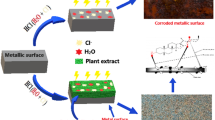
Abstract
Metallic materials have propelled human advancements for centuries. Nevertheless, the rapid degradation of metallic structures in corrosive environments is inevitable. To date, the demand for metal alloys in multiple sectors, including construction and transportation, continues to escalate, with the trend it is predicted to reach beyond 250% by 2050. Thus, the need for corrosion inhibitors (CI) with qualities of high efficiency, excellent solubility, low toxicity, and inexpensiveness drive researchers to examine various possibilities. Here, we review the novelty, methods, and outputs from the latest studies on organic CI with the primary purpose of protecting mild steel and stainless steel in salt and acidic solutions. We reviewed the effectiveness, synergism impacts, and technological availability of each inhibitor and also comprehensively discussed related mechanisms of metallic corrosion and inhibition of CI.






Similar content being viewed by others
References
Tamalmani K, Husin H (2020) Review on corrosion inhibitors for oil and gas corrosion issues. Appl Sci. https://doi.org/10.3390/app10103389
Xiaofei Z, Tao M, Xiaochun H, Jinde Z, Xiaoyi W, Sixian R (2020) Flow accelerated naphthenic acid corrosion during high acid crude oil refining. Eng Fail Anal. https://doi.org/10.1016/j.engfailanal.2020.104802
Zhang Q, Zheng M, Huang Y, Kunte HJ, Wang X, Liu Y, Zheng C (2019) Long term corrosion estimation of carbon steel, titanium and its alloy in backfill material of compacted bentonite for nuclear waste repository. Sci Rep. https://doi.org/10.1038/s41598-019-39751-9
Ganjeh M, Jafari SM, Amanjani M, Katouzian I (2017) Modeling corrosion trends in tin-free steel and tinplate cans containing tomato paste via adaptive-network-based fuzzy inference system. J Food Process Eng. https://doi.org/10.1111/jfpe.12580
Rajput A, Park JH, Hwan Noh S, Kee Paik J (2020) Fresh and sea water immersion corrosion testing on marine structural steel at low temperature. Ships Offshore Struct. https://doi.org/10.1080/17445302.2019.1664128
Wu J, Zhang W, Chai K, Yu A (2020) Corrosion behavior of AISI 1045 steel in seawater in the presence of Flavobacterium sp. Front Microbiol. https://doi.org/10.3389/fmicb.2020.00303
Askari M, Aliofkhazraei M, Afroukhteh S (2019) A comprehensive review on internal corrosion and cracking of oil and gas pipelines. J Nat Gas Sci Eng. https://doi.org/10.1016/j.jngse.2019.102971
Iloms E, Ololade OO, Ogola HJ, Selvarajan R (2020) Investigating industrial effluent impact on municipal wastewater treatment plant in Vaal, South Africa. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph17031096
Mali SA, Zhu D, Liu Y, Gilbert JL (2021) Fretting crevice corrosion of 316 L stainless steel in physiological phosphate buffered saline: load, potential and alloy counterface effects. Tribol Int. https://doi.org/10.1016/j.triboint.2021.107198
Palit S (2018). In: Kharisov B (ed) Recent advances in corrosion science: a critical overview and a deep comprehension. Elsevier, Amsterdam
P.H.M.S.A (2020) Pipeline incident 20 year trends. In: U.S.D. Transportation (ed) Pipeline and Hazardous Materials Safety Administration, Washington, United States
Koch G, Varney J, Thompson N, Moghissi O, Gould M, Payer J (2016) International measures of prevention, application, and economics of corrosion technologies study, In: Jacobson G (ed) NACE International, Texas, United States, pp 1–216
El-Sherik AM (2017) Trends in oil and gas corrosion research and technologies: production and transmission. Woodhead Publishing, Cambridge
El-Haddad MA, Radwan AB, Sliem MH, Hassan WM, Abdullah AM (2019) Highly efficient eco-friendly corrosion inhibitor for mild steel in 5 M HCl at elevated temperatures: experimental & molecular dynamics study. Sci Rep. https://doi.org/10.1038/s41598-019-40149-w
Huang CF, Yang CY, Tsai JR, Wu CT, Liu SH, Lan KC (2018) Low-dose tributyltin exposure induces an oxidative stress-triggered JNK-related pancreatic β-cell apoptosis and a reversible hypoinsulinemic hyperglycemia in mice. Sci Rep. https://doi.org/10.1038/s41598-018-24076-w
Simpson N, Maaijen K, Roelofsen Y, Hage R (2019) The evolution of catalysis for alkyd coatings: responding to impending cobalt reclassification with very active iron and manganese catalysts, using polydentate nitrogen donor ligands. Catalysts. https://doi.org/10.3390/catal9100825
Gharbi O, Thomas S, Smith C, Birbilis N (2018) Chromate replacement: what does the future hold? NPJ Mater Degrad. https://doi.org/10.1038/s41529-018-0034-5
Ćwieląg-Drabek M, Piekut A, Gut K, Grabowski M (2020) Risk of cadmium, lead and zinc exposure from consumption of vegetables produced in areas with mining and smelting past. Sci Rep. https://doi.org/10.1038/s41598-020-60386-8
Jiang S, Wang H, Wu Y, Liu X, Chen H, Yao M, Gault B, Ponge D, Raabe D, Hirata A (2017) Ultrastrong steel via minimal lattice misfit and high-density nanoprecipitation. Nature. https://doi.org/10.1038/nature22032
Raabe D, Springer H, Gutiérrez-Urrutia I, Roters F, Bausch M, Seol JB, Koyama M, Choi PP, Tsuzaki K (2014) Alloy design, combinatorial synthesis, and microstructure–property relations for low-density Fe-Mn-Al-C austenitic steels. JOM. https://doi.org/10.1007/s11837-014-1032-x
Li D, Wang J (2018) Study of supercritical power plant integration with high temperature thermal energy storage for flexible operation. J Energy Storage. https://doi.org/10.1016/j.est.2018.09.008
King F (2013) Container materials for the storage and disposal of nuclear waste. Corrosion. https://doi.org/10.5006/0894
Zhang LC, Chen LY (2019) A review on biomedical titanium alloys: recent progress and prospect. Adv Eng Mater. https://doi.org/10.1002/adem.201801215
Dunn BD (2015) Materials and processes: for spacecraft and high reliability applications. Springer, Switzerland
Allwood JM, Cullen JM, Carruth MA, Cooper DR, McBrien M, Milford RL, Moynihan MC, Patel AC (2012) Sustainable materials: with both eyes open. UIT Cambridge Limited, Cambridge
Prajila M, Ammal PR, Joseph A (2018) Comparative studies on the corrosion inhibition characteristics of three different triazine based Schiff’s bases, HMMT, DHMMT and MHMMT, for mild steel exposed in sulfuric acid. Egypt J Pet. https://doi.org/10.1016/j.ejpe.2017.07.011
Dwivedi D, Lepková K, Becker T (2017) Carbon steel corrosion: a review of key surface properties and characterization methods. RSC Adv. https://doi.org/10.1039/C6RA25094G
Cao Q, Esmaily M, Liu R, Birbilis N, Thomas S (2020) Corrosion of mild steel under insulation—the effect of dissolved metal ions. Corros Eng Sci Technol. https://doi.org/10.1080/1478422X.2020.1734737
Erna M, Herdini H, Futra D (2019) Corrosion inhibition mechanism of mild steel by amylose-acetate/carboxymethyl chitosan composites in acidic media. Int J Chem Eng. https://doi.org/10.1155/2019/8514132
Hou Y, Lei D, Li S, Yang W, Li C-Q (2016) Experimental investigation on corrosion effect on mechanical properties of buried metal pipes. Int J Corros. https://doi.org/10.1155/2016/5808372
Kim YS, Kim JG (2017) Corrosion behavior of pipeline carbon steel under different iron oxide deposits in the district heating system. Metals. https://doi.org/10.3390/met7050182
Jebaraj AV, Ajaykumar L, Deepak C, Aditya K (2017) Weldability, machinability and surfacing of commercial duplex stainless steel AISI2205 for marine applications—a recent review. J Adv Res. https://doi.org/10.1016/j.jare.2017.01.002
Tranchida G, Clesi M, Di Franco F, Di Quarto F, Santamaria M (2018) Electronic properties and corrosion resistance of passive films on austenitic and duplex stainless steels. Electrochim Acta. https://doi.org/10.1016/j.electacta.2018.04.058
Wang Z, Paschalidou E-M, Seyeux A, Zanna S, Maurice V, Marcus P (2019) Mechanisms of Cr and Mo enrichments in the passive oxide film on 316L austenitic stainless steel. Front Mater. https://doi.org/10.3389/fmats.2019.00232
Simcoe CR (2018) Stainless steel. In: Richards F (ed) The history of metals in America. ASM International, OH
Boillot P, Peultier J (2014) Use of stainless steels in the industry: recent and future developments. Procedia Eng. https://doi.org/10.1016/j.proeng.2014.09.015
Gary SW, Shigeharu U (2019) Austenitic stainless steels. In: Odette GR, Steven ZJ (ed) Structural alloys for nuclear energy applications. Newnes, Amsterdam, Netherlands
Klapper HS, Zadorozne NS, Rebak RB (2017) Localized corrosion characteristics of nickel alloys: a review. Acta Metall Sin. https://doi.org/10.1007/s40195-017-0553-z
Alwan AS, Fayyadh SK, Khalid EA (2019) A review: behavior of pitting corrosion in manufacturing food equipment. Iraqi J Agric Sci 50(4):1–7. https://doi.org/10.36103/ijas.v50i4.744
Niu LB, Okano K, Izumi S, Shiokawa K, Yamashita M, Sakai Y (2018) Effect of chloride and sulfate ions on crevice corrosion behavior of low-pressure steam turbine materials. Corros Sci. https://doi.org/10.1016/j.corsci.2017.12.017
Lindgren M, Huttunen-Saarivirta E, Peltola H, Romu J, Sarikka T, Hänninen H, Pohjanne P (2018) Crevice corrosion of stainless steels 904L, 2205, and 2507 in high-temperature sulfuric acid solution containing chlorides: influence of metal cations. Corrosion. https://doi.org/10.5006/2565
Gilbert JL, Zhu D (2020) A metallic biomaterial tribocorrosion model linking fretting mechanics, currents, and potentials: model development and experimental comparison. J Biomed Mater Res B: Appl Biomater. https://doi.org/10.1002/jbm.b.34643
Liu Y, Zhu D, Pierre D, Gilbert JL (2019) Fretting initiated crevice corrosion of 316LVM stainless steel in physiological phosphate buffered saline: potential and cycles to initiation. Acta Biomater. https://doi.org/10.1016/j.actbio.2019.07.051
Kuang W, Han E-H, Wu X, Rao J (2010) Microstructural characteristics of the oxide scale formed on 304 stainless steel in oxygenated high temperature water. Corros Sci. https://doi.org/10.1016/j.corsci.2010.07.015
Ziemniak S, Hanson M (2002) Corrosion behavior of 304 stainless steel in high temperature, hydrogenated water. Corros Sci. https://doi.org/10.1016/S0010-938X(02)00004-5
Lepingle V, Louis G, Allue D, Lefebvre B, Vandenberghe B (2008) Steam oxidation resistance of new 12% Cr steels: comparison with some other ferritic steels. Corros Sci. https://doi.org/10.1016/j.corsci.2007.11.033
Zurek J, Nieto-Hierro L, Piron-Abellan J, Niewolak L, Singheiser L, Quadakkers WJ (2004) Effect of alloying additions in ferritic 9–12% Cr steels on the temperature dependence of the steam oxidation resistance, 6th international symposium on high temperature corrosion and protection of materials. Trans Tech Publ, Embiez, France, pp 791–798
Nazari MH, Shi X (2018) Vehicle risks of winter road operations and best management practices. In: Shi X, Fu L (eds) Sustainable winter road operations. Wiley Online Library, Hoboken, NJ
Zhong ZW (2020) Advanced polishing, grinding and finishing processes for various manufacturing applications: a review. Mater Manuf Processes. https://doi.org/10.1080/10426914.2020.1772481
Brownlie F, Giourntas L, Hodgkiess T, Palmeira I, Odutayo O, Galloway A, Pearson A (2020) Effect of cathodic protection methods on ferrous engineering materials under corrosive wear conditions. Corros Eng Sci Technol. https://doi.org/10.1080/1478422X.2020.1742997
Oleiwi HM, Wang Y, Curioni M, Chen X, Yao G, Augusthus-Nelson L, Ragazzon-Smith A, Shabalin I (2018) An experimental study of cathodic protection for chloride contaminated reinforced concrete. Mater Struct. https://doi.org/10.1617/s11527-018-1273-1
Santos W, Brasil S, Santiago J, Telles J (2018) A new solution technique for cathodic protection systems with homogeneous region by the boundary element method. Eur J Comput Mech. https://doi.org/10.1080/17797179.2018.1439138
Yang M, Kainuma S, Zhuang S, Ishihara S, Kaneko A, Yamauchi T (2019) Cathodic protection system applied to steel using fiber sheet and Al-based alloy anode in atmospheric environment. Int J Electrochem Sci. https://doi.org/10.20964/2019.10.40
He J, Guo X, Qiao Y, Luo F (2020) A novel Zr-Y modified silicide coating on Nb-Si based alloys as protection against oxidation and hot corrosion. Corros Sci. https://doi.org/10.1016/j.corsci.2020.108948
Kinnunen-Raudaskoski K, Hjelt T, Kenttä E, Forsström U (2014) Thin coatings for paper by foam coating. Tappi J 13(7):9–19
Liu J, Cui Z, Ma D, Lu J, Cui Y, Li C, Liu W, Hao Z, Hu P, Yao M (2020) Investigation of oxidation behaviors of coated zircaloy as accident-tolerant fuel with CrAlN and CrAlSiN coatings in high-temperature steam. Corros Sci. https://doi.org/10.1016/j.corsci.2020.108896
Rajput A, Ak M, Kim SJ, Noh SH, Park JH, Paik JK (2019) Effects of the surface preparation on the life of epoxy coating in steel ship plates: an experimental study. Ships Offshore Struct. https://doi.org/10.1080/17445302.2019.1565072
Zhang F, Ju P, Pan M, Zhang D, Huang Y, Li G, Li X (2018) Self-healing mechanisms in smart protective coatings: a review. Corros Sci. https://doi.org/10.1016/j.corsci.2018.08.005
Zulkifli F, Yusof MSM, Isa M, Yabuki A, Nik WW (2017) Henna leaves extract as a corrosion inhibitor in acrylic resin coating. Prog Org Coat. https://doi.org/10.1016/j.porgcoat.2017.01.017
Parikh V, Badheka V, Badgujar A, Ghetiya N (2021) Fabrication and processing of aluminum alloy metal matrix composites. Mater Manuf Processes. https://doi.org/10.1080/10426914.2021.1914848
Iannuzzi M, Barnoush A, Johnsen R (2017) Materials and corrosion trends in offshore and subsea oil and gas production. npj Mater Degrad 1(1):1–1
Chigondo M, Chigondo F (2016) Recent natural corrosion inhibitors for mild steel: an overview. J Chem. https://doi.org/10.1155/2016/6208937
Faiz M, Zahari A, Awang K, Hussin H (2020) Corrosion inhibition on mild steel in 1 M HCl solution by Cryptocarya nigra extracts and three of its constituents (alkaloids). RSC Adv. https://doi.org/10.1039/C9RA05654H
Honarvar Nazari M, Shihab MS, Havens EA, Shi X (2020) Mechanism of corrosion protection in chloride solution by an apple-based green inhibitor: experimental and theoretical studies. J Infrastruct Preserv Resil. https://doi.org/10.1186/s43065-020-00007-w
Espinoza-Vázquez A, Rodríguez-Gómez F, Negrón-Silva G, González-Olvera R, Ángeles-Beltrán D, Palomar-Pardavé M, Miralrio A, Castro M (2020) Fluconazole and fragments as corrosion inhibitors of API 5L X52 steel immersed in 1M HCl. Corros Sci. https://doi.org/10.1016/j.corsci.2020.108853
Su H, Wang L, Wu Y, Zhang Y, Zhang J (2020) Insight into inhibition behavior of novel ionic liquids for magnesium alloy in NaCl solution: experimental and theoretical investigation. Corros Sci. https://doi.org/10.1016/j.corsci.2019.108410
Taghavikish M, Dutta NK, Roy Choudhury N (2017) Emerging corrosion inhibitors for interfacial coating. Coatings. https://doi.org/10.3390/coatings7120217
Goyal M, Kumar S, Bahadur I, Verma C, Ebenso EE (2018) Organic corrosion inhibitors for industrial cleaning of ferrous and non-ferrous metals in acidic solutions: a review. J Mol Liq. https://doi.org/10.1016/j.molliq.2018.02.045
Srivastava M, Tiwari P, Srivastava SK, Prakash R, Ji G (2017) Electrochemical investigation of Irbesartan drug molecules as an inhibitor of mild steel corrosion in 1 M HCl and 0.5 M H2SO4 solutions. J Mol Liq. https://doi.org/10.1016/j.molliq.2017.04.017
Tsoeunyane M, Makhatha M, Arotiba O (2019) Corrosion inhibition of mild steel by poly (butylene succinate)-L-histidine extended with 1, 6-diisocynatohexane polymer composite in 1 M HCl. Int J Corros. https://doi.org/10.1155/2019/7406409
Yaro AS, Khadom AA, Wael RK (2013) Apricot juice as green corrosion inhibitor of mild steel in phosphoric acid. Alex Eng J. https://doi.org/10.1016/j.aej.2012.11.001
Guo L, Qi C, Zheng X, Zhang R, Shen X, Kaya S (2017) Toward understanding the adsorption mechanism of large size organic corrosion inhibitors on an Fe (110) surface using the DFTB method. RSC Adv. https://doi.org/10.1039/C7RA04120A
Rani B, Basu BBJ (2012) Green inhibitors for corrosion protection of metals and alloys: an overview. Int J Corros. https://doi.org/10.1155/2012/380217
Tiu BDB, Advincula RC (2015) Polymeric corrosion inhibitors for the oil and gas industry: design principles and mechanism. React Funct Polym. https://doi.org/10.1016/j.reactfunctpolym.2015.08.006
Umoren SA, Solomon MM (2020) Polymeric corrosion inhibitors for oil and gas industry. In: Viswanathan SS, Saviour AU (eds) Corrosion inhibitors in the oil and gas industry. Wiley, Hoboken
Ali MM, Irawadi TT, Darmawan N, Khotib M, Mas’ud ZA (2019) Reaction products of crude palm oil-based fatty acids and monoethanolamine as corrosion inhibitors of carbon steel. Makara J Sci. https://doi.org/10.7454/mss.v23i3.11263
Elsharif AM, Abubshait SA, Abdulazeez I, Abubshait HA (2020) Synthesis of a new class of corrosion inhibitors derived from natural fatty acid: 13-docosenoic acid amide derivatives for oil and gas industry. Arab J Chem. https://doi.org/10.1016/j.arabjc.2020.03.015
Vázquez-Vélez E, Gonzalez-Rodriguez J, Escalante-Pérez M, Mendoza J, Martínez-Gómez L (2019) Use of fatty amide and anionic surfactant as corrosion inhibitors for carbon steel in different atmospheres. Int J Corros Scale Inhib. https://doi.org/10.17675/2305-6894-2019-8-1-11
Velazquez-Torres N, Martinez H, Porcayo-Calderon J, Vazquez-Velez E, Gonzalez-Rodriguez J, Martinez-Gomez L (2018) Use of an amide-type corrosion inhibitor synthesized from the coffee bagasse oil on the corrosion of Cu in NaCl. Green Chem Lett Rev. https://doi.org/10.1080/17518253.2017.1404645
Ahmed SK, Ali WB, Khadom AA (2019) Synthesis and investigations of heterocyclic compounds as corrosion inhibitors for mild steel in hydrochloric acid. Int J Ind Chem. https://doi.org/10.1007/s40090-019-0181-8
Petrunin M, Maksaeva L, Gladkikh N, Makarychev Y, Maleeva M, Yurasova T, Nazarov A (2020) Thin benzotriazole films for inhibition of carbon steel corrosion in neutral electrolytes. Coatings. https://doi.org/10.3390/coatings10040362
Xhanari K, Finšgar M (2019) The corrosion inhibition of AA6082 aluminium alloy by certain azoles in chloride solution: electrochemistry and surface analysis. Coatings. https://doi.org/10.3390/coatings9060380
Salim R, Ech-chihbi E, Oudda H, El Hajjaji F, Taleb M, Jodeh S (2019) A Review on the assessment of imidazo [1, 2-a] pyridines as corrosion inhibitor of metals. J Bio Tribo Corros. https://doi.org/10.1007/s40735-018-0207-3
Zhang W, Li HJ, Wang Y, Liu Y, Wu YC (2018) Adsorption and corrosion inhibition properties of pyridine-2-aldehyde-2-quinolylhydrazone for Q235 steel in acid medium: electrochemical, thermodynamic, and surface studies. Mater Corros. https://doi.org/10.1002/maco.201810252
Gonzalez-Rodriguez J, Porcayo-Calderon J, Vazquez-Velez E, de la Escalera LM, Canto J, Martinez L (2016) Palm oil-based imidazolines as corrosion inhibitor for copper in 1.0 M H2SO4. J Adv Electrochem 2:97–102
Ismail A, Irshad H, Zeino A, Toor I (2019) Electrochemical corrosion performance of aromatic functionalized imidazole inhibitor under hydrodynamic conditions on API X65 carbon steel in 1 M HCl solution. Arab J Sci Eng. https://doi.org/10.1007/s13369-019-03745-6
Olajire AA (2017) Corrosion inhibition of offshore oil and gas production facilities using organic compound inhibitors—a review. J Mol Liq. https://doi.org/10.1016/j.molliq.2017.10.097
Wang P, Wang Y, Zhao T, Xiong C, Xu P, Zhou J, Fan Z (2020) Effectiveness protection performance of an internal blending organic corrosion inhibitor for carbon steel in chloride contaminated simulated concrete pore solution. J Adv Concr Technol. https://doi.org/10.3151/jact.18.116
Deyab M, Guibal E (2020) Enhancement of corrosion resistance of the cooling systems in desalination plants by green inhibitor. Sci Rep. https://doi.org/10.1038/s41598-020-61810-9
Fouda A, Emam A, Refat R, Nageeb M (2019) Eco-friendly plant extract of Medicago sativa (Alfalfa) as corrosion inhibitor for carbon steel in marine environment. Surf Eng Appl Electrochem. https://doi.org/10.3103/S1068375519030074
Haddadi SA, Alibakhshi E, Bahlakeh G, Ramezanzadeh B, Mahdavian M (2019) A detailed atomic level computational and electrochemical exploration of the Juglans regia green fruit shell extract as a sustainable and highly efficient green corrosion inhibitor for mild steel in 3.5 wt% NaCl solution. J Mol Liq. https://doi.org/10.1016/j.molliq.2019.04.045
Devikala S, Kamaraj P, Arthanareeswari M, Patel MB (2019) Green corrosion inhibition of mild steel by aqueous Allium sativum extract in 35% NaCl. Mater Today. https://doi.org/10.1016/j.matpr.2019.04.182
Ikhmal WMKWM, Maria MFM, Rafizah WAW, Norsani WNWM, Sabri MGM (2019) Corrosion inhibition of mild steel in seawater through green approach using Leucaena leucocephala leaves extract. Int J Corros Scale Inhib. https://doi.org/10.17675/2305-6894-2019-8-3-12
Ikhmal WMKWM, Yasmin MYN, Fazira MFM, Rafizah WAW, Nik WBW, Sabri MGM (2018) Anticorrosion coating using Olea sp leaves extract. IOP Conf Ser Mater Sci Eng. https://doi.org/10.1088/1757-899X/344/1/012028
Maria M, Ikhmal W, Amirah M, Manja S, Syaizwadi S, Chan K, Sabri M, Adnan A (2019) Green approach in anti-corrosion coating by using Andrographis paniculata leaves extract as additives of stainless steel 316L in seawater. Int J Corros Scale Inhib. https://doi.org/10.17675/2305-6894-2019-8-3-13
Loto RT, Fajobi M, Oluwole O, Loto CA (2020) Corrosion inhibition effect of calcium gluconate on mild steel in artificial seawater. Cogent Eng. https://doi.org/10.1080/23311916.2020.1712155
Schmitzhaus TE, Ortega Vega MR, Schroeder R, Muller IL, Mattedi S, Malfatti CdF (2020) An amino-based protic ionic liquid as a corrosion inhibitor of mild steel in aqueous chloride solutions. Mater Corros. https://doi.org/10.1002/maco.201911347
Moradi M, Ye S, Song Z (2019) Dual role of Psudoalteromonas piscicida biofilm for the corrosion and inhibition of carbon steel in artificial seawater. Corros Sci. https://doi.org/10.1016/j.corsci.2019.02.025
Pakiet M, Tedim J, Kowalczyk I, Brycki B (2019) Functionalized novel gemini surfactants as corrosion inhibitors for mild steel in 50 mM NaCl: experimental and theoretical insights. Colloids Surf A Physicochem Eng Asp. https://doi.org/10.1016/j.molliq.2019.04.045
Wang C, Chen J, Hu B, Liu Z, Wang C, Han J, Su M, Li Y, Li C (2019) Modified chitosan-oligosaccharide and sodium silicate as efficient sustainable inhibitor for carbon steel against chloride-induced corrosion. J Clean Prod. https://doi.org/10.1016/j.jclepro.2019.117823
Somers AE, Hinton BR, de Bruin-Dickason C, Deacon GB, Junk PC, Forsyth M (2018) New, environmentally friendly, rare earth carboxylate corrosion inhibitors for mild steel. Corros Sci. https://doi.org/10.1016/j.corsci.2018.05.017
Peng Y, Hughes AE, Deacon GB, Junk PC, Hinton BR, Forsyth M, Mardel JI, Somers AE (2018) A study of rare-earth 3-(4-methylbenzoyl)-propanoate compounds as corrosion inhibitors for AS1020 mild steel in NaCl solutions. Corros Sci. https://doi.org/10.1016/j.corsci.2018.09.022
Talebian M, Raeissi K, Atapour M, Fernández-Pérez B, Salarvand Z, Meghdadi S, Amirnasr M, Souto R (2018) Inhibitive effect of sodium (E)-4-(4-nitrobenzylideneamino) benzoate on the corrosion of some metals in sodium chloride solution. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2018.04.073
Dehghani A, Bahlakeh G, Ramezanzadeh B (2019) A detailed electrochemical/theoretical exploration of the aqueous Chinese gooseberry fruit shell extract as a green and cheap corrosion inhibitor for mild steel in acidic solution. J Mol Liq. https://doi.org/10.1016/j.molliq.2019.03.011
Fernandes CM, Fagundes TdSF, dos Santos NE, Rocha TSdM, Garrett R, Borges RM, Muricy G, Valverde AL, Ponzio EA (2019) Ircinia strobilina crude extract as corrosion inhibitor for mild steel in acid medium. Electrochim Acta. https://doi.org/10.1016/j.electacta.2019.04.148
Divya P, Subhashini S, Prithiba A, Rajalakshmi R (2019) Tithonia diversifolia flower extract as green corrosion inhibitor for mild steel in acid medium. Mater Today. https://doi.org/10.1016/j.matpr.2019.05.252
Salhi A, Hamdani I, Bouyanzer A, Chahboun N, Amhamdi H, Warad I, Hammouti B, Bentiss F, Zarrouk A (2018) Phytochemical analysis, antioxidant and anticorrosive activities of Thymus algeriensis extracts. Anal Bioanal Electrochem 10:1587–1610
Ramezanzadeh M, Bahlakeh G, Sanaei Z, Ramezanzadeh B (2018) Studying the Urtica dioica leaves extract inhibition effect on the mild steel corrosion in 1 M HCl solution: complementary experimental, ab initio quantum mechanics, Monte Carlo and molecular dynamics studies. J Mol Liq. https://doi.org/10.1016/j.molliq.2018.09.059
Liao LL, Mo S, Luo HQ, Li NB (2018) Corrosion protection for mild steel by extract from the waste of lychee fruit in HCl solution: experimental and theoretical studies. J Colloid Interface Sci. https://doi.org/10.1016/j.jcis.2018.02.071
Alibakhshi E, Ramezanzadeh M, Bahlakeh G, Ramezanzadeh B, Mahdavian M, Motamedi M (2018) Glycyrrhiza glabra leaves extract as a green corrosion inhibitor for mild steel in 1 M hydrochloric acid solution: experimental, molecular dynamics, Monte Carlo and quantum mechanics study. J Mol Liq. https://doi.org/10.1016/j.molliq.2018.01.144
Cordeiro RF, Belati AJ, Perrone D, D’Elia E (2018) Coffee husk as corrosion inhibitor for mild steel in HCl media. Int J Electrochem Sci. https://doi.org/10.20964/2018.12.29
Singh P, Chauhan D, Chauhan S, Singh G, Quraishi M (2019) Chemically modified expired Dapsone drug as environmentally benign corrosion inhibitor for mild steel in sulphuric acid useful for industrial pickling process. J Mol Liq. https://doi.org/10.1016/j.molliq.2019.110903
Verma C, Obot I, Bahadur I, Sherif E-SM, Ebenso EE (2018) Choline based ionic liquids as sustainable corrosion inhibitors on mild steel surface in acidic medium: gravimetric, electrochemical, surface morphology, DFT and Monte Carlo simulation studies. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2018.06.035
Rabizadeh T, Khameneh-Asl S (2019) Chitosan as a green inhibitor for mild steel corrosion: thermodynamic and electrochemical evaluations. Mater Corros. https://doi.org/10.1002/maco.201810501
Ahmed MHO, Al-Amiery AA, Al-Majedy YK, Kadhum AAH, Mohamad AB, Gaaz TS (2018) Synthesis and characterization of a novel organic corrosion inhibitor for mild steel in 1 M hydrochloric acid. Results Phys. https://doi.org/10.1016/j.rinp.2017.12.039
Al-Fakih AM, Abdallah HH, Aziz M (2019) Experimental and theoretical studies of the inhibition performance of two furan derivatives on mild steel corrosion in acidic medium. Mater Corros. https://doi.org/10.1002/maco.201810221
Idouhli R, Koumya Y, Khadiri M, Aityoub A, Abouelfida A, Benyaich A (2019) Inhibitory effect of Senecio anteuphorbium as green corrosion inhibitor for S300 steel. Int J Ind Chem. https://doi.org/10.1007/s40090-019-0179-2
Liu L, Cao TT, Zhang QW, Cui CW (2018) Organic phosphorus compounds as inhibitors of corrosion of carbon steel in circulating cooling water: weight loss method and thermodynamic and quantum chemical studies. Adv Mater Sci Eng. https://doi.org/10.1155/2018/1653484
Xavier Stango SA, Vijayalakshmi U (2018) Studies on corrosion inhibitory effect and adsorption behavior of waste materials on mild steel in acidic medium. J Asian Ceram Soc. https://doi.org/10.1080/21870764.2018.1439608
Faisal M, Saeed A, Shahzad D, Abbas N, Larik FA, Channar PA, Fattah TA, Khan DM, Shehzadi SA (2018) General properties and comparison of the corrosion inhibition efficiencies of the triazole derivatives for mild steel. Corros Rev. https://doi.org/10.1515/corrrev-2018-0006
Varvara S, Găină L, Bostan R, Popa F, Grozav A (2018) Some phenothiazinyl-thiazolyl-hydrazine derivatives as corrosion inhibitors for carbon steel in 1.0 m HCl: electrochemical, SEM-EDX and DFT investigations. Int J Electrochem Sci. https://doi.org/10.20964/2018.09.32
Deng S, Li X, Xie X (2014) Hydroxymethyl urea and 1, 3-bis (hydroxymethyl) urea as corrosion inhibitors for steel in HCl solution. Corros Sci. https://doi.org/10.1016/j.corsci.2013.11.041
Verma C, Quraishi M, Olasunkanmi L, Ebenso EE (2015) L-Proline-promoted synthesis of 2-amino-4-arylquinoline-3-carbonitriles as sustainable corrosion inhibitors for mild steel in 1 M HCl: experimental and computational studies. RSC Adv. https://doi.org/10.1039/C5RA16982H
Verma C, Quraishi M, Singh A (2016) A thermodynamical, electrochemical, theoretical and surface investigation of diheteroaryl thioethers as effective corrosion inhibitors for mild steel in 1 M HCl. J Taiwan Inst Chem Eng. https://doi.org/10.1016/j.jtice.2015.06.020
Verma CB, Quraishi M, Singh A (2015) 2-Aminobenzene-1, 3-dicarbonitriles as green corrosion inhibitor for mild steel in 1 M HCl: electrochemical, thermodynamic, surface and quantum chemical investigation. J Taiwan Inst Chem Eng. https://doi.org/10.1016/j.jtice.2014.11.029
Frolenkova S, Overchenko T, Motronyuk T, Vorobyova V, Miroshnychenko I, Panchenko M (2019) Passivating anions effect on the anodic behavior of steel in a converting acetate solution. J Chem Technol Metall 54(2):443–446
Tang Z (2019) A review of corrosion inhibitors for rust preventative fluids. Curr Opin Solid State Mater Sci. https://doi.org/10.1016/j.cossms.2019.06.003
Yang Z, Guo M, Wang N, Ma C, Wang J, Han M (2017) A short review of cathode poisoning and corrosion in solid oxide fuel cell. Int J Hydrog Energy. https://doi.org/10.1016/j.ijhydene.2017.08.057
Kundu SS, Seiersten M (2017) Development of a non-sulphite oxygen scavenger for monoethylene glycol (MEG) used as gas hydrate inhibitor. J Pet Sci Eng. https://doi.org/10.1016/j.petrol.2017.07.041
Acknowledgements
Sincere gratitude to the Universiti Malaysia Terengganu for providing the necessary tools to complete the review.
Funding
This research was funded by the Ministry of Higher Education (Malaysia) through the Fundamental Research Grant Scheme (Award no. FRGS/1/2018/WAB09/UMT/02/2, Vot no. 59537).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author claims no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kamaruzzaman, W.M.I.W.M., Nasir, N.A.M., Hamidi, N.A.S.M. et al. Frontiers in Organic Corrosion Inhibitors for Chloride and Acidic Media: A Review. J Bio Tribo Corros 8, 37 (2022). https://doi.org/10.1007/s40735-022-00638-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-022-00638-4