bacteria repel negatively charged surfaces by electrostatic contact.
3.2.2 Hydrophobicity
Bacterial hydrophobicity varies by species and is regulated by factors, such as growth media, bacterium age, and bacterial surface architecture. Bacterial hydrophobicity refers to a bacterial cell’s preference for interacting with cells of comparable hydrophobicity rather than water, and in the case of bacterial cells, this relates to the hydrophobic qualities of the cell’s surface. The hydrophobicity of a bacterial cell is largely governed by the residues and structures on its surface, which can be either hydrophilic or hydrophobic [41]. This indicates that bacterial hydrophobicity changes between species and strains and even within a single strain, depending on the mode and stage of development, as well as the growth medium composition [42].
3.3 Material Surface Characteristics
Material surface energy, roughness, wettability, and zeta potential are all important factors for bacterial adhesion [43]. Material surface chemistry features determine the pattern and shape of proteins adsorbed onto the material surface, as well as the ligands exposed to the body and microbes [44].
3.3.1 Surface Free Energy
Van der Waals forces, electrostatic interactions, and acid-based interactions make up physicochemical interactions (non-specific), which determine a substratum’s surface free energy [45].
3.3.2 Surface Roughness
Some studies found no link between surface roughness and bacterial or spore adherence, whereas others found a significant link between bacterial adhesion and surface roughness. Roughness has an effect on biofilm growth which however appears to be a minor role when it comes to initial adhesion. A rough surface is a folded, flat surface with an enlarged surface area that produces equivalent contact forces as a smooth surface from a physicochemical and mechanistic standpoint [46].
The rough surface of the substratum encourages the formation of biofilms [47]. The velocity of biofilm growth, the number of entrapped microorganisms, and the texture of the biofilm are all influenced by roughness. Roughness may cause a homogenous biofilm to grow over the whole surface area, resulting in a thin and active biofilm. Roughness can also shield immobilised cells from shock loads and create an optimal environment for biofilm adhesion. As a result, roughness is important because it regulates biofilm attachment, growth, and separation. The impact of roughness on long-term use, on the other hand, has yet to be determined. Roughness has also been shown to impact biofilm detachment rates [48]. Despite the well-known fact that hydrophobicity is influenced by surface roughness, several studies have demonstrated that tiny differences in surface roughness have no meaningful impact on hydrophobicity values [49].
3.3.3 Surface Hydrophobicity or Wettability
Surface wettability is a critical property that controls the interactions between solid and liquid phases in biological systems. In a nutshell, the liquid phase “wets” a solid surface by increasing the amount of time it is in contact with it. As a result, the contact between the liquid and the solid surface improves [50]. Wettability (hydrophobicity or hydrophilicity) refers to the quantity of wastewater or biomass that comes into contact with the surface of a biofilm carrier. It has a significant impact on bacterial adhesion and interaction with a carrier surface. Wettability influences both the rate of initial biofilm attachment and the toughness of adherent biofilms. Microbial adhesions and interactions between biofilm carriers and bacteria are thoroughly affected by hydrophilicity or hydrophobicity [51]. The hydrophobicity of a metal oxide surface might promote bacterial adherence. Bacterial adherence is highest to the most hydrophobic surfaces. Microorganisms adhere more quickly to hydrophobic, non-polar surfaces (Teflon and other polymers) than to hydrophilic surfaces, according to previous research (stainless steel). This demonstrates the presence of hydrophobic contact, which allows the repulsive forces between cells to be reduced. Thus, the initial adhesion of bacteria is determined by the charge and hydrophobicity of surfaces [52].
4 Techniques Used in Determining Bacteria–Material Adhesion
-
(i)
CFU plate counting, radiolabeling, 5-cyano-2,3-ditolyl tetrazolium chloride (CTC) staining, resazurin test, and fluorescein diacetate (FDA) assay are all viable bacterial counting techniques.
-
(ii)
Microscopical techniques for counting and morphological observation of adherent bacteria include Light microscopy, Electron microscopy, Image-analysed epifluorescence microscopy, Atomic force microscopy (AFM), and Confocal laser scanning microscopy (CLSM).
-
(iii)
Spectrophotometry, Biochemical markers (ATP) and Coulter count, are amongst the other direct and indirect approaches [53].
5 Biofilm
Microbial infestation of metal surfaces and the development of biofilms cause MIC, which affects the metal–environment system’s electrochemical nature [54]. Corrosion is infinitely more destructive when a multispecies biofilm is present. Interactions between different species in such biofilms may trigger a chain reaction of metabolic processes in the oxic and anoxic regions of the biofilm, exacerbating corrosion [55]. Multiple corrosion-causing organisms in a biofilm can work together to cause more serious corrosion than if only one species is present [56]. In natural, industrial, and medicinal contexts, microbial biofilms are common and may be found on biotic and abiotic surfaces which are exposed to bulk liquid environments. Biofilms have a role in a variety of illnesses and disorders, as well as biofouling and corrosion, which may be harmful to both human health and business. Biofilms, on the other hand, may be used in a variety of biotechnological processes, such as bioremediation, biological fuel cells, and biofertilizer synthesis. As a result, it is critical to control microbial biofilms both to optimise their production and to prevent infections, biofouling, and pollution [57]. Water (80%), bacteria, and extracellular polymeric compounds (EPSs) make up biofilms. EPSs comprise a mixture of polysaccharides, extracellular DNA (eDNA), lipids, and proteins that remain long after biofilm-forming bacteria have been removed from a mature biofilm [58]. Biofilms are known to cause MIC as a result of their metabolic activity or released metabolite of entrenched microbes. Biofilms are behind MIC because electroactive sessile cells in biofilms, not planktonic cells, can harvest electrons from energetic metals, and biofilms can harbour high concentrations of corrosive agents such as organic acids underneath. Although the sessile cell number may be just a tiny fraction of the total planktonic cell number, sessile cells are usually packed in a thin, often 50–200 µm, biofilm. This alone means that a biofilm provides a different biochemical environment for corrosion [59]. Some microbes can produce metabolites that are acidic (for instance, sulphuric acid by sulphur oxidising bacteria) or facilitate the local depassivation or dissolution of the protective films or corrosion products on the metal surface (for instance, biogenic sulphides destabilising the copper oxide film on Cu–Ni alloys, a marine Vibrio reducing insoluble corrosion product to soluble Fe2+). Microorganisms can consume substances in biofilm and lead to formation of concentration gradients of chemical species that are important for their metabolic activities, which are also electrochemical reactants (oxygen and protons) relevant to the underlying substratum [60].
Anaerobic metabolism is categorised into two. One, anaerobic respiration, in which an exogenous (non-oxygen) oxidant such as sulphate, thiosulfate, sulphite, sulphur, nitrate, CO2, and nitrite acts as a terminal electron acceptor to absorb the electrons produced by organic carbon oxidation. Two, anaerobic fermentation—the second form of anaerobic metabolism. Fermentative microorganisms capable of fermentative growth, such as APB and some SRB strains, oxidise an organic carbon and generate ATPs by substrate-level phosphorylation in the absence of an external electron acceptor [61].
Pitting, crevice corrosion, under deposit corrosion, stress corrosion cracking, and selective dealloying are all common outcomes of biofilm growth. Certain microorganisms’ metabolic products may be corrosive to metal substrates, and released enzymes may act as corrosion catalysts [62]. The creation of a conditioning layer, bacterial adhesion, slime production, and three-dimensional growth, followed by maturation and separation, are all phases in biofilm formation [63]. Biofilm bacteria do not behave like individuals with separate unicellular lifestyles. Many bacteria instead control cooperative behaviour by releasing, detecting, and reacting to diffusible signal molecules [64].
One-way biofilms impact metal corrosion is by devouring oxygen (the cathodic reactant). Another method is to increase the mass transfer of corrosion reactants and products, hence modifying the corrosion kinetics. Other possible ways are by producing corrosion-causing compounds and substances that serve as auxiliary cathodic reactants [65].
Different techniques, such as confocal laser scanning microscopy (CLSM), scanning electron microscopy, atomic force microscopy, and field emission scanning electron microscopy, can be used to determine the thickness (or depth) of a biofilm developed on a metal surface. The thickness of a biofilm can be used to determine how the biofilm affects the material surface, as well as the direct correlation between thickness and corrosion [66]. Biofilm depth profiles are also investigated in connection to the variables that may influence them (such as temperature and media replenishment).
For example, the thickness of the biofilm was evaluated using CLSM in a study conducted by Jayaram et al. involving the corrosion inhibition behaviour of Pseudomonas fragi and Escherichia coli on stainless steel. They found that the thickness of the biofilms did not alter significantly across growth temperatures, media, or medium replenishment [67].
Biofilm-influenced corrosion on cast iron pipes in reclaimed water was researched by Zhang et al. They discovered that as the biofilm thickness increased over the course of 30 days, the corrosion rate fell dramatically, resulting in an inverse relationship between biofilm thickness and general corrosion rate. They concluded that the biofilm thickness grew dramatically because of bacterial proliferation and the accumulation of corrosion and metabolic products on the cast iron surface [68].
In artificial Beijing soil, Liu et al. investigated the effects of biofilm-influenced corrosion on X80 pipeline steel by a nitrate-reducing bacterium, Bacillus cereus. CLSM was used to determine the biofilm thickness of the samples. The nitrate reduction of bacteria beneath the biofilm was observed to drive X80 pipeline steel pitting corrosion, which was attributed to B. cereus biofilm. The findings also revealed that dense biofilms had a minor tendency to prevent general corrosion compared to its production and exfoliation, which was confirmed using a scanning Kelvin probe [69].
5.1 Development of a Biofilm
Biofilm generation is accomplished by following the processes shown in Fig. 4.
-
i.
Attachment,
-
ii.
Microcolony formation,
-
iii.
Matrix formation,
-
iv.
Macrocolony formation (maturation of biofilm), and
-
v.
Dispersal [70].
Simply put, Biofilm development has four stages:
(1) Biofilm Adhesion, (2) Biofilm Formation, (3) Biofilm Maturation, and (4) Biofilm Dispersal.
Stage 1 Biofilm adhesion is the first stage. After an instant interaction with an abiotic surface, free-floating bacteria cling to it. Bacterial adherence to the implant surface is influenced by physiochemical variables, such as polar and ionic interactions, van der Waals forces, and hydrophobicity. The creation of a conditioning film following insertion as a result of host protein adsorption improves bacterial adherence via interactions between bacterial and host proteins. At the conclusion of this step, the bacteria monolayer attaches firmly to the binding sites [71].
Stage 2 (Biofilm formation): At this point, bacteria in the monolayer begin to multiply locally, generating microcolonies. A self-secreted extracellular polymeric material matrix organises many multilayers of bacteria into an ordered structure. Microbial surface components that recognise sticky matrix molecules and polysaccharide intercellular adhesion facilitate this process [71].
Stage 3 Biofilm maturation is the third stage. Microcolonies become macrocolonies as a result of cell adaptation and development. Regulation of exopolysaccharides, pili, flagellae, fimbriae, and glycocalyx leads to biofilm development. Surfactant peptides and shear force created by flowing bodily fluids on biofilm determine biofilm maturation and form. The strength of surface adhesion is determined by these parameters. After a biofilm has matured, removing it is incredibly challenging.
Stage 4 Dispersal of biofilms is the fourth stage. After detaching from the biofilm, bacteria revert to planktonic state and float freely in the surrounding liquids, spreading infection. Dispersion of biofilms leads to the selection of new places or greater local invasion, thereby initiating a new cycle.
There are “active dispersal,” which depends on cell motility or degradation of EPS and “passive dispersal,” which depends on physical factors such as shearing force under liquid flow conditions. Active dispersal is triggered by changes in environmental conditions, such as temperature change, starvation, oxygen deficiency, and metabolite accumulation [72].
Detachment of microorganisms from biofilm can be caused by bacteria themselves, such as enzymatic degradation of the biofilm matrix, such as dissolution of adhesins by proteases, nucleases, and a group of small amphiphilic α-helical peptides, known as phenol-soluble modulins (PSMs) functioning as surfactants, and quorum sensing or by external forces, such as fluid shear forces, corrosion, and human intervention [73]. After cell proliferation, the next stage is maturation and later dispersion occurs.
5.2 Biofilm for Corrosion Acceleration
Biofilms can play a variety of roles in increasing corrosion at a biologically conditioned metal–solution interface, and it can happen in a number of ways, including (a) Modifications in the transport of chemical species towards the metal surface. (b) Making it easier to remove protective films when the biofilm separates. (c) Inducing unequal aeration effects as a result of the biofilm’s uneven distribution. (d) At the metal–solution interface, changing oxidation–reduction conditions. (e) Changing the structure of inorganic passive layers and speeding up their decomposition and removal from metal surfaces. The metabolic activities of bacteria within the biofilm may have a significant impact on the MIC [74,75,76]. Temperature, pH level, salinity, external inputs of new species, availability of nutrients and, most importantly, the composition of the metal under attack will all influence the makeup of the species that will comprise the mature biofilm. In a biofilm developed on metal infrastructures, different microbial metabolic groups have varied impacts on the metal [77].
5.3 Biofilm for Corrosion Inhibition
The following are the most often stated methods for biofilm corrosion inhibition:
-
(i)
The biofilm acts as a diffusion barrier for corrosion agents, preventing metal disintegration.
-
(ii)
Respiring aerobic microorganisms within the biofilm consume oxygen, lowering the concentration of that reactant at the metal surface.
-
(iii)
Microorganisms create metabolic products that suppress corrosion (e.g. siderophores) [78].
Gao et al. investigated the Microbiologically Influenced Corrosion Inhibition of aluminium alloy 5083 by three representative Vibrio species—V. parahaemolyticus, V. alginolyticus, and V. EF187016 in 2021. They noticed that matured biofilms improved corrosion resistance. When irreversible attachment was accomplished in the case of V. parahaemolyticus, the biofilm began to prevent corrosion. The thicker biofilm may operate as a better diffusion barrier against aggressive ion penetration, lowering corrosion rates and reducing the danger of localised attack. Vibrio species are known to be able to breathe oxygen in an aerobic environment, which is a potent electron acceptor and corrosive agent. Keeping that in mind, they established the fact that the Vibrio species depleted the oxygen beneath the biofilm, resulting in a local anaerobic environment. They also noted that the biofilm could prevent the diffusion of chloride ions to the coupon surface [79].
The corrosion behaviour of Bacillus cereus on stainless steel (SS) was researched by Li et al. They revealed that a biofilm of B. cereus might prevent SS pitting corrosion. This could be related to the role of biofilms and oxidative material accumulation on the surface of SS. The electron transmission between SS and the cathodic depolarizer (oxygen) was hampered by a B. cereus biofilm. Because of the barrier effect of biofilm on electron transfer (which suppresses the process of metal breakdown), biofilm formation may delay SS corrosion. They also learned that the additional polymeric substances (EPS) generated during bacterial cell attachment (and development) combine with the metal ion to form an organometallic complex that prevents steel corrosion [80].
Jayaraman et al. genetically constructed Bacillus subtilis biofilms that secreted antimicrobials indolicidin, bactenecin, and probactenecin. It lead to the discovery that these biofilms could inhibit the growth of corrosion-causing SRB (Desulfovibrio vulgaris and D. gigas) and reduce corrosion rates significantly in continuous culture conditions. This is the first time that beneficial, genetically engineered antimicrobial-expressing biofilms have been used in situ to prevent corrosion. This method has the benefit of creating antibacterials from inside the biofilm, avoiding any transportation barriers that biocide therapy may meet. Another advantage, according to the authors, is the exopolymeric material that forms the biofilm matrix, which can help keep local antibacterial concentrations relatively high by preventing them from migrating into bulk fluids [75].
Because of biological activity, the major mechanisms of bacterial corrosion inhibition are invariably linked to a considerable change in the environment at the metal/solution interface. Then why do the metals beneath the biofilm still corrode if these biofilms successfully suppress corrosion? In 1997, Jayaram et al. presented a solution to this problem. They stated that even in the presence of a dense biofilm, oxygen can permeate to the metal surface via the biofilm’s water channels. This might explain why, in the presence of metabolically active biofilms, there are still a limited amount of corrosion [81].
Microbes are shown to have a lot of potential in terms of preserving metals against corrosion. (1) The removal of corrosive substances via microbial activity, such as microbes consuming reactive oxygen via aerobic respiration, (2) growth inhibition of corrosion-causing microbes, such as antimicrobial production by non-corrosive microorganisms, and (3) the formation of a protective layer are all potential mechanisms for inhibiting biofilm-induced metal corrosion [56].
A new surface with new qualities is produced as the biofilm covers the surface of a substance. The wetting qualities of a freshly formed surface are critical for both maximising the benefits of biofilms and avoiding the negative repercussions of their negative impacts. Surface characteristics and wettability of biofilms are garnering more attention, especially given that novel surface coatings show promise for avoiding biofilm development and its negative consequences, which is one of the developing solutions for battling biofilms [82].
6 Microbes Involved in Mic
Many microorganisms, including eukaryotes, like fungus, algae, and diatoms, as well as microbes from the domains archaea and bacteria, are involved in MIC. Archaea may seem like bacteria, yet they have a lot of genes in common with eukaryotes. Bacteria have been the focus of the great bulk of MIC research thus far. Sulphate-reducing bacteria (SRB), Sulphur-oxidising bacteria (SOB), iron-oxidising/reducing bacteria (IB), manganese-oxidising bacteria, and bacteria that release organic acids or extracellular polymeric compounds are expected to have the greatest influence on corrosion [83]. The capacity of iron-oxidising bacteria to oxidise iron from the ferrous to the ferric state and precipitate it as a coating distinguishes them. Sulphur-oxidising bacteria can be divided into two categories, albeit they come in a variety of forms: (1) aerobic (colourless sulphide oxidisers) and (2) anaerobic (colourful sulphide oxidisers) [84].
7 Mechanisms of Mic
When a metal is submerged in water, moist soil, or another aquatic environment, the metal disintegrates into metallic cations, leaving an excess of electrons behind. The balancing process, which in near neutral solution is generally the reduction of oxygen to hydroxyl ions, consumes these electrons at near cathodic sites. In the absence of oxygen, the most common cathodic reaction for corrosion is hydrogen ion reduction. The generation of oxidising agents by microbes through their metabolism is required for them to participate in the reaction. Microorganisms play a role in starting or worsening the electrochemical processes on metals by directly participating in one or both of the reactions [85].
There have been several suggested mechanisms for MIC, and it is conceivable that none of them are dominant. Kuhr et al. proposed the cathodic depolarization hypothesis, commonly known as the classical theory, in 1934 in an attempt to explain the exceptionally high incidence of corrosion failures observed on subterranean cast iron pipelines in the Dutch countryside [16].
The circumstances that cause MIC may be divided into two categories: aerobic and anaerobic, with the latter being believed to be the more economically damaging. As mechanisms for MIC, galvanic cells, bacterial secretion of H2S, NH3, or PH3, differential aeration or chemical concentration cells, enzymatic oxygen reduction reactions, organic acid acidity derived from bacterial metabolites, and direct extraction and consumption of electrons from iron have all been proposed [86]. Based on the two metabolic processes of anaerobic bacteria: (i) respiration and (ii) fermentation, microbial corrosion produced by anaerobic microorganisms is categorised into two types: type I and type II. Microbial corrosion of type I occurs as a result of electron transfer processes in microbial respiration. Corrosive metabolites generated by anaerobic microbial activity cause Type II microbial corrosion [87]. It contains microorganisms such as acid-producing bacteria secreting oxidants, such as protons. Without biocatalysis, the biofilms in this condition provide locally high concentrations of corrosive oxidants [88].
EET-MIC is the name for Type I MIC (extracellular electron transfer). When the EET direction is reversed to transfer electrons from organic carbon oxidation in the cytoplasm to an external anode, microbes capable of EET are active in MFC because they are electrogenic. If there are a local scarcity of organic carbon, these electrogenic microorganisms can produce MIC (electron donor). EET-MIC includes the SRB-MIC and NRB-MIC processes mentioned above. Extracellular electrons generated by iron oxidation must be delivered to the cytoplasm for oxidant reduction in Type I biocorrosion. Electrons, unlike ions, cannot “swim” in water. Because planktonic cells cannot transfer electrons from a metal surface through an aqueous liquid media, they cannot directly contribute to Type I biocorrosion. This is exactly why sessile cells in a biofilm generate Type I biocorrosion rather than planktonic cells [89].
Metabolite MIC is a kind of Type II MIC (M-MIC). Because “chemical corrosion” refers to the direct contact of a metal with an oxidant, generally at high temperatures, without separate oxidation and reduction processes, unlike an electrochemical corrosion process, M-MIC uses the term “metabolite” instead of “chemical” [90]. The biocorrosion processes of Types I and II are both electrochemical. Microbes attacking an extracellular organic material such as polyurethane and its organic plasticizer with the goal of extracting organic carbon and energy may be classified as Type III biocorrosion [91].
There are certain mechanisms that are universally acknowledged where MIC is concerned; they can be summarised as follows:
-
Cathodic depolarization, in which microbiological activity accelerates the cathodic rate limiting step.
-
Microorganisms create patchy surface colonies due to the formation of occluded surface cells. Sticky polymers attract and aggregate biological and non-biological organisms, resulting in fissures and concentration cells, which serve as the foundation for accelerated assault.
-
Anodic reaction site fixation, in which microbiological surface colonies cause corrosion pits to develop, which is triggered by microbial activity and linked to the position of these colonies.
-
Under deposit acid attack, in which acidic products of the MIC community metabolism, primarily short-chain fatty acids, intensify corrosive attack [92].
8 Mic Measurement Techniques
General pitting, stress corrosion cracking, crevice attack, intergranular stress cracking and hydrogen embrittlement, acceleration of corrosion fatigue, and cracking are just a few of the types of corrosion that may be encouraged by microorganisms interacting with metals. A majority of MIC cases are linked to a localised attack. Because of the intricacy of MIC reactions, a variety of approaches must be used to link corrosion processes to microbial activity at surfaces [93].
New approaches for laboratory and field assessment of MIC in industrial systems have been developed thanks to significant advancements in microbiological, analytical, microscopy and electrochemical, techniques and apparatus. Chemical analysis within the biofilm using microsensors is one of the most intriguing advances in instrumentation [94]. Several authors have critically reviewed a wide range of electrochemical techniques for use in biocorrosion, including corrosion and redox potential measurements, linear polarisation, Tafel and Potentiodynamic polarisation, and electrical resistance probes, as well as several modern electrochemical techniques, such as alternating current methods or electrochemical noise [95]. However, electrochemical methods do not allow for a precise assessment of the corrosion acceleration (total corrosion rate augmentation) produced by corrosion-promoting bacteria strains, which might result in severe industrial equipment damage. The difference between microbially induced corrosion (MIC) and general corrosion (GC) rates is critical for selecting an appropriate MIC inhibitor/suppressor. Demonstrating how microbial reactions interfere with corrosion processes and, as a result, finding products of these reactions on the surfaces of corroding metals using appropriate analytical techniques are critical components of characterising the mechanisms of microbially influenced corrosion. The presence of these compounds, which is linked to an increase in corrosion rate, is used as proof that the microbially influenced corrosion process is operational. MIC does not have a universal mechanism [96].
The development of the many approaches used to investigate microbial populations in MIC is seen in Fig. 5. Whilst characterising the microbial community composition at MIC-affected areas has been found to be informative and useful in predicting the putative function of specific species in the community, it does not provide information on the microbial community’s actual functional activity. Because of the development of analytical tools for large-scale studies of metabolites and small compounds, it is now possible to analyse both the composition and functional output of microbial communities in MIC [97]. Bacterial adhesion research techniques have been established around three key steps: bacterial contact with the surface, bacterial removal, and bacterial counting [98].
9 Conclusion
MIC has been studied from both a biological perspective (due to the complex nature of bacteria consortia and biofilm formation) and a materials corrosion standpoint (considering the evidence and mitigation of metal pitting under controlled conditions). The current scenario demands a combined approach to tackle the problem of MIC. MIC accounts for up to 20% of all corrosion in aqueous systems, with significant ambiguity around its contribution. To fully understand MIC, one must have in depth knowledge on bacterial adhesion, biofilms, and the factors that influence this process. Several researchers have critically examined a wide range of electrochemical methods in regard to their usage in biocorrosion assessment. Understanding the difference between microbially induced corrosion, which can much surpass general corrosion as determined by electrochemical methods, and general corrosion is critical for selecting an appropriate MIC inhibitor/suppressor. Despite the fact that passivity phenomena have been studied extensively in corrosion literature, the exact nature or origin of these effects is still unknown, hence further study in this field is essential.
Data Availability
N/A.
Code Availability
N/A.
References
Gaines RH (1910) Bacterial activity as a corrosive influence in the soil. Ind Eng Chem 2(4):128–130. https://doi.org/10.1021/ie50016a003
Zarasvand KA, Rai RV (2014) Microorganisms: induction and inhibition of corrosion in metals. Int Biodeterior Biodegrad 87:66–74. https://doi.org/10.1016/j.ibiod.2013.10.023
Dao VH, Ryu HK, Yoon KB (2021) Leak failure at the TP316L welds of a water pipe caused by microbiologically influenced corrosion. Eng Fail Anal 122:105244. https://doi.org/10.1016/j.engfailanal.2021.105244
Lavanya M (2021) A brief insight into microbial corrosion and its mitigation with eco-friendly inhibitors. J Bio- Tribo-Corros 7(3):1–9. https://doi.org/10.1007/s40735-021-00563-y
Abdolahi A, Hamzah E, Ibrahim Z, Hashim S (2014) Application of environmentally-friendly coatings toward inhibiting the microbially influenced corrosion (MIC) of steel: a review. Polym Rev 54(4):702–745. https://doi.org/10.1080/15583724.2014.946188
Little BJ, Lee JS (2007) Microbiologically influenced corrosion, vol. 3. John Wiley & Sons, USA
Beech IB, Cheung CS (1995) Interactions of exopolymers produced by sulphate-reducing bacteria with metal ions. Int Biodeterior Biodegrad 35(1–3):59–72. https://doi.org/10.1016/0964-8305(95)00082-G
Khandeparker RD, Bhosle NB (2001) Extracellular polymeric substances of the marine fouling diatom Amphora rostrata Wm. Sm Biofouling 17(2):117–127. https://doi.org/10.1080/08927010109378471
Yuan B, Liu G, Chen Y (2005) The research overview of material microbiological corrosion. Material Protection 38(4):38
Omran BA, Abdel-Salam MO (2020) The catastrophic battle of biofouling in oil and gas facilities: impacts, history, involved microorganisms, biocides and polymer coatings to combat biofouling. In: A new era for microbial corrosion mitigation using nanotechnology. Springer International Publishing, pp 47–99
Qi Fu et al (2021) The effect of nitrate reducing bacteria on the corrosion behavior of X80 pipeline steel in the soil extract solution of Shenyang. Int J Press Vessels Pip 190:104313. https://doi.org/10.1016/j.ijpvp.2021.104313
Barker J, Bloomfield SF (2000) Survival of Salmonella in bathrooms and toilets in domestic homes following salmonellosis. J Appl Microbiol 89(1):137–144. https://doi.org/10.1046/j.1365-2672.2000.01091.x
Koch G (2017) Cost of corrosion. In: Trends in oil and gas corrosion research and technologies. pp 3–30. https://doi.org/10.1016/B978-0-08-101105-8.00001-2
Iverson WP (1987) Microbial corrosion of metals. Adv Appl Microbiol 32:1–36. https://doi.org/10.1016/S0065-2164(08)70077-7
Gu T (2014) Theoretical modeling of the possibility of acid producing bacteria causing fast pitting biocorrosion. J Microb Biochem Technol 6(2):068–074. https://doi.org/10.4172/1948-5948.1000124
Li K, Whitfield M, Van Vliet KJ (2013) Beating the bugs: roles of microbial biofilms in corrosion. Corros Rev 31(3–6):73–84. https://doi.org/10.1515/corrrev-2013-0019
Korenblum E, Sebastián GV, Paiva MM, Coutinho CMLM, Magalhães FCM, Peyton BM, Seldin L (2008) Action of antimicrobial substances produced by different oil reservoir Bacillus strains against biofilm formation. Appl Microbiol Biotechnol 79(1):97–103. https://doi.org/10.1007/s00253-008-1401-x
Xu LC, Siedlecki CA (2012) Submicron-textured biomaterial surface reduces staphylococcal bacterial adhesion and biofilm formation. Acta Biomater 8(1):72–81. https://doi.org/10.1016/j.actbio.2011.08.009
Kreve S, Dos Reis AC (2021) Bacterial adhesion to biomaterials: what regulates this attachment? A review. Jpn Dent Sci Rev 57:85–96
Van Loosdrecht MC, Lyklema NW, Zehnder AJ (1990) Hydrophobic and electrostatic parameters in bacterial adhesion. Aquat Sci 52(1):103–114. https://doi.org/10.1007/BF00878244
Marshall KC, Stout R, Mitchell R (1971) Mechanism of the initial events in the sorption of marine bacteria to surfaces. Microbiology. https://doi.org/10.1099/00221287-68-3-337
Armentano I et al (2014) The interaction of bacteria with engineered nanostructured polymeric materials: a review. Sci World J 2014:410423. https://doi.org/10.1155/2014/410423
Cheng Y, Guoping F, Carmen MI (2019) Micro-and nanotopography sensitive bacterial attachment mechanisms: a review. Front Microbiol. https://doi.org/10.3389/fmicb.2019.00191
Toyofuku M, Inaba T, Kiyokawa T, Obana N, Yawata Y, Nomura N (2016) Environmental factors that shape biofilm formation. Biosci Biotechnol Biochem 80(1):7–12. https://doi.org/10.1080/09168451.2015.1058701
Katsikogianni M, Missirlis YF (2004) Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur Cell Mater 8(3):37–57
van Loosdrecht MC, Lyklema J, Norde W, Zehnder AJ (1989) Bacterial adhesion: a physicochemical approach. Microb Ecol 17(1):1–15. https://doi.org/10.1007/bf02025589
Ninham BW (1999) On progress in forces since the DLVO theory. Adv Colloid Interface Sci 83:1–17
Carniello V, Peterson B, van der Mei W, H. C. & Busscher H. J. (2018) Physico-chemistry from initial bacterial adhesion to surface-programmed biofilm growth. Adv Colloid Interface Sci 261:1–14. https://doi.org/10.1016/j.cis.2018.10.005
Perni S, Preedy EC, Prokopovich P (2014) Success and failure of colloidal approaches in adhesion of microorganisms to surfaces. Adv Colloid Interface Sci 206:265–274. https://doi.org/10.1016/j.cis.2013.11.008
Hori K, Matsumoto S (2010) Bacterial adhesion: from mechanism to control. Biochem Eng J 48(3):424–434. https://doi.org/10.1016/j.bej.2009.11.014
An YH, Friedman RJ (1998) Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J Biomed Mater Res 43(3):338–348. https://doi.org/10.1002/(sici)1097-4636(199823)43:3%3C338::aid-jbm16%3E3.0.co;2-b
Hemdan BA, El-Taweel GE, Goswami P, Pant D, Sevda S (2021) The role of biofilm in the development and dissemination of ubiquitous pathogens in drinking water distribution systems: an overview of surveillance, outbreaks, and prevention. World J Microbiol Biotechnol 37(2):1–18. https://doi.org/10.1007/s11274-021-03008-3
Garrett TR, Bhakoo M, Zhang Z (2008) Bacterial adhesion and biofilms on surfaces. Prog Nat Sci 18(9):1049–1056. https://doi.org/10.1016/j.pnsc.2008.04.001
Achinas S, Charalampogiannis N, Euverink GJW (2019) A brief recap of microbial adhesion and biofilms. Appl Sci 9(14):2801. https://doi.org/10.3390/app9142801
Chavant P, Martinie B, Meylheuc T, Bellon-Fontaine MN, Hebraud M (2002) Listeria monocytogenes LO28: surface physicochemical properties and ability to form biofilms at different temperatures and growth phases. Appl Environ Microbiol 68(2):728–737. https://doi.org/10.1128/aem.68.2.728-737.2002
Krmanovic M et al (2021) Hydrodynamics and surface properties influence biofilm proliferation. Adv Colloid Interface Sci 288:102336. https://doi.org/10.1016/j.cis.2020.102336
Tian RM et al (2014) Effect of copper treatment on the composition and function of the bacterial community in the sponge Haliclona cymaeformis. MBio 5(6):e01980-e2014. https://doi.org/10.1128/mbio.01980-14
Foster TJ et al (2014) Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol 12(1):49–62. https://doi.org/10.1038/2Fnrmicro3161
Rivas L, Fegan N, Dykes GA (2005) Physicochemical properties of shiga toxigenic Escherichia coli. J Appl Microbiol 99(4):716–727. https://doi.org/10.1111/j.1365-2672.2005.02688.x
González-Rivas F, Ripolles-Avila C, Fontecha-Umaña F, Ríos-Castillo AG, Rodríguez-Jerez JJ (2018) Biofilms in the spotlight: detection, quantification, and removal methods. Compr Rev Food Sci Food Saf 17(5):1261–1276. https://doi.org/10.1111/1541-4337.12378
Krekeler C, Ziehr H, Klein J (1989) Physical methods for characterization of microbial cell surfaces. Experientia 45(11):1047–1055. https://doi.org/10.1007/bf01950157
Goulter RM, Gentle IR, Dykes GA (2009) Issues in determining factors influencing bacterial attachment: a review using the attachment of Escherichia coli to abiotic surfaces as an example. Lett Appl Microbiol 49(1):1–7. https://doi.org/10.1111/j.1472-765x.2009.02591.x
Bohinc K, Kukić L, Štukelj R, Zore A, Abram A, Klačić T, Kovačević D (2021) Bacterial adhesion capacity of uropathogenic Escherichia coli to polyelectrolyte multilayer coated urinary catheter surface. Coatings 11(6):630. https://doi.org/10.3390/coatings11060630
Keselowsky BG, Collard DM, García AJ (2003) Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J Biomed Mater Res A 66(2):247–259. https://doi.org/10.1002/jbm.a.10537
Grivet M, Morrier JJ, Benay G, Barsotti O (2000) Effect of hydrophobicity on in vitro streptococcal adhesion to dental alloys. J Mater Sci Mater Med 11(10):637–642. https://doi.org/10.1023/a:1008913915399
Bos R, Van der Mei HC, Busscher HJ (1999) Physico-chemistry of initial microbial adhesive interactions–its mechanisms and methods for study. FEMS Microbiol Rev 23(2):179–230. https://doi.org/10.1111/j.1574-6976.1999.tb00396.x
Pagán R, García-Gonzalo D (2015) Influence of environmental factors on bacterial biofilm formation in the food industry: a review. Postdoc J 3(6):4–13
Al-Amshawee S, Yunus MYBM (2021) Geometry of biofilm carriers: a systematic review deciding the best shape and pore size. Groundw Sustain Dev 12:100520. https://doi.org/10.1016/j.gsd.2020.100520
Wassmann T, Kreis S, Behr M, Buergers R (2017) The influence of surface texture and wettability on initial bacterial adhesion on titanium and zirconium oxide dental implants. Int J Implant Dentistry 3(1):1–11. https://doi.org/10.1186/s40729-017-0093-3
Zheng S, Bawazir M, Dhall A, Kim H, He EL, Heo J, Hwang G (2021) Implication of surface properties, bacterial motility, and hydrodynamic conditions on bacterial surface sensing and their initial adhesion. Front Bioeng Biotechnol 9:82. https://doi.org/10.3389/fbioe.2021.643722
Farber R, Dabush-Busheri I, Chaniel G, Rozenfeld S, Bormashenko E, Multanen V, Cahan R (2019) Biofilm grown on wood waste pretreated with cold low-pressure nitrogen plasma: utilization for toluene remediation. Int Biodeterior Biodegradat 139:62–69. https://doi.org/10.1016/j.ibiod.2019.03.003
Maurya A, Raj A (2020) Recent advances in the application of biofilm in bioremediation of industrial wastewater and organic pollutants. In: Microorganisms for sustainable environment and health. pp 81–118. https://doi.org/10.1016/B978-0-12-819001-2.00005-X
Armentano I et al (2014) The interaction of bacteria with engineered nanostructured polymeric materials: a review. Sci World J
Lata S, Sharma C, Singh AK (2012) Microbial influenced corrosion by thermophilic bacteria. Central Eur J Eng 2(1):113–122. https://doi.org/10.2478/s13531-011-0056-z
Videla HA, Herrera LK (2009) Understanding microbial inhibition of corrosion. A comprehensive overview. Int Biodeterior Biodegrad 63(7):896–900. https://doi.org/10.1016/j.ibiod.2009.02.002
Kip N, Van Veen JA (2015) The dual role of microbes in corrosion. ISME J 9(3):542–551. https://doi.org/10.1038/ismej.2014.169
Jia R, Yang D, Xu D, Gu T (2017) Anaerobic corrosion of 304 stainless steel caused by the Pseudomonas aeruginosa biofilm. Front Microbiol 8:2335. https://doi.org/10.3389/fmicb.2017.02335
Berne C, Ellison CK, Ducret A, Brun YV (2018) Bacterial adhesion at the single-cell level. Nat Rev Microbiol 16(10):616–627. https://doi.org/10.1038/s41579-018-0057-5
Dou W, Xu D, Gu T (2021) Biocorrosion caused by microbial biofilms is ubiquitous around us. Microb Biotechnol 14(3):803–805. https://doi.org/10.1111/2F1751-7915.13690
Rawat J, Sharma N, Khandewal A (2014) Microbiological causes of corrosion. Digital Refining, Bharat Petroleum Corporate R&D Center. https://www.digitalrefining.com/article/1000999/microbiological-causes-of-corrosion
Akiko O et al (2020) Biofilm formation plays a crucial rule in the initial step of carbon steel corrosion in air and water environments. Materials 13(4):923. https://doi.org/10.3390/ma13040923
Shuler ML, Kargi F (2002) Bioprocess engineering. Prentice Hall, New York
Coetser SE, Cloete TE (2008) Biofouling and biocorrosion in industrial water systems. Crit Rev Microbiol 31(4):213–232. https://doi.org/10.1080/10408410500304074
Stoodley P, Sauer K, Davies DG, Costerton JW (2002) Biofilms as complex differentiated communities. Ann Rev Microbiol 56(1):187–209. https://doi.org/10.1146/annurev.micro.56.012302.160705
Lewandowski Z, Beyenal H (2009) Mechanisms of microbially influenced corrosion. In: Marine and industrial biofouling. Springer, Heidelberg, pp 35–64
Zuo R, Kus E, Mansfeld F, Wood TK (2005) The importance of live biofilms in corrosion protection. Corros Sci 47(2):287. https://doi.org/10.1016/j.corsci.2004.09.006
Jayaraman A, Sun AK, Wood TK (1998) Characterization of axenic Pseudomonas fragi and Escherichia coli biofilms that inhibit corrosion of SAE 1018 steel. J Appl Microbiol 84(4):485–492. https://doi.org/10.1046/j.1365-2672.1998.00359.x
Zhang H, Tian Y, Wan J, Zhao P (2015) Study of biofilm influenced corrosion on cast iron pipes in reclaimed water. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2015.09.021
Bo L et al (2021) Study of biofilm-influenced corrosion on X80 pipeline steel by a nitrate-reducing bacterium, Bacillus cereus, in artificial Beijing soil. Colloids Surf B: Biointerfaces 197:111356. https://doi.org/10.1016/j.colsurfb.2020.111356
Sagar SS, Kumar R, Kaistha SD (2016) Biofilm: an eternalchronicle of bacteria. Indian J Comp Microbiol Immunol Infect Dis 37(2):45–56. https://doi.org/10.5958/0974-0147.2016.00010.6
Narayana SVVS, Srihari SVV (2019) A review on surface modifications and coatings on implants to prevent biofilm. Regen Eng Transl Med 6:330–346
Toyofuku M et al (2015) Environmental factors that shape biofilm formation. Biosci Biotehcnol Biochem 80:7–12. https://doi.org/10.1080/09168451.2015.1058701
Kırmusaoğlu S (2016) Staphylococcal biofilms: pathogenicity, mechanism and regulation of biofilm formation by quorum sensing system and antibiotic resistance mechanisms of biofilm embedded microorganisms. In: Microbial biofilms: importance and applications. IntechOpen. pp 189–209. https://doi.org/10.5772/62943
Videla HA, Herrera LK (2005) Microbiologically influenced corrosion: looking to the future. Int Microbiol 8(3):169
Zuo R (2007) Biofilms: strategies for metal corrosion inhibition employing microorganisms. Appl Microbiol Biotechnol 76(6):1245–1253. https://doi.org/10.1007/s00253-007-1130-6
Zuo R, Wook TK (2004) Inhibiting mild steel corrosion from sulfate-reducing and iron-oxidizing bacteria using gramicidin-S-producing biofilms. Appl Microbiol Biotechnol 65(6):747–753. https://doi.org/10.1007/s00253-004-1651-1
Procópio L (2019) The role of biofilms in the corrosion of steel in marine environments. World J Microbiol Biotechnol 35(5):1–8. https://doi.org/10.1007/s11274-019-2647-4
Little B, Lee J, Ray R (2007) A review of ‘green’strategies to prevent or mitigate microbiologically influenced corrosion. Biofouling 23(2):87–97. https://doi.org/10.1080/08927010601151782
Gao Y et al (2021) Inhibiting corrosion of aluminum alloy 5083 through Vibrio species biofilm. Corros Sci 180:109188. https://doi.org/10.1016/j.corsci.2020.109188
Li S et al (2019) Extracellular electron transfer of Bacillus cereus biofilm and its effect on the corrosion behaviour of 316L stainless steel. Colloids Surf B: Biointerfaces 173:139–147. https://doi.org/10.1016/j.colsurfb.2018.09.059
Jayaraman A et al (1997) Importance of biofilm formation for corrosion inhibition of SAE 1018 steel by axenic aerobic biofilms. J Indus Microbiol Biotechnol 18(6):396–401. https://doi.org/10.1038/sj.jim.2900396
Zabiegaj D et al (2021) Wetting/spreading on porous media and on deformable, soluble structured substrates as a model system for studying the effect of morphology on biofilms wetting and for assessing anti-biofilm methods. Curr Opin Colloid Interface Sci. https://doi.org/10.1016/j.cocis.2021.101426
Usher KM, Kaksonen AH, Cole I, Marney D (2014) Critical review: microbially influenced corrosion of buried carbon steel pipes. Int Biodeterior Biodegrad 93:84–106. https://doi.org/10.1016/j.ibiod.2014.05.007
Loto CA (2017) Microbiological corrosion: mechanism, control and impact: a review. Int J Adv Manuf Technol 92(9):4241–4252
Dubey RS, Dubey RS, Upadhyay SN (1999) A review of electrochemical techniques applied to microbiologically influenced corrosion in recent studies. http://nopr.niscair.res.in/handle/123456789/16927
Little BJ, Hinks J, Blackwood DJ (2020) Microbially influenced corrosion: towards an interdisciplinary perspective on mechanisms. Int Biodeterior Biodegradat 154:105062. https://doi.org/10.1016/j.ibiod.2020.105062
Victoria SN, Sharma A, Manivannan R (2021) Metal corrosion induced by microbial activity: mechanism and control options. J Indian Chem Soc https://doi.org/10.1016/j.jics.2021.100083
Xu D, Li Y, Gu T (2016) Mechanistic modeling of biocorrosion caused by biofilms of sulfate reducing bacteria and acid producing bacteria. Bioelectrochemistry 110:52–58
Du Z, Li H, Gu T (2007) A state of the art review on microbial fuel cells: a promising technology for wastewater treatment and bioenergy. Biotechnol Adv 25(5):464–482. https://doi.org/10.1016/j.biotechadv.2007.05.004
Li Y et al (2018) Anaerobic microbiologically influenced corrosion mechanisms interpreted using bioenergetics and bioelectrochemistry: a review. J Mater Sci Technol 34(10):1713–1718. https://doi.org/10.1016/j.jmst.2018.02.023
Gu JD (2003) Microbiological deterioration and degradation of synthetic polymeric materials: recent research advances. Int Biodeterior Biodegrad 52(2):69–91
Roberge P (2008) Corrosion engineering. McGraw-Hill Education
Beech IB, Gaylarde CC (1999) Recent advances in the study of biocorrosion: an overview. Rev Microbiol 30(3):117–190
Lewandowski Z (1994) Dissolved oxygen gradients near microbially colonized surfaces. Biofouling and biocorrosion in industrial water systems. 175–188
Videla HA (1995) Electrochemical aspects of biocorrosion. Bioextraction and biodeterioration of metals
Zlatev R et al (2013) Microbially induced corrosion rate determination applying clark amperometric sensor. Int J Electrochem Sci 8:1079–1094
Kotu SP, Mannan MS, Jayaraman A (2019) Emerging molecular techniques for studying microbial community composition and function in microbiologically influenced corrosion. Int Biodeterior Biodegradation 144:104722. https://doi.org/10.1016/j.ibiod.2019.104722
Murga R, Forster T, Brown SE, Pruckler JM, Fields B, Donlan RM (2001) Role of biofilms in the survival of Legionella pneumophila in a model potable-water system. Microbiology 147(11):3121–3126. https://doi.org/10.1099/00221287-147-11-3121
Acknowledgements
None.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. The above written work is entirely non-funded.
Author information
Authors and Affiliations
Contributions
The above written work was carried out by MKP under the guidance of ML.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Approval
The following review work does not involve experimentation on animals or humans.
Consent for Publication
N/A.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pal, M.K., Lavanya, M. Microbial Influenced Corrosion: Understanding Bioadhesion and Biofilm Formation. J Bio Tribo Corros 8, 76 (2022). https://doi.org/10.1007/s40735-022-00677-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-022-00677-x