Abstract
Purpose of Review
Approximately 40 years ago, key papers indicating that volatile chemicals released by damaged plants elicited defense-related changes in their neighbors, brought prominence to the idea of plant communication. These studies were conducted with several tree species and the phenomenon observed was dubbed “talking trees.” Today there is a wealth of evidence supporting the idea that plants can send and receive information both above and belowground. However, while early reports of plant-plant communication concerned trees, the literature is now heavily biased towards herbaceous plants. The purpose of this review is to highlight recent research on tree-tree communication with an emphasis on synthesizing knowledge on the ecological relevance of the process.
Recent Findings
Aboveground, information is often provided in the form of biogenic volatile organic compounds (VOCs), which are released by both undamaged and damaged plants. The blends of VOCs released by plants provide information on their physiological condition. Belowground, information is conveyed through mycorrhizal networks and via VOCs and chemical exudates released into the rhizosphere. Recent findings have indicated a sophistication to tree communication with more effective VOC-mediated interactions between trees of the same versus a different genotype, kin-group, or chemotype. Moreover, common mycorrhizal networks have been shown to convey stress-related signals in intra- and interspecific associations. Together these two forms of communication represent “wireless” and “wired” channels with significance to facilitating plant resistance to herbivores.
Summary
In this review, we examine tree-tree communication with a focus on research in natural forest ecosystems. We particularly address the effects of tree-tree communication on interactions with herbivorous insects. Aboveground and belowground interactions are both reviewed and suggested implications for forest management and future research are presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, there has been a proliferation of scientific research on the topic of plant communication and abundant articles in the popular press. The body of literature has fascinated the public and provided numerous metaphors that can extend to guiding human activities, including economic policy and societal organization [1]. Contemporary rethinking of relations between human self-interest and shared flourishing in the face of financial crashes, social destabilization, and ecological catastrophe can potentially draw on the “lessons” of the altruistic forest [1]. However, whether plant communication represents altruism, mutualism, or a competitive or even pernicious interaction remains open for debate. Indeed, even the definition of plant communication has been extensively dissected and reformulated during the last few decades [e.g., [2, 3]. It is now commonly accepted that true plant-plant communication would require both the signal sender and the receiver to benefit, which is often difficult to determine. However, meaningful plant-plant interactions that fall short of the true plant-plant communication classification have been reported in abundance and they occur both above and belowground. Here we look at plant-plant communication in trees with a particular emphasis on their effects on plant–herbivore interactions.
Interactions between trees and herbivores are key processes shaping life history traits and dynamics of forest ecosystems [4,5,6]. By consuming plant tissues, herbivorous insects can reduce tree survival and growth, as well as affecting nutrient and carbon fluxes [7, 8]. Ultimately, herbivory can affect tree fitness—defined as the ability to contribute the tree’s genotype to succeeding generations [9]—and forest regeneration. Chronic background insect herbivory is estimated to cause biomass loss of 1 to 15% annually, depending on geographic region [10]. Insect herbivore population dynamics are influenced by multiple ecological drivers. For example, tree diversity is well known to affect the abundance of insects at the landscape scale [11] and more diverse forests are often associated with lower herbivore pressure [12, 13]. In addition, insect herbivory is influenced by nutrients, and physical and chemical defensive traits of trees [14, 15]. Plant strategies to combat herbivores include an array of constitutive defenses that are continually present, e.g., alkaloids and phenolic compounds in plant tissue, and inducible defenses that are activated when plants detect a threat, e.g., by being fed on by an herbivore, by sensing herbivore eggs or herbivore induced plant volatiles (HIPVs) [16, 17]. The defenses can also be direct, such as toxic defense metabolites that are expressed constitutively [18], or indirect, such as HIPVs that attract natural enemies of herbivores [19]. Appropriate responses to reliable information from neighboring trees should in principle enable raising of effective defenses against herbivores and reduce biomass loss and potential fitness costs.
Plant-plant communication has been shown to play a key role in defense against herbivore attack [20]. Initial reports on chemical-based plant-plant communication and defense against herbivores were published in the 1980s [21,22,23]. The understanding of plant communication has advanced substantially since and it is now widely established that plants recognize and interact with each other and the surrounding community of herbivorous and beneficial arthropods [20, 24,25,26,27]. Recognition of herbivores via herbivore-associated molecular patterns (HAMPs) is an example of how plants perceive their environment [28, 29], while HIPV emission and detection enable plants to interact with other organisms [30]. Plant-plant communication through informative chemical cues or signals—also known as infochemicals—can occur above- and belowground [31, 32]. The aboveground interactions are largely mediated by biogenic volatile organic compound (BVOC) emissions and belowground interactions by root-associated pathways involving common mycorrhizal networks (CMNs), volatile emissions, and chemical exudates [25, 33, 34]. The CMN-based communication in the rhizosphere can be considered “wired” and the BVOC-based communication aboveground considered “wireless,” indicating a complexity of information processing that can depend on the medium through which signals traverse.
Plant-plant communication has been reviewed in the context of the diversity of communication signals and its role in crop and produce plantations [34,35,36]. In this review, we focus on addressing how these processes can affect herbivory in forests which are more diverse in species richness (both plant and animal) compared to agricultural systems. Moreover, it is important to note that trees are usually long-lived species that may have different priorities for resource partitioning than short-lived plants and may consequently respond differently to damage-induced infochemicals. We synthesize current knowledge on tree-tree communication via “wireless” aboveground BVOC emissions and “wired” CMN-mediated belowground processes. Discoveries in the field of chemical ecology provide compelling evidence that trees may cooperate by both sharing their resources and by emitting stress signals that induce defenses in nearby trees. We identify some potential considerations for forest management, highlight gaps in the current knowledge, and suggest potential directions for future research.
Aboveground BVOC-Mediated Communication In Trees
BVOCs as a “Wireless” Plant Communication Medium
BVOCs are low molecular mass compounds with a high vapor pressure. Plant-emitted BVOCs can be released above or belowground [37] and most are classified into compound groups that include isoprenoids (or terpenoids), green leaf volatiles, and aromatic compounds [31] (Fig. 1). The global estimate of BVOCs released by plants to the atmosphere is about 1 Pg carbon (C) year−1, of which isoprene is the most dominant compound [38, 39]. Isoprene is a small (C5), highly reactive compound with an atmospheric lifetime of about 1–2 h. It is the building block of larger isoprenoids such as the volatile monoterpenes (C10) and sesquiterpenes (C15) [26, 39]. Isoprenoids are synthesized in the plastids via the mevalonate (MVA) pathway and in the cytosol (Fig. 1) via the methylerythritol phosphate (MEP) pathway from the C5 precursor molecules isopentenyl diphosphate (IPP) and its interconvertible isomer dimethylallyl diphosphate (DMAPP) [31, 40]. Isoprene is the single most important BVOC in the Earth system [26, 41] and the isoprenoid group is structurally and functionally the most diverse in living organisms [40]. Besides isoprenoids, plants also emit green leaf volatiles (GLV), which are a group of diverse C6 compounds comprising alcohols, esters, and aldehydes [42]. Membrane lipids and fatty acids are used to synthesize the most common GLVs via the hydroperoxide lyase (HPL) branch of the oxylipin pathway [42,43,44] (Fig. 1). Aromatic BVOCs include phenolic and benzenoid compounds that are synthesized via the shikimate pathway [45] (Fig. 1).
Schematic representation of the two main communication channels reviewed here. Aboveground, plants release blends of volatile organic compounds (VOCs) including green leaf volatiles, phenylpropanoids and benzenoids, sesquiterpenes and monoterpenes, which are formed by different biochemical pathways in different parts of the cell, as indicated on the right. Belowground, different mycorrhizal structures are depicted that function in producing common mycorrhizal networks that mediate signaling between plants. The VOCs represent the wireless communication channel, while mycorrhizae represent a wired channel
Plants constitutively release BVOCs from all above and belowground parts such as leaves, flower buds, flowers, fruits [e.g., 46], needles [47], and roots [37, 48, 49]. The release of the BVOCs is regulated by plant hormones, notably jasmonates, salicylic acid, and ethylene [50,51,52]. The sensitivity of BVOC emissions to biotic and abiotic stresses has been extensively reviewed [31, 53, 54]. Blends of HIPVs can be specific to a plant species and the herbivores feeding on it [51]. GLVs are usually the first HIPVs to be detected, occurring within minutes of insect feeding starting, while terpenoids and other volatile compounds such as methyl-jasmonate and methyl-salicylate are emitted several hours after damage initiation [55]. The active signals within volatile blends have been investigated extensively with diverse ecological interactions shown to be mediated by these chemical cues and signals.
Mechanisms of Aboveground BVOC-Mediated Communication in Trees
Initial reports of aboveground volatile-mediated plant-plant communication were made on tree species [21, 22]. These studies were conducted under laboratory [21] and natural forest conditions [22]. In the latter, caterpillar-infested willows (Salix sitchensis) were suggested to have released signals, described as “pheromonal substances,” that induced increases in resistance to subsequent herbivory in their neighbors [22]. Although this work was controversial for several years, communication between plants is now accepted as a common process in plant biology. Much of this acceptance was due to extensive laboratory work on crop plants, leaving natural forest ecosystems underexplored [56]. To date, tree-tree communication has been reported for sugar maple (Acer saccharum) [21], alder (Alnus glutinosa) [57, 58], birch (Betula pendula and Betula pubescens) [59, 60], poplar (Populus euromericana; P. deltoides × P. nigra; P. tremula × P. tremuloides) [21, 61, 62], willows (S. sitchensis, S. exigua, and S. lemmonii) [22, 63, 64], and more recently in lodgepole pine (Pinus contorta) [65••], beech (Fagus crenata) [66•], and Scots pine (Pinus sylvestris) [67•].
These studies have revealed that BVOC-mediated interactions could occur via two broad mechanisms. Constitutively emitted volatiles and HIPVs from neighboring plants could enhance tree resistance to herbivores by (1) conferring an odor-based camouflage [59] and (2) by triggering or priming tree defenses [58, 62]. The first mechanism is a passive process that may not strictly fit requirements to be called communication, but clearly represents an interaction between plants based on BVOCs. It was described in a natural European subarctic forest with mountain birch trees growing with Rhododendron tomentosum in the understory [59]. Characteristic sesquiterpenes emitted by the rhododendron, notably ledene, ledol, and palustrol, were found to adhere to leaf surfaces of neighboring birches at low temperatures and were then released by the birches when temperatures raised [60]. The release of adhered rhododendron sesquiterpenes by birches was found to act as an odorous camouflage imparting an odor that was not characteristic of birch and facilitated a reduction in the density of aphids (Euceraphis betulae) on birch leaves [59].
Other studies have demonstrated clear active BVOC-mediated interactions that better fit the definition of communication. In an active interaction, the receiver plant is required to undergo a change of its own making, which may include the induction or priming of defenses in plants upon receipt of BVOCs that indicate the presence of herbivores. Defense induction is defined as the physiological change observable in plants upon exposure to BVOCs and includes overexpression of defense-related genes and/or production and emission of secondary defense compounds [30]. Plant priming is defined as plants being in a state of readiness [68, 69]. Thus, after exposure to BVOCs, physiological changes may not be visible, but plants will respond more quickly and/or more intensely to future stress [70, 71]. These phenomena have been demonstrated in many herbaceous species in the laboratory and in the field e.g. [72,73,74,75]. Research has shown that HIPV-exposed trees had enhanced expression of herbivore defense-related genes, and increased levels of protease inhibitors, phenolic compounds, jasmonic acid, and linolenic acid [58, 61]. They have also been shown to have elevated levels of primary and secondary metabolites with potent activity in plant defense against pathogens and invading organisms [76] and to respond with greater BVOC emissions upon feeding than plants not exposed to HIPVs [62]. The responses can result in a reduction in the attractiveness of trees to herbivorous insects [57, 58, 66•]. Another study found that exposure to HIPVs enhances the production of extrafloral nectar in hybrid aspen saplings [62], which may attract more natural enemies of herbivorous insects [77,78,79]. A recent observation has shown that potted pine saplings exposed to neighboring plants damaged by pine weevils (Hylobius abietis) responded to subsequent herbivory by inducing higher emissions of volatile terpenes and were fourfold less fed upon by weevils [67•]. Considering the extensive damage caused to saplings by pine weevils, estimated at €140 million annually in Europe [80], such observations could help to identify methods to induce the defenses of young trees rendering them more resistant to damage caused by weevils.
Effects of BVOC-Mediated Communication on Herbivores in Forest Ecosystems
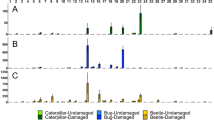
Tree-tree communication mediated by BVOCs plays a significant role in shaping plant–insect interactions (Fig. 2). By inducing or priming defenses of neighboring trees, interactions between trees can enhance resistance of neighbors to subsequent herbivory, reduce the damage caused by herbivores, and reduce the density of insect pests. For example, in a study of two willow species, exposure to wounded neighbors reduced herbivore damage by 21% for S. exigua and 41% for S. lemmonii in experiments conducted at two different sites [63]. Exposure to damaged neighbors was also shown to halve the presence of phytophagous specialist arthropods in black alder [58]. By comparison, an increase in temperature of 2 °C was found to increase insect herbivory by 21% in willows [81], and drought was found to increase herbivore-induced damage by 68.5% in female Populus yunnanensis [82], emphasizing the ecological importance of tree-to-tree communication on insect herbivory. To date, few studies have investigated the magnitude of the effects of between-tree communication in field and natural forest ecosystems. More work should examine the biological relevance of tree communication to herbivore populations if we want to better understand the significance of communication between trees.
Schematic representation of different interactions trees and insects might have in forest ecosystems through the emission of BVOCs and via mycorrhizal networks. Aboveground interactions are mediated by BVOC emissions. BVOC cues from an infested tree can be eavesdropped upon by nearby (up to 5 m) conspecific neighbors (A) and enhance their resistance to herbivory (B). Those receivers might also be more attractive to natural enemies of herbivorous insects (C). Concentrations of BVOC cues might be reduced with distance and BVOCs might be degraded or transformed with tropospheric pollutants (D). BVOCs can also be perceived by heterospecific neighbors (E) but might not have enhanced pest resistance due to not sharing the same insect community and potentially possessing different chemotypes. BVOCs emitted from understory species on forest floors might also confer odor camouflage to trees making them less attractive to herbivorous insects (F). An infested tree might also send signals of herbivore presence through CMNs to nearby (at least up to 20 cm) conspecific or heterospecific trees (G). The trees share nutrients as well as stress signals via CMNs across conspecific (H, I) and heterospecific (J) associations. Ericaceae associated with ERM are not yet reported to communicate through CMNs (K) connections. Grey arrows represent interactions observed in the literature while orange arrows represent hypothetical interactions
Interactions between trees may also affect higher trophic levels such as the natural enemies of herbivores (Fig. 2). To date, this phenomenon has not been demonstrated either in a controlled environment or in a natural forest ecosystem. However, field studies with annual plants have shown that parasitism and non-consumptive effects of parasitic wasps can contribute up to 80% in reducing damage by herbivorous insects (Fig. 2), which contributes to promoting plant biomass production, and improving plant fitness [83,84,85,86]. While the effects of attracting parasitoids on net herbivory can be unclear, studies have shown that avian predators are also attracted by HIPVs emitted by trees [87, 88]. Predation rates of artificially modelled caterpillars were 50% greater than in non-damaged trees [87] (Fig. 2). Thus, since trees exposed to HIPVs can respond to subsequent herbivory with higher HIPV emissions [62, 67•], they may attract more natural enemies and reduce herbivore pressure.
Ecological Relevance of BVOC-Mediated Communication in Forest Ecosystems
Since most research on tree-tree communication has been conducted under controlled environments, the ecological relevance of BVOC-mediated interactions in forest ecosystems remains uncertain. However, two recent studies have addressed volatile-mediated tree-tree interactions in natural forest ecosystems. The first study tested whether BVOCs emitted by lodgepole pine trees attacked by the mountain pine beetle (Dendroctonus ponderosae) affected neighboring trees across a heterogeneous forest of the Jasper National Park, Alberta [65••]. The authors also tested whether communication was more efficient between trees with similar chemotypes. They characterized high and low β-phellandrene chemotypes based on their constitutive BVOCs and reported sub-chemotypes within those chemotypes based on quantities of induced monoterpenes. Further BVOC analyses on non-attacked focal trees situated from 0 to 10 m away from D. ponderosae-attacked trees showed that communication was more effective among trees with similar chemotypes supporting the hypothesis of BVOC-mediated kin recognition by trees in natural ecosystems. The second field-based study tested whether BVOCs emitted from clipped mature beech trees (approx. 31 years old) enhance the resistance of neighboring trees to herbivores and pathogens and over what distance [66•]. Ninety days after exposure, the number of leaves damaged by chewing herbivores and pathogens was recorded on trees located at 3, 5, 7, 9, or 11 m from a clipped tree. It was found that beech trees < 5 m from clipped neighbors had significantly less leaf damage than nonexposed ones.
Natural ecosystems have several factors that can complicate or obscure the observation of tree-tree communication. These factors include the forest conditions (e.g., mixed forest, monocrop forest), heterogeneous environmental factors (e.g., temperature, wind), distance between trees and density of the forest, tree characteristics (e.g., age, size), tree kinship, and intra-specific/individual variation in BVOC emission profiles and defense induction [31, 89]. For example, the release and dispersal of airborne BVOCs is known to be influenced by tree size with greater BVOC dispersal in tall trees [90]. In addition, some BVOCs, such as oxidized terpenoids (e.g., α-terpinene, β-caryophyllene, α-humulene), are known to react in a few minutes with atmospheric pollutants, which can alter the composition of the chemicals that receivers are exposed to [91, 92]. This may affect the quality and concentration of BVOCs reaching neighbors and affect the responses of the receivers [93]. As BVOCs convey information more efficiently over short distances, it has been proposed that the release of VOCs may have evolved to coordinate defenses within a plant rather than between plants and that neighbors may be eavesdropping on cues that help them to pre-empt danger [94, 95]. The effective distance of communication between plants has typically been shown to be rather short under field conditions. Fieldwork on sagebrush (Artemisia tridentata) has demonstrated that HIPVs could induce resistance in neighbors at distances of up to 60 cm [96], while earlier work on black alder similarly showed induced resistance in trees situated at 1.3 m from a defoliated emitter tree, while trees farther away (10.6 m) did not respond as clearly [57, 58]. In 2021, Hagiwara et al. [66•] found that damage levels were lower in beech trees located less than 5 m away from clipped emitter trees than in trees exposed to undamaged emitters, but this was no longer the case in trees located from 7 to 11 m away from clipped trees (Fig. 2).
In addition, plants may respond more effectively to volatile cues emitted by genetically identical kin than non-kin as shown in sagebrush and lodgepole pine [97, 98, 65••]. Individuals of the same species may emit highly variable BVOC profiles, but within that variation, there may be a number of characteristic chemotypes that have been shown to be heritable [99, 100]. Each plant chemotype was also shown to have coevolved with a similar arthropod community [100], suggesting that future herbivory is more likely to be similar within plants of a certain chemotype than between plants of different chemotypes [100, 101], which could explain the more effective communication between kin observed. Thus, determining the genetic relatedness among trees in forest ecosystems is necessary to correctly assess tree-tree communication and draw the right conclusions.
Investigating aboveground BVOC-mediated tree-tree communication in natural forest ecosystems is extremely challenging and the researchers that have attempted to do so should be commended for their efforts. Elucidating mechanisms in a forest setting is even more challenging than determining whether interactions occur. For example, in forests, the variation in genotype, phenotype, and physical factors leads to variation in intra-specific and intra-individual BVOC emissions [102]. Moreover, most trees that communicate by the emission of BVOCs could also communicate via the roots and mycorrhizae [103•], which may facilitate information transfer between plants [104, 105••]. Thus, studying the effects of aboveground volatile signals from an insect-infested tree to other trees can only be confirmed mechanistically by eliminating belowground contact and ascertaining that an underground wired network is not responsible for defense induction. In the next section, we examine the role of mycorrhizae in communication between plants.
Mycorrhizal Fungi and Common Mycorrhizal Network (CMN)-Mediated Plant Communication
Mycorrhizal Fungi
About 80% of terrestrial plant species (92% of families) form mycorrhizal associations [106, 107]. Mycorrhizal associations are symbiotic whereby plants act as a source of carbon (C) in exchange for increased access to nutrients from the soil, such as nitrogen (N) and phosphorus (P) [108]. Mycorrhizae are obligate symbiotic fungi; a single mycorrhizal fungus can colonize several trees maximizing its access to C sources [106, 107]. Based on their structure and function, mycorrhizal fungi can be categorized into several groups. The most widely distributed are the arbuscular mycorrhizal fungi (AMF), ericoid mycorrhizal fungi (ERM), and ectomycorrhizal fungi (EMF) [34, 106, 109, 111] (Fig. 1). Mycorrhizal fungi can be host specific, but most are generalists colonizing numerous species [33]. Similarly, plants can form mycorrhizal associations with a certain group of mycorrhizal fungi, or in some cases form symbiotic associations with more than one group (e.g., both AMF and EMF) [109]. It has been further shown that conspecific seedlings grow better with CMNs and that the mycorrhizal association pattern follows the kin selection theory, whereby mycorrhizal associations are greater in the presence of kin compared to when in the presence of non-kin [112, 113].
AMF are the most common symbionts of terrestrial plants, and EMF are the dominant mycorrhizal fungi in boreal forests [106]. The AMF and EMF differ in the structure of the association they form with the host plant with EMF forming a Hartig net, which is where fungal hyphae grow between the root cortical cells, and AMF forming arbuscules in the root cortical cells (Fig. 1) [106, 114, 115]. Ericoid mycorrhizal fungi mostly form associations with the Ericaceae, e.g., in R. tomentosum [106, 115]. The AMF and EMF can form long hyphae of up to hundreds of meters per meter of root length depending on the fungal and host species, while the ERM generally form hyphae close to the host plant that likely operate only in the localized space in the host rhizosphere [116].
Common Mycorrhizal Networks
The mycorrhizal hyphae, both alone and in chord-like clusters called rhizomorphs, may form external bridges between the root tips of the same tree and link different trees belowground [106] (Fig. 2). These mycorrhizal connections are referred to as common mycorrhizal networks (CMNs). It has been shown that sugars (monosaccharides), amino acids, lipids, water, and micronutrients can all be transported via CMNs [33, 34, 117]. In addition to nutrient exchange, CMNs have been shown to carry infochemicals, including plant hormones, enzymes, allelochemicals, and secondary compounds not involved in growth and development of the plant [118,119,120]. Stress-induced signals from herbivore or pathogen infested plants have been shown to be carried through mycorrhizae to nearby plants, which prime their own defenses against attack [120,121,122,123]. Not only do CMNs mediate resource sharing and stress signal transmission between conspecific associates, but they have also been demonstrated to facilitate heterospecific connections [123,124,125] (Fig. 2). Most of the research on communication via CMNs has been conducted using crop plants and the mechanisms by which infochemicals are used in mycorrhizal networks are largely unclear [126, 127].
To date, no membrane transporters likely to be responsible for transporting infochemicals into the cytosol have been identified at the interface of the root-mycorrhizal associations. It has been proposed that nutrients and infochemicals may be transported through the cytosol or cell walls, via surface adhesion, or via rhizomorphs that are formed by twining of several hyphae [33]. It has also been proposed that the hollow space inside rhizomorphs could carry BVOCs [33]. Action potentials or electrical signals across cell membranes have been suggested to transmit stress signals across CMNs [108]. In addition to providing a physical means of transport across the hyphae, fungal presence in the rhizosphere alters the soil morphology by forming and maintaining soil aggregates enabling better transport of root exudates and even belowground BVOCs through the soil matrix [33, 128].
Effects of Mycorrhizal Associations on Plant Physiology, Nutrient Status, and Defense
Mycorrhizal fungi are associated with increased rates of photosynthesis and growth in plants [129], which plays a compensatory role in the event of herbivore attack [130]. In addition to better access to nutrition and increased photosynthesis, several studies have shown that mycorrhizal association may increase herbivore tolerance, induce plant resistance against herbivores, and alter the gene expression and secondary compound composition of plant tissues [107, 128, 131,132,133]. In a recent study with gray poplar, mycorrhizal associations have also been shown to alter the entire plant defense strategy against herbivores by inducing constitutive phenol-based defenses [134]. More recently, a non-targeted analysis of secondary compounds in poplars revealed that mycorrhizal association induces substantial changes in plant defense [135•], which may indicate plant defense as a further evolutionary benefit of mycorrhizal association.
It is well established that plants exchange nutrients through CMNs and that the exchange is controlled by the plants [112]. The exchange of nutrients may not appear to be balanced, i.e., one species may appear to extract more nutrients from the CMN network and provide little in return or vice versa. A good example of unequal resource sharing is in the achlorophyllous plants that depend on nutrients extracted through CMNs connecting them to nearby plants [112]. It is worth noting that a plant which may seem at a disadvantage in resource sharing might still receive benefits from the CMNs that are sufficient to maintain the symbiosis, e.g., improved defense against herbivores through altered secondary compound composition [135•]. It is also likely that the plants’ role as a net donor/receiver of nutrients to/from the CMNs may change over time; i.e., a plant that is a receiver as a sapling may become a donor of nutrients into the CMNs after a few years. The exchange of nutrients through CMNs among plants has not been shown to decrease individual plant fitness; in fact, mycorrhizal association with its integral resource sharing is understood to improve overall plant fitness in the forest be it monodominant or mixed forest [112, 129, 136]. EMFs, for example, have been shown to increase seedling survival through resource sharing from mature trees, and to transfer labile carbon and nitrogen between Douglas fir [137].
In an experiment conducted with crop plants, it has been shown that AMF increase the host attractiveness to herbivores while the herbivore feeding inhibits further development of the mycorrhizal associations [138]. Recently, in a field experiment conducted with Scots pines associated with EMF, herbivory was shown to decrease mycorrhizal colonization; however, the changes in the BVOC emissions due to mycorrhizal associations were not studied in the experiments [139, 140]. Previously, it has been suggested that the increased attractiveness of trees with mycorrhizal associations for oviposition by herbivores compared to non-mycorrhizal trees is due to mycorrhizal trees providing better nutrition to herbivores and being more attractive due to their size [130]. It is also well established and widely reported that herbivores perform better on mycorrhizal plants whereby herbivore performance was estimated based on growth rate, consumption, survival, oviposition performance, and herbivore density [141]. Padmanaban et al. [135•], however, have reported that herbivore performance is significantly reduced by mycorrhizal association. Therefore, it remains inconclusive if plant fitness or plant defense post mycorrhizal colonization determines the attractiveness of the plant to herbivores and the herbivore fitness. However, the attractiveness and susceptibility of mycorrhizal plants to herbivores may vary extensively based on the plant, fungal, and herbivore species.
Propagation of Stress Signals Through CMNs
The first account of mycorrhizal transmission of stress signals was reported in tomato plants [120]. The study used pathogenic fungi Alternaria solani to infect “donor” plants that produced signals that induced disease resistance in “receiver” plants connected via AMF CMNs. Soon after, stress signal transmission through AMF CMNs was reported in studies that had used herbivorous insects to damage “donor” plants that induced defenses in crop plant “receivers” [121, 122]. Undamaged bean plants connected to aphid-infested bean plants via AMF CMNs responded by changing their BVOC emissions, which attracted an aphid natural enemy [121]. BVOC emissions from bean plants not connected by CMNs to aphid infested bean plants remained unchanged [121]. Song et al. [122] showed that caterpillar (Spodoptera litura) feeding on tomato plants induced both defense-related genes and the jasmonate pathway in undamaged receiver plants connected through AMF CMN.
A landmark publication on CMN-based communication through stress signals was conducted with interior Douglas fir and Ponderosa pine whereby it was shown that stress signals can be transmitted via EMF and that they can cross heterospecific mycorrhizal connections [123]. The experiment was conducted with 4-month-old sterilized interior Douglas fir as “donor” and Ponderosa pine as “receiver” seedlings. EMF spores were acquired from the soil of a mono-specific stand of interior Douglas fir, which was mixed with autoclaved potting soil. The EMF CMNs in this experiment were comprised entirely of the single fungal taxon Wilcoxina rehmii (Ascomycota, Pezizales order). The donor seedlings received either (i) no defoliation treatment, (ii) manual defoliation, or (iii) defoliation by the third instar larvae of the western spruce budworm. The receivers were grown (i) directly into the soil with the donor seedlings, (ii) in a mesh bag using a 35 mm pore size (which blocks root growth but enables hyphal growth through the pores), or (iii) in a mesh bag with a 0.5 mm pore size (which blocks both root and hyphal growth through the pore but allows nutrient exchange). In response to herbivory and manual defoliation of interior Douglas fir, there was increased activity of the defense-related enzymes peroxidase (POD), polyphenol oxidase (PPD), and superoxide dismutase (SOD) in both the donor plants and receiver plants that were connected via CMNs to the donor plant. The damaged seedlings also transferred labile C to receiver seedlings that were connected via CMNs. Neither Song et al. [120, 123] nor Babikova et al. [121] identified the nature or composition of the stress signal being transmitted via the CMNs. However, Song et al. [122] identified the jasmonate pathway as important for transmission of stress signals across the CMN, which remains a promising avenue for further research.
Responses in Receiver Plants
Stress signal transmission across CMNs has been shown to affect BVOC emissions of receiver plants. In a study by Babikova et al. [121], BVOC emissions from receiver plants were similar to those of infested donor plants. This resulted in the uninfested receiver plants repelling herbivores and attracting parasitoid wasps despite not actually being exposed to herbivores [121]. In other studies, receiver plants were shown to upregulate several defense-related genes including those for pathogen-related proteins PR1, PR-2, PR-3, phenylalanine ammonia-lyase (PAL), lipoxygenase (LOX), lipoxygenase D (LOXD), allene oxide cyclase (AOC), PPD, SOD, and two serine protease inhibitors (PI-I and PI-II) [120, 122, 123]. The increased activity of enzymes in receiver plants was observed within 24 h, which was approximately the same time scale as for the donor plants, and much faster than the C-transfer across the CMNs [122, 123]. It can be speculated that during stressful events, the first factors to cross CMNs from herbivore-infested donor plants are stress signals rather than nutrients. LOX and AOC are two important enzymes of the jasmonic acid pathway, which has been studied extensively as a response to tissue injury and activation of defense against herbivores [142]. Based on this rather limited amount of literature, it can be predicted that defense upregulation in uninfested “receiver” plants connected via CMNs to infested “donor” plants is rather similar.
Challenges in Designing CMN-Based Communication Studies in Natural Systems
There have been no further advances in the field of CMN-mediated stress signal transmission to our knowledge, but the aforementioned literature presents sufficient evidence to conclude that stress signals can be transmitted across AMF and ECM. It is not certain if the CMN-based stress signaling is an altruistic act of active communication on the part of the donor tree, if it is a clever trick utilized by mycorrhizal fungi to protect its C-supplying symbionts from excess damage, or if it is eavesdropping by the receiver plant that hijacks the CMN for information. There are numerous challenges in designing experiments to study CMN-based communication in fields and forests. It is not plausible to cut off mycorrhizal connections of trees in natural systems and it is challenging to ascertain if trees are connected belowground via the same CMN. Prevention of BVOC-mediated communication is also another challenge in natural systems. In the experiments conducted by Song et al. [123], BVOC-mediated “wireless” communication aboveground was prevented by containing the damaged Douglas fir trees in clear plastic bags. Such methods are hard to implement in natural or near natural ecosystems and with large trees. It is yet another challenge to prevent belowground BVOC or exudate-based communication while still maintaining the CMNs. The nature of stress signals transmitted via CMNs is yet unknown. Plant hormones are a plausible candidate for signaling as they do mediate signaling within plants, are involved in establishing root-mycorrhizal fungi “synapses,” and are transported across the CMNs [34]. It is not confirmed that the hormones are involved in stress signaling from an emitter plant, across the CMN, to a receiver plant. Stable isotope labelling has been used to study nutrient sharing across CMNs [124, 143]. Synthetic hormones labelled with stable isotopes could potentially be used to study the involvement of plant hormones in stress signaling across CMNs in carefully designed experiments. It is likely that the stress signals transmitted across the CMNs do not involve molecules crossing the root-mycorrhizal fungi “synapse.” Rather, it is predicted that stress signals from the emitter are stimuli that induce production of infochemicals in the CMNs that are detected by the receiver plant; if so, isotope labelling will not reveal the nature of stress signals. Secondary compounds are a promising key to understanding the belowground plant signaling network [144]. A non-targeted metabolite analysis of the root exudates and the secondary compounds in the CMNs suspected of signal transmission may reveal the nature of the signaling compounds. Presently, our inability to separate secondary metabolites from fungal hyphae limits our ability to study the signaling molecules across the CMNs.
To study the role of mycorrhizal fungi in herbivory-related stress signal transmission poses an extra challenge. As discussed above, mycorrhizal association changes the secondary compound composition, including the composition of BVOC emissions [134, 135•]. This means that comparison of herbivore performance on mycorrhizal “receiver” trees versus non-mycorrhizal “receiver” trees of the same species is invalidated by changes in induced and constitutive defenses as direct effects of mycorrhization and the cascading effects on plant–herbivore interactions. The upcoming research of mycorrhizologists should look to better understand the nature of stress signals transmitted through CMNs and compare the robustness of BVOC-mediated communication with CMN-mediated communication in laboratory and natural forest systems.
Forest Management in the Light of Plant-Plant Communication
About 31% of the planet’s land area is forested, 30% of which (1.15 billion ha) is managed for production [145]. With climate change, herbivory has become a more potent threat to global forests with increases in the frequency of outbreaks, earlier emergence of herbivores, and overall increases in feeding period and the number of generations per summer [146, 147]. Understanding the defenses of forests can be a key to mitigating the direct and indirect effects of climate change. At present, sustainable forest management (SFM) in the European Union (EU) represents one of the most thorough forest management protocols in the world, but the functional pragmatism of the SFM is more socio-political than ecological [148, 149]. Much of the European forests are being excessively fertilized and receive atmospheric nitrogen deposition, which has been shown to have negative impact on mycorrhizal symbionts [139, 140, 150]. Clear-cutting is being practiced globally to harvest forests, which also leads to changes in soil microbiota [151]. Clear-cutting is often advocated as being analogous to forest fires whereby all the large trees are lost. However, the changes induced in the soil microbiota in response to forest fires are completely different from the effects of clear cutting [151]. The newly planted seedlings after a clear-cut are exposed to a plethora of dangers from pathogens and herbivores. Newly planted stands are also unnatural in the way they are monodominant and of the same age.
Our understanding of forest interactions at the community level mediated by “wired” and “wireless” communication is in the preliminary stages. What we already know is that trees in mixed forests are more fit compared to single species stands [143], and young seedlings benefit from resource flow from established trees through CMNs [34]. Tree diversity [12] and diversity of BVOCs [152] also negatively correlates with herbivore damage. Forest management guidelines in the future should prioritize tree fitness in light of recent advances in ecology. Pest prevention techniques could encompass increasing tree genetic diversity and age and avoiding clear cutting, e.g., by leaving behind a few old trees [152]. In addition, guidelines for forest management should promote tree resistance to herbivorous insects by encouraging tree associations that enhance positive interactions. For example, it has been shown that BVOCs from Rhododendron tomentosum may adsorb to and be re-released from the surface of birch (Betula spp.) providing resistance to herbivores [154]. Trees in managed forests could be matched with lower story plants that may provide them with odorous camouflage. Moreover, modification of the “push–pull” strategy utilized in subsistence farming could be developed for forests by choosing companion plants that release infochemicals that avert insect pests from trees combined with trap plants that act as a “pull” component [155]. The effectiveness of this system has been proven in agricultural systems and is increasingly adopted by farmers [156] suggesting potential for management of pests in forest ecosystems. These strategies could be combined with promoting natural enemy habitats and attraction via infochemicals above and belowground, as well as promoting the exchange of information between trees. However, before we can attempt to implement these methods in forests, considerable research must be conducted to better understand these interactions. Even though more work seems to have investigated interactions of trees with herbivorous insects aboveground than interactions occurring belowground, we do not know whether trees receiving cues attract more natural enemies, or if there are interactions with other factors. In addition, little research has shown the effect of transmitting alarm signals via mycorrhizae on receiver tree resistance to herbivorous insects, nor whether these interactions occur over long distances, or are more effective between kin or distantly related plant species [104, 105••]. Therefore, we provide some further directions for future research to address these issues.
Future Directions
Through the synthesis above, it has become clear that plants have the means to provide information to and receive information from their communities, but substantial knowledge gaps remain. Evidence of plant-plant interactions has been obtained from numerous plant species and shown convincingly to occur both above and belowground. However, there is relatively little information from actual forest environments, and mechanisms occurring above and belowground can be difficult to separate. As a consequence, the ecological significance of plant-plant interactions has not been adequately determined. Laboratory and field studies have indicated a complexity in the information encoded within VOCs to the extent that plants with a shared chemotype signal more effectively than plants with different chemotypes [98]. Other studies have indicated greater signaling or receiving capacities of particular species or cultivated plant varieties [96, 157]. However, these nuances have mostly been observed in the laboratory or with the widespread shrub, Artemisia tridentata e.g. [97, 99], with very few studies on forest trees, aside from a recent study on Lodgepole pine [65••]. Most studies focusing on kin recognition among trees have been addressed in studies of belowground communication mediated by mycorrhizae. For example, it has been demonstrated that trees have greater mycorrhizal connections between kin and that older trees provide resources that aid the growth and survival of conspecific younger trees. However, our knowledge of whether these phenomena occur through aboveground BVOC-mediated communication is limited and this should be a focus of further research.
The effects of tree-tree communication on organisms of higher trophic levels should also be considered. In this review, we focused on the effects of tree-tree interactions on herbivores. However, there is relatively scant information about the benefits or costs of tree-tree communication on interactions with herbivores and even less on the underlying mechanisms. Future studies in natural forest settings should determine the effects of tree-tree interactions on the community of herbivores and should extend to examining the effects on herbivore natural enemies. The contribution of combined tree-tree interactions and natural enemy responses in pest control should be determined to develop new strategies for effective and sustainable pest management.
In order to more completely understand plant communication in forest ecosystems, there is a need to scale up research to better include mature trees and responses in all parts of the canopy. It is challenging to conduct carefully manipulated experiments within a mature forest with tall trees, but equipment for accessing canopies to make measurements and take samples from the upper reaches of a canopy would enhance progress in this area. Infrastructures to enable canopy accessibility do exist. For example, forest canopy walkways, towers, and cranes enable accessibility to the upper forest reaches [158]. So far, these tools have been utilized for important advances into canopy ecology, but tree-tree interactions have not typically been studied. This is at least partially due to the limited replication that such facilities allow, with reach limited to where the facilities are constructed. We do not view equipment for climbing trees to be appropriate for studying tree-tree communication due to the need to have minimal contact with the subject trees and produce minimal amounts of damage. However, mobile elevated working platforms could be used for accessing tree canopies and may represent an excellent tool for better investigating volatile-mediated interactions between mature trees and acquiring sufficient replication.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Nixon R. The less selfish gene: Forest altruism, neoliberalism, and the tree of life. Environ Humanit. 2021. https://doi.org/10.1215/22011919-9320189.
Karban R. Plant sensing and communication. The University of Chicago Press; 2015. https://www.perlego.com/book/1851952/plant-sensing-and-communication-pdf.
Blande JD. Plant communication with herbivores. In Becard G (Ed) How plants communicate with their biotic environment. Adv Botanic Res. 2017. https://doi.org/10.1016/bs.abr.2016.09.004.
Cook-Patton SC, LaForgia M, Parker JD. Positive interactions between herbivores and plant diversity shape forest regeneration. Proc R Soc B-Biol Sci. 2014. https://doi.org/10.1098/rspb.2014.0261.
Leroux SJ, Wiersma YF, Vander WE. Herbivore impacts on carbon cycling in boreal forests. Trends Ecol Evol. 2020. https://doi.org/10.1016/j.tree.2020.07.009.
Beguin J, Côté SD, Vellend M. Large herbivores trigger spatiotemporal changes in forest plant diversity. Ecology. 2022. https://doi.org/10.1002/ecy.3739.
Stadler B, Mühlenberg E, Michalzik B. The ecology driving nutrient fluxes in forests. In: Weisser WW, Siemann E. (Eds.), Insects and Ecosystem Function. Ecological Studies (Analysis and Synthesis). Springer, Heidelberg, 213–239; 2008.
Lemoine NP, Burkepile DE, Parker JD. Insect herbivores increase mortality and reduce tree seedling growth of some species in temperate forest canopy gaps. PeerJ. 2017. https://doi.org/10.7717/peerj.3102.
Molina R, Horton TR. Mycorrhizal Specificity: its role in the development and function of common mycelial networks. In: Horton TR. (Eds.), Mycorrhizal Networks. Ecological Studies (Analysis and Synthesis). Springer, Heidelberg, 1–40; 2015.
Shestakov AL, Filippov BY, Zubrii NA, Klemola T, Zezin I, Zverev V, Zvereva EL, Kozlov MV. Doubling of biomass production in European boreal forest trees by a four-year suppression of background insect herbivory. For Ecol Manage. 2020. https://doi.org/10.1016/j.foreco.2020.117992.
Shao X, Cheng K, Kong Y, Zhang Q, Yang X. Effects of tree diversity on insect herbivory. J Forest Res. 2021. https://doi.org/10.1007/s11676-020-01274-9.
Jactel H, Moreira X, Castagneyrol B. Tree diversity and forest resistance to insect pests: patterns, mechanisms, and prospects. Annu Rev Entomol. 2021. https://doi.org/10.1146/annurev-ento-041720-075234.
Espelta JM, Cruz-Alonso V, Alfaro-Sánchez R, Hampe A, Messier C. Pino J Functional diversity enhances tree growth and reduces herbivory damage in secondary broadleaf forests but does not influence resilience to drought. J Appl Ecol. 2020. https://doi.org/10.1111/1365-2664.13728.
Cardenas RE, Valencia R, Kraft NJ, Argoti A, Dangles O. Plant traits predict inter-and intraspecific variation in susceptibility to herbivory in a hyperdiverse Neotropical rain forest tree community. J Ecol. 2014. https://doi.org/10.1111/1365-2745.12255.
Caldwell E, Read J, Sanson GD. Which leaf mechanical traits correlate with insect herbivory among feeding guilds? Ann Bot. 2016. https://doi.org/10.1093/aob/mcv178.
Stuefer JF, Gomez S, Mölken TV. Clonal integration beyond resource sharing: implications for defense signaling and disease transmission in clonal plant networks. Evol Ecol. 2004. https://doi.org/10.1007/s10682-004-5148-2.
Kaplan I, Halitschke R, Kessler A, Sardanell S, Denno RF. Constitutive and induced defenses to herbivory in above- and belowground plant tissues. Ecology. 2008. https://doi.org/10.1007/s11103-021-01203-2.
Wittstock U, Gershenzon J. Constitutive plant toxins and their role in defense against herbivores and pathogens. Curr Opin Plant Biol. 2002. https://doi.org/10.1016/S1369-5266(02)00264-9.
Joo Y, Schuman MC, Goldberg JK, Kim SG, Yon F, Brütting C, Baldwin IT. Herbivore-induced volatile blends with both “fast” and “slow” components provide robust indirect defence in nature. Funct Ecol. 2018. https://doi.org/10.1111/1365-2435.12947.
Heil M, Karban R. Explaining evolution of plant communication by airborne signals. Trends Ecol Evol. 2010. https://doi.org/10.1016/j.tree.2009.09.010.
Baldwin IT, Schultz JC. Rapid changes in tree leaf chemistry induced by damage: evidence for communication between plants. Science. 1983. https://doi.org/10.1126/science.221.4607.277.
Rhoades DF. Responses of alder and willow to attack by tent caterpillars and webworms: evidence for pheromonal sensitivity of willows. ACS Sym Ser. 1983. https://doi.org/10.1021/bk-1983-0208.ch004.
Haukioja E, Suomela J, Neuvonen S. Long-term inducible resistance in birch foliage: triggering cues and efficacy on a defoliator. Oecologia. 1985. https://doi.org/10.1007/BF00378910.
Bouwmeester H, Schuurink RC, Bleeker PM, Schiesti R. The role of volatiles in plant communication. Plant J. 2019. https://doi.org/10.1111/tpj.14496.
Rahman MK, Zhou X, Wu F. The role of root exudates, CMNs and VOCs in plant-plant interaction. J. Plant Interact. 2019; https://doi.org/10.1080/17429145.2019.1689581
Lluisà J, Estiarte M, Peñuelas J. Terpenoids and plant communication. Butll Inst Cat Hist Nat. 1996;64:125–33.
Zu PJ, Garcia-Garcia R, Schuman MC, Saavedra S, Melian CJ. Plant-insect chemical communication in ecological communities: an information theory perspective. J Syst Evol. 2022;00:1–9. https://doi.org/10.1111/jse.12841.
Jones AC, Felton GW, Tumlinson JH. The dual function of elicitors and effectors from insects: reviewing the ‘arms race’ against plant defenses. Plant Mol Biol. 2021. https://doi.org/10.1007/s11103-021-01203-2.
Felton GW, Tumlinson JH. Plant-insect dialogs: complex interactions at the plant-insect interface. Curr Opin Plant Biol. 2020. https://doi.org/10.1016/j.pbi.2008.07.001.
Brosset A, Blande JD. Volatile-mediated plant-plant interactions: volatile organic compounds as modulators of receiver plant defense, growth, and reproduction. J Exp Bot. 2022. https://doi.org/10.1093/jxb/erab487.
Simpraga M, Ghimire RP, Straeten DVD, Blande JD, Kasurinen A, Sorvari J, Holopainen T, Adriaenssens S, Holopainen JK, Kivimäenpää M. Unravelling the functions of biogenic volatiles in boreal and temperate forest ecosystems. Eur J For Res. 2019. https://doi.org/10.1007/s10342-019-01213-2.
Checcucci A, Marchetti M. The rhizosphere talk show: the Rhizobia on stage. Front Agron. 2020. https://doi.org/10.3389/fagro.2020.591494.
Barto EK, Weidenhamer JD, Cipollini D, Rillig MC. Fungal superhighways: do common mycorrhizal networks enhance belowground communication? Trends Plant Sci. 2012. https://doi.org/10.1016/j.tplants.2012.06.007.
Boyno G, Demir S. Plant-mycorrhiza communication and mycorrhizae in inter-plant communication. Symbiosis. 2022;86:155–68. https://doi.org/10.1007/s.13199-022-00837-0.
Sharifi R, Ryu C-M. Social networking in crop plants; wired and wireless cross-plant communications. Plant Cell Environ. 2020;44:1095–110. https://doi.org/10.1111/pce.13966.
Meents AK, Mithöfer A. Plant-plant communication: is there a role for volatile damage-associated molecular patterns? Front Plant Sci. 2020;11:583275. https://doi.org/10.3389/fpls.2020.583275.
Dehimeche N, Buatois B, Bertin N, Staudt M. Insights into the intraspecific variability of the above and belowground emissions of volatile organic compounds in tomato. Molecules. 2021. https://doi.org/10.3390/molecules26010237.
Guenther AB, Jiang X, Sakulyanontvittaya T, Dujl T, Emmons LK, Wang X. The model of emissions of gases and aerosols from nature version 2.1 (MEGAN2.1): and extended and updates framework for modeling biogenic emissions. Geosci Model Dev. 2012. https://doi.org/10.5194/gmd-5-1471-2012.
Wells KC, Millet DB, Payne VH, Deventer MJ, Bates KH, de Gouw JA, Graus M, Warneke C, Wisthaler A, Fuentes JD. Satellite isoprene retrievals constrain emissions and atmospheric oxidation. Nature. 2020. https://doi.org/10.1038/s41586-020-2664-3.
Zhou F, Pichersky E. More is better: the diversity of terpene metabolism in plants. Curr Opin Plant Biol. 2020. https://doi.org/10.1016/j.pbi.2020.01.005.
Laothawornkitkul J, Taylor JE, Paul ND, Heiwitt CN. Biogenic volatile organic compounds in the earth system. New Phytol. 2009. https://doi.org/10.1111/j.1469-8137.2009.02859.x.
Ameye M, Allmann S, Verwaeren J, Smagghe G, Haesaert G, Schuurink RC, Audenaert K. Green leaf volatile production by plants: a meta-analysis. New Phytol. 2017. https://doi.org/10.1111/nph.14671.
Matsui K. Green leaf volatiles: hydroperoxide lipase pathway of oxylipin metabolism. Curr Opin Plant Biol. 2006. https://doi.org/10.1016/j.pbi.2006.03.002.
Scala A, Allmann S, Rossana M, Haring MA, Schuurink RC. Green leaf volatiles: a plant’s multifunctional weapon against herbivores and pathogens. Intl J Mol Sci. 2013. https://doi.org/10.3390/ijms140917781.
Santos-Sanchez NF, Salas-Coronado R, Hernandez-Carlos B, Villanueva-Canongo C. Shikimate acid pathway in biosynthesis of phenolic compounds. In Soto-Hernandez M, Garcia-Mateos R, Palma-Tenango M (Eds) Plant Physiological aspects of phenolic compounds;2019. https://doi.org/10.5772/intechopen.77494
Najar B, Ferri B, Cioni PL, Pistelli L. Volatile emission and essential oil composition of Sambucus nigra L organs during different developmental stages. Plant Biosystems-An Int: J. Plant Biol; 2021. https://doi.org/10.1080/11263504.2020.1779841.
Runyon JB, Gray CA Jenkins MJ. Volatiles of high-elevation five-needle pines: chemical signatures through ratios and insight into insect and pathogen resistance. J Chem Ecol 2020. https://doi.org/10.1007/s10886-020-01150-0.
Trowbridge AM, Stoy PC, Phillips RP. Soil biogenic volatile organic compound flux in a mixed hardwood forest: net uptake at warmer temperatures and the importance of mycorrhizal associations. J Geophys Res Biosci. 2020. https://doi.org/10.1029/2019JG005479.
Rasheed MU, Kivimäenpää M, Kasurinen A. Emissions of biogenic volatile organic compounds (BVOCs from the rhizosphere of Scots pine (Pinus sylvestris) seedlings exposed to warming, moderate N addition and bark herbivory by large pine weevil (Hylobius abietis). Plant Soil. 2021. https://doi.org/10.1007/s11104-021-04888-y.
Ozawa R, Arimura G, Takabayashi J, Shimoda T, Nishioka T. Involvement of jasmonate- and salicylate-related signaling pathways for the production of specific herbivore-induced volatiles in plants. Plant Cell Physiol. 2000. https://doi.org/10.1093/pcp/41.4.391.
War AR, Sharma HC, Paulraj MG, War MY, Ignacimuth S. Herbivore induced plant volatiles: their role in plant defense for pest management. Plant Signal Behav. 2011. https://doi.org/10.4161/psb.6.12.18053.
de Castro Tobaruela E, Gomes BL, de Barros Bonato VC, de Lima ES, Freschi L, Purgatto E. Ethylene and auxin: hormonal regulation of volatile compound production during tomato (Solanum lycopersicum L.) fruit ripening. Front Plant Sci, 2021. https://doi.org/10.3389/fpls.2021.765897
Holopainen JK, Virjamo V, Ghimire RP, Blande JD, Julkunen-Tiitto R, Kivimäenpää M. Climate change effects on secondary compounds of forest trees in the northern hemisphere. Front Plant Sci. 2018. https://doi.org/10.3389/fpls.2018.01445.
Yu H, Holopainen JK, Kivimäenpää M, Virtanen A, Blande JD. Potential of climate change and herbivory to affect the release and atmospheric reactions of BVOCs from boreal and subarctic forests. Molecules. 2021. https://doi.org/10.3390/molecules2608228.
Turlings TC, Erb M. Tritrophic interactions mediated by herbivore-induced plant volatiles: mechanisms, ecological relevance, and application potential. Annu Rev Entomol. 2018. https://doi.org/10.1146/annurev-ento-020117-043507-043507.
Arimura GI, Pearse IS. From the lab bench to the forest: ecology and defence mechanisms of volatile-mediated ‘talking trees’. In Advances in Botanical Research 2017; Academic Press. https://doi.org/10.1016/bs.abr.2016.08.001.
Dolch R, Tscharntke T. Defoliation of alders (Alnus glutinosa) affects herbivory by leaf beetles on undamaged neighbours. Oecologia. 2000. https://doi.org/10.1007/s004420000482.
Tscharntke T, Thiessen S, Dolch R, Boland W. Herbivory, induced resistance, and interplant signal transfer in Alnus glutinosa. Biochem Sys Ecol. 2001. https://doi.org/10.1016/S0305-1978(01)00048-5.
Himanen SJ, Blande JD, Klemola T, Pulkkinen J, Heijari J, Holopainen JK. Birch (Betula spp.) leaves adsorb and re‐release volatiles specific to neighbouring plants–a mechanism for associational herbivore resistance? New Phytol. 2010. https://doi.org/10.1111/j.1469-8137.2010.03220.x.
Mofikoya A, Miura K, Holopainen T, Holopainen JK. Passive adsorption of neighbouring plant volatiles linked to associational susceptibility in a subarctic ecosystem. Biogeosciences Discuss. 2016. https://doi.org/10.5194/bg-2016-464
Frost CJ, Mescher MC, Dervinis C, Davis JM, Carlson JE, De Moraes CM. Priming defense genes and metabolites in hybrid poplar by the green leaf volatile cis-3-hexenyl acetate. New Phytol. 2008. https://doi.org/10.1111/j.1469-8137.2008.02599.x.
Li T, Holopainen JK, Kokko H, Tervahauta AI, Blande JD. Herbivore-induced aspen volatiles temporally regulate two different indirect defences in neighbouring plants. Funct Ecol. 2012. https://doi.org/10.1111/j.1365-2435.2012.01984.x.
Pearse IS, Hughes K, Shiojiri K, Ishizaki S, Karban R. Interplant volatile signaling in willows: revisiting the original talking trees. Oecologia. 2013. https://doi.org/10.1007/s00442-013-2610-2.
Hughes KM, Pearse IS, Grof-Tisza P, Karban R. Individual-level differences in generalist caterpillar responses to a plant–plant cue. Ecol Entomol. 2015. https://doi.org/10.1111/een.12224.
•• Hussain A, Rodriguez-Ramos JC, Erbilgin N. Spatial characteristics of volatile communication in lodgepole pine trees: evidence of kin recognition and intra-species support. Sci Tot Env. 2019. https://doi.org/10.1016/j.scitotenv.2019.07.211. (This paper shows that in natural forest ecosystems, trees of a single species with different chemotypes coexist and that BVOC-mediated interactions are stronger between trees of the same chemotype. This study supports the hypothesis of kin recognition as a phenomenon occurring in plants and driven by natural selection.)
• Hagiwara T, Ishihara MI, Takabayashi J, Hiura T, Shiojiri K. Effective distance of volatile cues for plant–plant communication in beech. Ecol Evol. 2021. https://doi.org/10.1002/ece3.7990. (This study investigated the distances over which BVOC cues from clipped beech trees (Fagus crenata) can enhance resistance in neighbouring trees. This study is one of few investigating this question in forest ecosystems and shows that communication is more effective among trees located within 5m of each other.)
• Yu H, Kivimäenpää M, Blande JD. Volatile-mediated between-plant communication in Scots pine and the effects of elevated ozone. 2022. https://doi.org/10.1098/rspb.2022.0963. (This study showed that Scots pine (Pinus sylvestris) seedlings damaged by stem feeding weevils (Hylobius abietis L.) release volatiles that induce defense responses and enhanced pest resistance in neighbouring conspecific seedlings. Among other defense-related responses, receiver plants had increased stomatal conductance and photosynthesis, which has not previously been reported.)
Pastor V, Balmer A, Gamir J, Flors V, Mauch-Mani B. Preparing to fight back: generation and storage of priming compounds. Front Plant Sci. 2014. https://doi.org/10.3389/fpls.2014.00295.
Hilker M, Schmülling T. Stress priming, memory, and signalling in plants. Plant, Cell Environ. 2019. https://doi.org/10.1111/pce.13526
Frost CJ, Mescher MC, Carlson JE, De Moraes CM. Plant defense priming against herbivores: getting ready for a different battle. Plant physiol. 2008. https://doi.org/10.1104/pp.107.113027.
Kim J, Felton GW. Priming of antiherbivore defensive responses in plants. Insect Sci. 2013. https://doi.org/10.1111/j.1744-7917.2012.01584.x.
Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH. Airborne signals prime plants against insect herbivore attack. Proc Natl Acad Sci U S A. 2004. https://doi.org/10.1073/pnas.0308037100.
Pashalidou FG, Eyman L, Sims J, Buckley J, Fatouros NE, De Moraes CM, Mescher MC. Plant volatiles induced by herbivore eggs prime defences and mediate shifts in the reproductive strategy of receiving plants. Ecol Lett. 2020. https://doi.org/10.1111/ele.13509.
Su Q, Yang F, Zhang Q, Tong H, Hu Y, Zhang X, Xie W, Wang S, Wu Q, Zhang Y. Defence priming in tomato by the green leaf volatile (Z)-3-hexenol reduces whitefly transmission of a plant virus. Plant Cell Environ. 2020. https://doi.org/10.1111/pce.13885.
Michereff MFF, Grynberg P, Togawa RC, Costa MMC, Laumann RA, Zhou JJ, Schimmelpfeng PHC, Borges M, Pickett JA, Birkett MA, Blassioli-Moraes MC. Priming of indirect defence responses in maize is shown to be genotype-specific. Arthropod Plant Interact. 2021. https://doi.org/10.1007/s11829-021-09826-4.
Erb M, Kliebenstein DJ. Plant secondary metabolites as defenses, regulators, and primary metabolites: the blurred functional trichotomy. Plant physiol. 2020. https://doi.org/10.1104/pp.20.00433.
Holopainen JK, Blande JD, Sorvari J. Functional role of extrafloral nectar in boreal forest ecosystems under climate change. Forests. 2020. https://doi.org/10.3390/f11010067.
Choh Y, Kugimiya S, Takabayashi J. Induced production of extrafloral nectar in intact lima bean plants in response to volatiles from spider mite-infested conspecific plants as a possible indirect defense against spider mites. Oecologia, 2006. https://doi.org/10.1007/s00442-005-0289-8.
Kost C, Heil M. Herbivore-induced plant volatiles induce an indirect defence in neighbouring plants. J Ecol. 2006. https://doi.org/10.1111/j.1365-2745.2006.01120.x.
Långström B, Day KR. Damage, control and management of weevil pests, especially Hylobius abietis. In Bark and wood boring insects in living trees in Europe, a synthesis (415–444). Springer, Dordrecht; 2007. https://doi.org/10.1007/978-1-4020-2241-8_19.
Veteli TO, Kuokkanen K, Julkunen-Tiitto R, Roininen H, Tahvanainen J. Effects of elevated CO2 and temperature on plant growth and herbivore defensive chemistry. Glob Change Biol. 2002. https://doi.org/10.1046/j.1365-2486.2002.00553.x.
Zhang R, Liu J, Liu Q, He H, Xu X, Dong T. Sexual differences in growth and defence of Populus yunnanensis under drought stress. Can J For Res. 2019. https://doi.org/10.1139/cjfr-2018-0270.
van Loon JJ, de Boer JG, Dicke M. Parasitoid-plant mutualism: parasitoid attack of herbivore increases plant reproduction. Entomol Exp Appl. 2000. https://doi.org/10.1046/j.1570-7458.2000.00733.x.
de Lange ES, Farnier K, Degen T, Gaudillat B, Aguilar-Romero R, Bahena-Juárez F, Oyama K, Turlings TC. Parasitic wasps can reduce mortality of teosinte plants infested with fall armyworm: support for a defensive function of herbivore-induced plant volatiles. Front Ecol Evol. 2018. https://doi.org/10.3389/fevo.2018.00055.
Ingerslew KS, Finke DL. Non-consumptive effects stabilize herbivore control over multiple generations. PLoS ONE. 2020. https://doi.org/10.1371/journal.pone.0241870.
Tan CW, Peiffer ML, Ali JG, Luthe DS, Felton GW. Top-down effects from parasitoids may mediate plant defence and plant fitness. Funct Ecol. 2020. https://doi.org/10.1111/1365-2435.13617.
Mäntylä E, Kipper S, Hilker M. Insectivorous birds can see and smell systemically herbivore-induced pines. Ecol Evol. 2020. https://doi.org/10.1002/ece3.6622.
Nguyen M, McGrath C, McNamara C, Van Huynh A. Tritrophic interactions with avian predators: the effect of host plant species and herbivore-induced plant volatiles on recruiting avian predators. J Field Ornithol. 2022. https://doi.org/10.5751/JFO-00050-930104.
Volf M, Volfova T, Seifert CL, Ludwig A, Engelmann RA, Jorge LR, Richter R, Schedl A, Weinhold A, Wirth C, van Dam NM. A mosaic of induced and non-induced branches promotes variation in leaf traits, predation and insect herbivore assemblages in canopy trees. Ecol Lett. 2021. https://doi.org/10.1111/ele.13943.
Lowman MD, Schowalter TD. Plant science in forest canopies–the first 30 years of advances and challenges (1980–2010). New Phytol. 2012. https://doi.org/10.1111/j.1469-8137.2012.04076.x.
Atkinson R, Arey J. Atmospheric degradation of volatile organic compounds. Chem Rev. 2003. https://doi.org/10.1021/cr0206420.
Holopainen JK, Blande JD. Where do herbivore-induced plant volatiles go? Front Plant Sci. 2013. https://doi.org/10.3389/fpls.2013.00185.
Blande JD, Holopainen JK, Li T. Air pollution impedes plant-to-plant communication by volatiles. Ecol Lett. 2010. https://doi.org/10.1111/j.1461-0248.2010.01510.x.
Frost CJ, Mescher MC, Carlson JE, De Moraes CM. Why do distance limitations exist on plant-plant signaling via airborne volatiles? Plant signal behave. 2008. https://doi.org/10.4161/psb.3.7.5675.
Heil M, Adame-Alvarez RM. Short signalling distances make plant communication a soliloquy. Biol Lett. 2010. https://doi.org/10.1098/rsbl.2010.0440.
Karban R, Shiojiri K, Huntzinger M, McCall AC. Damage‐induced resistance in sagebrush: volatiles are key to intra‐and interplant communication. Ecology 2006. https://doi.org/10.1890/0012-9658(2006)87[922:drisva]2.0.co;2.
Karban R, Shiojiri K. Self-recognition affects plant communication and defense. Ecol Lett. 2009. https://doi.org/10.1111/j.1461-0248.2009.01313.x.
Karban R, Shiojiri K, Ishizaki S, Wetzel WC, Evans RY. Kin recognition affects plant communication and defence. Proc Roy Soc B: Biol Sci. 2013. https://doi.org/10.1098/rspb.2012.3062.
Karban R, Wetzel WC, Shiojiri K, Ishizaki S, Ramirez SR, Blande JD. Deciphering the language of plant communication: volatile chemotypes of sagebrush. New Phytol. 2014. https://doi.org/10.1111/nph.12887.
Shiojiri K, Ishizaki S, Ando Y. Plant–plant communication and community of herbivores on tall goldenrod. Ecol Evol. 2021. https://doi.org/10.1002/ece3.7575.
Kalske A, Shiojiri K, Uesugi A, Sakata Y, Morrell K, Kessler A. Insect herbivory selects for volatile-mediated plant-plant communication. Curr Biol. 2019. https://doi.org/10.1016/j.cub.2019.08.011.
Lämke JS, Unsicker SB. Phytochemical variation in treetops: causes and consequences for tree-insect herbivore interactions. Oecologia. 2018. https://doi.org/10.1007/s00442-018-4085-5.
• Tedersoo L, Bahram M, Zobel M. How mycorrhizal associations drive plant population and community biology. Science. 2020. https://doi.org/10.1126/science.aba1223. (This review synthesizes current knowledge on how arbuscular mycorrhiza, ectomycorrhiza, ericoid mycorrhiza, and orchid mycorrhiza shape plant populations and communities, and ecosystem processes.)
Bennett JA, Maherali H, Reinhart KO, Lekberg Y, Hart MM, Klironomos J. Plant-soil feedbacks and mycorrhizal type influence temperate forest population dynamics. Science. 2017. https://doi.org/10.1126/science.aai8212.
•• Montesinos-Navarro A, Valiente-Banuet A, Verdú M. Plant facilitation through mycorrhizal symbiosis is stronger between distantly related plant species. New Phytol. 2019. https://doi.org/10.1111/nph.16051. (This meta-analysis provides evidence that the strength of plant facilitative interactions via mycorrhizal fungi depends on plants phylogenetic relatedness, and revealed that in heterospecific interactions, mycorrhizal symbiosis facilitation increases with the phylogenetic distance between nurse and facilitated plant species.)
Smith SE, Read DJ. Mycorrhizal symbiosis. 3rd ed. Academic Press, London; 2008. https://doi.org/10.1016/B978-012652840-4/500001-2.
Dreischhoff S, Das IS, Jakobi M, Kasper K, Polle A. Local responses and systemic induced resistance mediated by ectomycorrhizal fungi. Front Plant Sci. 2020. https://doi.org/10.3389/fpls2020.590063.
Gilbert L, Johnson D. Plant-plant communication through common mycorrhizal networks. Adv Bot Res. 2017. https://doi.org/10.1016/bs.abr.2016.09.001.
van der Heijden MGA, Martin FM, Selosse M-A, Sanders IR. Mycorrhizal ecology and evolution: the past, the present and the future. New Phytol. 2015. https://doi.org/10.1111/nph.13288.
Kuijper TW. Carbon and energy sources of mycorrhizal fungi: obligate symbionts or latent saprotrophs? In Johnson C (Eds.) Mycorrhizal mediation of soil. Elsevier Inc. Academic Press. https://doi.org/10.1016/B978-0-12-804312-7.00020-6.
Genre A, Lanfranco L, Perotto S, Bonfante P. Unique and common traits in mycorrhizal symbiosis. Nat Rev Microbiol. 2020. https://doi.org/10.1038/s41579-020-0402-3.
File AL, Klironomos J, Maherali H, Dudley SA. Plant kin recognition enhances abundance of symbiotic microbial partner. PLoS ONE. 2012. https://doi.org/10.1371/journal.pone.0045648.
Asay A. Mycorrhizal fascilitation of kin recognition in interior Douglas-fir (Pseudotsuga menziesii var. glauca). UBC Masters Thesis. 2013. http://hdl.handle.net/2429/45400. Accessed 24.06.2022.
Peterson RL, Massicotte. Exploring structural definitions of mycorrhizas, with emphasis on nutrient-exchange interfaces. Can J Bot. 2004. https://doi.org/10.1139/b04-071.
Perotto S, Daghino S, Martino E. Ericoid mycorrhizal fungi and their genomes: another side to the mycorrhizal symbiosis? New Phytol. 2018. https://doi.org/10.1111/nph.15218.
Leake JR, Johnson D, Donnelly DP, Muckle G, Boddy L, Read DJ. Networks for power and influence: the role of mycorrhizal mycelium in controlling plant communities and agroecosystem functioning. Can J Bot. 2004. https://doi.org/10.1139/b04-060.
Simard SW, Asay AK, Beiler KJ, Bingham MA, Deslippe, JR, He, X, Philip LJ, Song Y, Teste F. Resource transfer between plants through ectomycorrhizal fungal networks. In Horton TR (Eds.) Mycorrhizal networks. Springer, Germany. 2015. https://doi.org/10.1007/978-94-017-7395-9_5.
Hause B, Mrosk C, Isayenkov S, Strack D. Jasmonates in arbuscular mycorrhizal interactions. Phytochem. 2007. https://doi.org/10.1016/j.phytochem.2006.09.025.
Barto EK, Hilker M, Muller F, Mohney BK, Weidenhamer JD, Rillig MC. The fungal fast lane: common mycorrhizal networks extend bioactive zones of allelochemicals in soils. PLoS ONE. 2011. https://doi.org/10.1371/journal.pone.0027195.
Song YY, Zeng RS, Xu JF, Li J, Shen X, Yihdego WG. Interplant communication of tomato plants through underground common mycorrhizal networks. PLoS ONE. 2010. https://doi.org/10.1371/journal.pone.0013324.
Babikova Z, Gilbert L, Bruce TJA, Birkett M, Caulfield JC, Woodcock C, Pickett JA, Johnson D. Underground signals carried through common mycelial networks warn neighboring plants of aphid attack. Ecol Lett. 2013. https://doi.org/10.1111/ele.12115.
Song YY, Ye M, Li C, He X, Zhu-Salzman K, Wang RL, Su YJ, Luo SM, Zeng RS. Hijacking common mycorrhizal networks for herbivore induced defense signal transfer between tomato plants. Sci. 2014. https://doi.org/10.1038/srep03915.
Song YY, Simard SW, Carrol A, Mohn WW, Zeng RS. Defoliation of interior Douglas-fir elicits carbon transfer and stress signalling to ponderosa pine neighbors through ectomycorrhizal networks. Sci. 2015. https://doi.org/10.1038/srep08495.
He X, Xu M, Qiu GY, Zhou J. Use of 15N stable isotope to quantify nitrogen transfer between mycorrhizal plants. J Plant Ecol. 2009. https://doi.org/10.1093/jpe/rtp015.
Simard SW, Beiler KJ, Bingham MA, Deslippe JR, Philip LJ, Teste FP. Mycorrhizal networks: mechanisms, ecology and modeling. Fung Biol Rev. 2012. https://doi.org/10.1016/j.fbr.2012.01.001.
Alaux P-L, Naveau F, Declerck S, Cramembrouck S. Common mycorrhizal network induced JA/ET gene expression in healthy potato plants connected to potato plants infected by Phytophthora infestans. Front Plant Sci. 2020. https://doi.org/10.3389/fpls.2020.00602.
Alaux P-L, Zhang Y, Gilbert L, Johnson D. Can common mycorrhizal fungal networks be managed to enhance ecosystem functionality? Plants People Planet 2021; 10:1002/ppp3.10178
Paudel S, Longcore T, MacDonald B, McCormick MK, Szlavecz K, Wilson GWT, Loss SR. Belowground interactions with aboveground consequences: invasive earthworms and arbuscular mycorrhizal fungi. J Ecol. 2016. https://doi.org/10.1890/15-1085.
Gorzelak MA, Asay AK, Pickles BJ, Simard SW. Inter-plant communication through mycorrhizal networks mediates complex adaptive behavior in plant communities. AoBP. 2014. https://doi.org/10.1093/aobpla/plv050.
Gange AC. Insect-mycorrhizal interaction: patterns, processes and consequences. In Ohgushi T, Craig TP, Price PW (Eds.) Ecological Communities, Plant Mediation in Indirect Interaction Webs. Cambridge University Press; 2007. https://doi.org/10.1017/CBO9780511542701.007.
Kempel A, Schmidt AK, Brandl R, Schädler M. Support from the underground: Induced plant resistance depends on arbuscular mycorrhizal fungi. Func Ecol. 2010: 24. https://doi.org/10.1111/j.1365-2435.2009.01647.
Bacht M, Tarkka M, Lopez IF, Bönn M, Brandl R, Feldahln L, Grams TEE, Herrmann S, Schädler M. Tree response to herbivore is affected by endogenous rhythmic growth and attenuation by cotreatment with a mycorrhizal fungus. Mol Plant-Micro Interact. 2019. https://doi.org/10.1094/MPMI-10-18-0290-R.
Jiang D, Tam M, Wu S, Zheng L, Wang Q, Wang G, Yan S. Defense responses of arbuscular mycorrhizal fungus-colonized poplar seedlings against gypsy moth larvae: a multiomics study. Hortic Res. 2021. https://doi.org/10.1038/s41438-021-00671-3.
Kaling M, Schmidt A, Moritz F, Rosenkranz M, Witting M, Kasper K, Janz D, Schmitt-Kopplin P, Schnitzler J-P, Polle A. Mycorrhiza-triggered transcriptomic and metabolomic networks on herbivore fitness. Plant Physiol. 2018. https://doi.org/10.1104/pp.17.0181.
• Padmanaban PBS, Rozenkranz M, Zhu P, Kaling M, Schmidt A, Schmitt-Koppln P, Polle A, Schnitzler J-P. Mycorrhiza-tree-herbivore interactions: alterations in poplar metabolome and volatilome. Metabolites. 2022. https://doi.org/10.3390/metabo12020093. (This is groundbreaking work on the changes to secondary compounds due to mycorrhiza-tree-herbivore interactions.)
Finley RD, Ek H, Odham G, Suderström B. Uptake, translocation and assimilation of nitrogen from 15N-labelled ammonium and nitrate sources by intact ectomycorrhizal systems of Fagus sylvatica infected with Paxillus involutus. New Phytol. 1989. https://doi.org/10.1111/j.1469-8137.1989.tb02394.x
Teste FP, Simard SW, Durall DM, Guy RD, Jones MD, Schoonmaker AL. Access to mycorrhizal networks and roots of trees: importance for seedling survival and resource transfer. Ecology. 2009. https://doi.org/10.1890/08-1884.1.
Babikova Z, Gilbert L, Bruce T, Dewhirst SY, Pickett JA, Johnson D. Arbuscular mycorrhizal fungi and aphids interact by changing host plant quality and volatile emission. Func Ecol. 2014. https://doi.org/10.1111/1365-12181.
Rasheed MU, Kasurinen A, Kivimäenpää M, Ghimire R, Häikiö E, Mpamah P, Holopainen JK, Holopainen T. The responses of shoot-root-rhizosphere continuum to simultaneous fertilizer addition, warming, ozone and herbivory in young Scots pine seedlings in a high altitude field experiment. Soil Biol Biochem. 2017. https://doi.org/10.1016/j.soilbiol.2017.07.024.
Rasheed MU, Julkunen-Tiitto R, Kivimäenpää M, Riikonen J, Kasurinen A. Responses of soil-grown Scots pine seedlings to experimental warming, moderate nitrogen addition and bark herbivory in a three-year field experiment. Sci Tot Env. 2020. https://doi.org/10.1016/j.scitotenv.2020.139110.
Koricheva J, Gange AC, Jones T. Effects of mycorrhizal fungi on insect herbivores: a meta-analysis. Ecology. 2009. https://doi.org/10.1890/08-1555.1.
Howe G, Jander G. Plant immunity to insect herbivores. Ann Rev Plant Biol. 2008. https://doi.org/10.1146/annurev.arplant.59.032607.092825.
Simard SW, Durall DM, Jones MD. Carbon allocation and carbon transfer between Betula papyrifera and Pseudostuga manziesii seedlings using a 13C pulse-labelling method. Plant Soil. 1997. https://doi.org/10.1023/A:1004205727882.
Hong Y, Zhou Q, Hao Y, Huang AC. Crafting the plant root metabolome for improved microbe-assisted stress resilience. New Phytol. 2021. https://doi.org/10.1111/nph.17908.
FAO. Global Forest Resources Assessment 2020 – key findings. Rome; 2020. https://doi.org/10.4060/ca8753en.
Logan JA, Regniere J, Powell JA. Assessing the impacts of global warming on forest pest dynamics. Front Ecol Env. 2003. https://doi.org/10.1890/1540-9295(2003)001[0130:ATIOGW]2.0.CO;2.
Schwartzberg EG, Jamieson MA, Raffa KF, Reich PB, Montgomery RA, Lindroth RL. Simulated climate warming alters phenological synchrony between an outbreak insect herbivore and host trees. Oecologia. 2014. https://doi.org/10.1007/s0042-014-2960-4.
Linser S, Wolfslehner B, Bridge SRJ, Gritten D, Johnson S, Payn T, Prins K, Rasi R, Robertson G. 25 years of criteria and indicators for sustainable forest management: how intergovernmental C&I process have made a difference. Forests. 2018. https://doi.org/10.3390/f9090578.
Lazdinis M, Angelstam P, Pulzl H. Towards sustainable management in the European Union through polycentric forest governance and an integrated landscape approach. Landscape Ecol. 2019. https://doi.org/10.1007/s10980-019-00864-1.
Eztold S, Ferretti M, Reinds GJ, Solberg S, Gessler A, Waldner P, Schuab M, Simpson D, Benha S, Hansen K, et al. Nitrogen deposition is the most important environmental driver of growth of pure, even-aged and managed European forests. Forest Ecol Manag. 2020. https://doi.org/10.1016/j.foreco.2019.117762.
Rodringez-Ramos JC, Cale JA, Cahill JF Jr, Simard SW, Karst J, Erbilgin N. Changes in soil fungal community composition depend on functional group and forest disturbance type. New Phytol. 2021. https://doi.org/10.1111/nph.16749.
Grof-Tisza P, Karban R, Rasheed MU, Saunier A, Blande JD. Risk of herbivory negatively correlates with the diversity of volatile emissions involved in plant communication. Proc R Soc B. 2021. https://doi.org/10.1098/rspb.2021.1790.
Hlásny T, Zimová S, Merganičová K, Štěpánek P, Modlinger R, Turčáni M. Devastating outbreak of bark beetles in the Czech Republic: drivers, impacts, and management implications. For Ecol Manag. 2021. https://doi.org/10.1016/j.foreco.2021.119075.
Himanen SJ, Blande JD, Klemola T, Pulkkinen J, Heijari J, Holopainen JK. Birch (Betula spp.) leaves adsorb and re-release volatiles specific to neighbouring plants-a mechanism for associational herbivore resistance) New Phytol. 2010; 186: 722–732; https://doi.org/10.1111/j.1469-8137.2010.03220.x
Byers JA, Levi‐Zada. Modelling push‐pull management of pest insects using repellents and attractive traps in fruit tree orchards. Pest Manag. Sci. 2022; https://doi.org/10.1002/ps.7005
Pickett JA, Khan ZR. Plant volatile-mediated signalling and its application in agriculture: successes and challenges. New Phytol. 2016. https://doi.org/10.1111/nph.14274.
Glinwood R, Ahmed E, Qvarfordt E, Ninkovic V, Pettersson J. Airborne interactions between undamaged plants of different cultivars affect insect herbivores and natural enemies. Arthropod Plant Interact. 2009. https://doi.org/10.1007/s11829-009-9072-9.
Lowman M. Life in the treetops – an overview of forest canopy science and its future directions. Plants People Planet. 2021. https://doi.org/10.1002/ppp3.10125.
Funding
Open access funding provided by University of Eastern Finland (UEF) including Kuopio University Hospital. This work was funded by the Academy of Finland decision number 339575.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Forest Entomology.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rasheed, M.U., Brosset, A. & Blande, J.D. Tree Communication: the Effects of “Wired” and “Wireless” Channels on Interactions with Herbivores. Curr Forestry Rep 9, 33–47 (2023). https://doi.org/10.1007/s40725-022-00177-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40725-022-00177-8