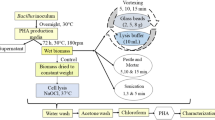
Abstract
The increased global population has concurrently increased waste disposal, whose majority is conventional plastic. In this study, polyhydroxyalkanoates (PHA), an alternative biopolymer to conventional plastics, were extracted from bacteria Bacillus sp., using response surface methodology (RSM), a statistical approach. To design, optimize and study the relationship between the parameters (glass beads weight, incubation time, water volume, incubation temperature, and shaker speed) Box-Behnken Design of response surface methodology was applied in Design Expert 10.0 software package. The solvent method is known in PHA extraction; however, this approach is environmentally hazardous on a large scale. The current study used a physical extraction method using glass beads for bacterial cell lysis. As a characterization, FTIR, 1HNMR, and DSC confirmed the recovered polymer as Polyhydroxy butyrate (PHB). 31.53% (w/v) of PHB was recovered for 1 g/L biomass. PHB is known to be widely applied in various fields, specifically in medical applications. Genetically modified isolate, low-cost substrate, and recovery without solvent assure a cost-effective and increased PHA production. Glass beads can be reused in extraction, reducing overall production cost. Therefore, this work used a reduced amount of chemicals during extraction to recover the PHB. Thus, sustainability assures a better scope for the future promotion of PHA production in academia and industries.
Highlights
-
Sustainable PHA extraction alternatives, reducing environmental impact, were studied.
-
Glass beads are used to lyse the cell to release the PHA from the bacteria Bacillus sp.
-
Box-Behnken design optimized extraction parameters for maximum recovery of PHA.
-
The cellular lysis approach yields higher recovery than solvent-method recovery and is eco-friendly.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With the rising population, the ubiquitous use of conventional plastics is causing alarming signs of global pollution. The marine ecosystem is encountering significant effects from waste disposals, specifically single-time-use plastics (Xanthos and Walker 2017; Haward 2018). Biopolymers, an alternative replacement, are catching global attention for being biodegradable, widely applicable, and biocompatible. Polyhydroxyalkanoates (PHAs) are one such biopolymer positioning highest for their biodegradability and a broad range of thermoplastic properties (Chen 2010; Geethu et al. 2019). PHA, produced by bacterial fermentation with cost-effective substrates as a carbon source, marks it as bio-based and renewable (Braunegg et al. 1998). Cost-intensive downstream process (DSP) involved in the polymer recovery and the problems applied during purification has made companies pay less attention to PHAs.
On the contrary, the downstream processes of PHA recovery and purification, which have the most significant economic impact, are among the aspects of the entire PHA production chain that have received less investigation (Jiang and Chen 2016; Koller 2017; Saavedra del Oso et al. 2021a, b). The overall PHA production life cycle inherently entails high energy consumption, particularly during PHA downstream processing, as highlighted by life cycle assessment (LCA) studies comparing PHA with fossil plastics. Despite being biobased and biodegradable, PHAs exhibit a higher carbon footprint than fossil plastics (Saavedra del Oso et al. 2021a, b).
Indeed, today several novel recovery methods are emerging as a boon to PHA production. Earlier, the solvents used in extraction have been recently replaced with 'green solvents' and other 'green methods' of extraction. Green solvents involve non-chlorinated solvents, ionic-liquids (ILs), deep eutectic solvents (DESs), and other physical green extraction methods like Aqueous two-phase system (ATPS) (Ryan et al. 2018; Pérez-Rivero et al. 2019; Colombo et al. 2020). Several extraction methods have been reported to date. These methods can be categorized as chemical, physical, and biologically-based on the source involved in extraction. Though these methods result in higher productivity of PHA, few are cost-intensive and environmentally hazardous, involving harsh chemicals and other process parameters (Jacquel et al. 2008; Laura de Donno et al. 2021).
Industrial-scale production of bacterial PHA reduces overall cost production concerning DSP, including recovery and purification. The intracellular presence of PHA and the company of a hydrophobic, amorphous core enveloped within a phospholipid layer makes it harder for isolation. Studies have been reported on its improvisation, but low-cost recovery techniques and standardization are much of a strategic asset to retain competency on an industrial scale. The development of standard research techniques in DSP is still in greater need.
The cells store PHA intracellularly as PHA granules (Koller and Niebelschütz 2013; Madkour et al. 2013). Cellular lysis can be achieved by directly applying additives, resulting in PHA degradation and reduced molecular weight (Kosseva and Rusbandi 2018; Mannina et al. 2020). Additionally, these agents are often non-recyclable, leading to the wastage of remaining aqueous solutions that require treatment. The investigation for more sustainable alternatives for PHA extraction has been conducted to decrease the environmental and economic impact of the current approaches.
Although several physical methods are available in the literature, there is a lack of literature concerning applying glass beads in cell lysis and releasing PHA from bacterial cells. To release total protein and interferon-α2b, glass beads were used to disrupt the cells of E. coli (Ramanan et al. 2008). The present study attempted PHA recovery by using 0.5 mm glass beads during cell lysis of Bacillus sp. The novelty of the current work focuses on the effectiveness of using glass beads in cell lysis and polymer release, which was otherwise used as pretreatment by other researchers (Samorì et al. 2015). Other factors assisting in cell lysis with glass beads included incubation time, temperature, the volume of water, and incubator shaker speed. Optimization of these factors involved in polymer recovery was made using Design Expert-10 statistical tool. Box-Behnken design (BBD), as a response surface methodological (RSM) tool, was used to optimize experimental trials. Hence the method is novel.
The principle involves the simple abrasive force exerted by glass beads on bacterial cells, hence lysing the cells and releasing PHA. The feasibility and reusability of glass beads make them a promising material in PHA extraction. The present study used 8 g of 0.5 mm glass beads in a pilot scale for 5 mL water media with 1 g/L cell dry weight (CDW)%. Approximately 1600 g of glass beads are required to scale up for a 1000 L reactor volume. These glass beads can be reused after treatment, as mentioned in this study. But the only challenge faced during scale-up is the treatment of glass beads with an acid wash. However, this step can be modified as per the industrial needs, considering the availability of glass beads in clean and dried condition for their re-usage in the extraction.
Production of PHB from Bacillus megaterium was first observed by Maurice Lemoigne in 1923. Several extraction methods were developed using several Bacillus sp. (Mohapatra et al. 2017; Palmeiro-Sánchez et al. 2022). Bacillus sp. offers a wide range of advantages, such as having the capability of utilizing a broad substrate range, fast growth rate and high biomass production, faster PHA accumulation on nutrient-depleted conditions, production of a wide range of PHA copolymers (using different substrates and extraction methods), and robustness and tolerance to various environmental conditions. Therefore, this study isolated, produced, and extracted PHA using Bacillus sp. cells.
2 Materials and Methods
2.1 Experimental Setup for PHA Extraction Using Glass Beads
2.1.1 Shake Flask Cultivation of Bacillus sp. for Production of PHA
Previously isolated Bacillus sp. was subcultured and maintained on nutrient agar plates at 4 °C for further studies. 10% inoculum was prepared by growing cells overnight at room temperature in a sterile nutrient broth. As discussed in the introductory part of this article, Bacillus sp. was used as the first organism to produce and extract PHA. This study isolated Bacillus sp. to recover PHA for its advantages. Inoculum contained 2.1 × 10–8 viable cells/mL. The inoculum was transferred into 200 mL sterile PHA production media and was incubated at 30 °C and 180 rpm for three days (Geethu et al. 2019). All the required chemicals were procured from Loba, Maharashtra, India.
2.1.2 Batch Studies
After three days of incubation, samples from flasks were centrifuged at 10,000 rpm for 5 min to obtain biomass. The biomass for control was dried in a hot air oven at 95 ˚C to a consistent weight. Test samples were taken as wet biomass. Wet biomass of known dry weight was taken as test samples (Divyashree and Shamala 2009), as shown in Fig. 1.
Experiments were performed in batches to opt for the best conditions for RSM studies. Effects of glass beads weight, incubation time, water volume, incubation temperature, and shaker speed were studied individually by keeping other factors constant. Factorial conditions were chosen based on literature studies (Ramanan et al. 2008; Geethu et al. 2019). Each element that showed maximum PHA yield was selected for further studies of optimization using statistical design. PHA of control was extracted using 4% sodium hypochlorite. Both control and test samples were centrifuged using acetone and dissolved in chloroform to extract PHA. This study has replaced sodium hypochlorite (except control) with glass beads for cell lysis. However, chloroform used in the dissolution of PHA can be replaced with other methods like non-chlorinated solvents or ILs, DESs, and ATPS (Yeh and Lan 2014; Prasad and Sharma 2019; Torres-Acosta et al. 2019). Therefore, by replacing both solvents, the method can be considered eco-friendly or "green recovery" and cost-effective. The experimental flowchart for PHA extraction using glass beads is presented in Fig. 1.
2.1.3 Box-Behnken Design
Five factors showing maximum PHA production in batch studies were considered for further studies. In this experiment, five factors coded values (independent variables) of glass beads weight, incubation time, water volume, incubation temperature, and shaker speed, and three levels (-1, 0, + 1) were set, as shown in Table 1. The experimental design was established, and various factors involved were optimized. Response surface methodology was applied using Design-Expert-10 software. The second-order polynomial equation (Eq. 1) was used to explain the yield of PHA:
The box-Behnken design was used as an experimental design type, which showed 43 runs in total, which included three central points as in Table 2.
The experiments were performed in 1 L Erlenmeyer flasks containing 200 mL production media. PHA yield was considered as a response in the experimental design. Analysis of variance (ANOVA) was employed to check the statistical significance of the fitted model and analyze the results.
2.1.4 Response Optimization
The optimum conditions for maximum yield response were determined. The variables were generated in Design Expert-10, a response optimizer tool, as shown in Table 3. Using these variables, PHA was extracted, and the experiment was performed in triplicate.
2.1.5 Glass Bead Washing and Reusing
According to the procedures outlined by (Ramanan et al. 2008), glass beads were removed from the disrupted medium and acid washed, with the sterilization and the drying stage being slightly altered. After acid wash, beads were rinsed with water and autoclaved. The autoclaved beads were dried in a hot air oven at 70 °C until completely dried.
2.2 Polymer Characterization
2.2.1 Fourier Transform Infra-Red (FTIR)
About 5 mg of polymer film obtained was directly analyzed by FTIR IRSpirit, QATR-S single reflection ATR accessory (Shimadzu, Japan) spectroscopic Analysis. Infra-red radiation in the 4000–400 cm-1 range was used in the Analysis (Shamala et al. 2009).
2.2.2 Nuclear Magnetic Resonance (1HNMR)
Elucidation of the chemical structure of PHA film produced by Bacillus strain, 1HNMR spectroscopic analysis was made in AMX 400 (Bruker, Germany) NMR spectrophotometer at 400 MHz 5 mg of PHA film was dissolved in 1 mL of deuterated chloroform (CDCl3) and the spectral reading was recorded (Jan et al. 1996).
2.2.3 Gas Chromatography (GC)
About 1 mg of the polymer was added to the GC ampules with 1 mL chloroform. 850 µL methanol and 150 µL conc. H2SO4. The ampules were sealed and kept in a boiling water bath for 140 min. The program settings were taken per the literature (Anil Kumar et al. 2007).
2.2.4 Differential Scanning Calorimetry (DSC)
To analyze the melting temperature (Tm) of the polymer, differential scanning calorimetry (DSC-60 plus Shimadzu) was used. Analysis of 6–8 mg PHA film was carried out. It was heated to a temperature in the range of 0–350 °C. Temperature was increased from 0 to 350 °C to the rate of 10 °C min−1 (Shrivastav et al. 2011).
3 Results and Discussion
3.1 Extraction of PHA Using Glass Beads by Statistical Optimization
3.1.1 RSM Modeling
As part of the experimental control, PHA extracted by 4% sodium hypochlorite was 37% (w/v) for 1 g/L biomass. Sodium hypochlorite methods, the standard method used in the production of bioplastics, are known to be hazardous to the environment (Saavedra del Oso et al. 2021a, b). However, this method does remove endotoxins but contributes as one of the significant parameters in increasing the total production cost. The other cost-intensive processing involves using expensive carbon sources (Furrer et al. 2007; Chaudhry et al. 2011). Extraction methods must be optimized to get good yield and productivity (Kunasundari and Sudesh 2011).
In the current investigation, 43 Box-Behnken design (BBD) experiments were performed for five variables (Table 2). PHA yield ranged from 6.91 to 27.70% at the 20th and 38th experimental runs. Analysis and experimental runs were fitted to Eq. (2). The Design Expert software provides an equation for the relationship between yield and five factors as follows:
In the above equation, as discussed, A, B, C, D, and E represent coded values of parameters glass beads weight, incubation time, water volume, incubation temperature, and shaker speed, respectively (Table 1). The effect of the factor on yield was signified by the signs present in the equation. The impact of each element as positive or negative is represented by + or – signs, respectively (Eq. 2). Among the five factors, glass bead weight (A), incubation time (B), and water volume (C) possess + signs, therefore, having a positive effect on PHA yield, whereas, incubation temperature (D), and shaker speed (E) has a negative impact on PHA yield due to – sign.
The impact of various factors on PHA yield is shown by the perturbation plot (Fig. 2). The factors A, B, and C present positive slopes. For glass bead weight (A), the positive slope indicated a positive effect on the PHA yield, increasing from 18.15 to 20.26% when the glass bead weight changed from 2 to 8 g. Incubation time (B) also showed a positive slope from 20.85 to 22.19% when the incubation time was changed from 20 to 60 min. Water volume (C) is not playing a significant role in the PHA yield, as evidenced by the relatively flat line for this factor; the water volume in Eq. (2) has a low coefficient value of 0.55, corroborating this behavior. The effect of glass bead shaking, cell suspension, and agitation speed was studied for E.coli cell disruption to release total protein and interferon-α2b (Ramanan et al. 2008) supporting the present study results. Factors D and E showed negative slopes. The PHA yield is particularly sensitive to variations in incubation temperature, as evidenced by the steeply declining slope of factor D (incubation temperature) line. The plot shows that going from an incubation temperature of 55 to 60 °C reduces PHA yield from 22.9 to 12.34%. As a result, low incubation temperature encourages cell lysis to release PHA; hence, it can be considered as a combined effect with time duration and other factors. An increase in incubation time showed a positive impact on yield. The literature indicates that varied temperatures have been studied with heat as a pretreatment. Temperatures ranging from 50 to 120 °C were checked at various time intervals. As per the literature, the higher the temperature the lower will be the duration of the cell in contact with the temperature (Ren et al. 2007; Jacquel et al. 2008; Kunasundari and Sudesh 2011). Shaker speed (E) also showed a steep declining slope with decreased yield from 21.25 to 14.26% when the shaker speed changed from 150 to 250 rpm. Literature reports the effect of the rate of agitation on PHA yield as well as biomass. Also, biomass increases with higher agitation of 250 rpm (Ramanan et al. 2008; Geethu et al. 2019; Madhusoodanan et al. 2022). Surprisingly, lower agitation showed maximum yield. Such a condition may be due to the abrasive force of glass beads and incubation time as positive factors, as shown in the plot. In conclusion, the most critical factor affecting PHA yield is incubation temperature, shaker speed, glass bead weight, and incubation time.
3.1.2 ANOVA
In Table 4, the ANOVA result for PHA yield is observed. The adequacy of the proposed model with a 95% confidence level of statistical significance was monitored. A more significant effect on the yield of PHA was observed, with the parameters having p-values < 0.05. From the ANOVA table, factors that show < 0.05 p-values are glass bead weight, incubation temperature, and shaker speed, hence representing a more significant effect on PHA yield. Also, parameters-interaction was studied in BBD as in Table 4 (ANOVA).
Interactions like AC, BC, BD, BE, and CE were significant. B2, D2, and E2 also showed significance. The "Lack of Fit F-value" of 14.77 implies that lack of fit is insignificant with the pure error. There is a 6.52% chance that a “Lack of Fit F-value” may happen because of noise. The R2 was estimated as 0.9462 which implied that 94.62% of the experimental and predicted data can be explained by the proposed model. Also, each variable influence was compared to model success as the predicted R2 (R2 pred) of 0.7855 matches well with the adjusted R2 (R2 adj) of 0.8973. The excellent precision value obtained was 19.843, an estimate of the signal-to-noise ratio, which was adequate for a good-fit model.
3.1.3 Optimization
As in Table 3, optimum conditions were obtained by solving Eq. (1). After three days of fermentation, the predicted yield of PHA for optimum conditions was 32.70% (w/v) for 1 g/L biomass dry weight. The wet biomass of known dry weight was used in the experiment. By modifying optimized values (glass beads: 8 g; incubation time: 60 min; water volume: 5 mL; incubation temperature: 55 °C; shaker speed: 160 rpm), the experimental yield of PHA was about 30.53% (w/v). Since the observed and predicted values for PHA yield are close to each other, the suggested model is adequate and validated.
The extraction procedure, regardless of whether it uses a solvent-based or a cellular lysis technique, has been shown to play a significant impact in determining the properties of the recovered PHA, including its purity and crystallinity, and it can also change the PHA granules' original morphology. This alteration of the original morphology of PHA granules is worth noting, considering that PHAs are currently utilized as chemically extracted bulk material. Notably, the intracellular accumulation of storage PHB and related PHA, which are high molecular weight polymers with over 103 3-HB units, occurs in granules surrounded by a significant number of proteins (approximately 1.9%wt). The protein content exceeds that required for PHA synthesis (Jendrossek and Pfeiffer 2014).
The lipid monolayer surrounding the polymer core of PHB/PHA granules, consisting of various structural, biosynthetic, catabolic, and regulatory proteins (Merrick and Doudoroff 1964; Mayer and Hoppert 1997), indicates a broader functionality of these granules, which have been termed "carbonosomes" (Pagliano et al. 2021). Alongside serving as energy and carbon storage, PHA granules undergo denaturation processes and increase in crystallinity when exposed to (bio)chemical (solvents, enzymes, alkaline compounds) or physical treatments (centrifugal pelleting, freezing) (Merrick and Doudoroff 1964). Native PHA granules, in contrast, have polymer chains in an amorphous state due to the presence of water, which acts as a plasticizer and prevents crystallization (Pagliano et al. 2021). Additionally, the extracted PHA granules tend to maintain the proteinaceous surface layer characteristic of native PHB granules (Kuchta et al. 2007), making it challenging to achieve 100% purity of the granules.
To prevent denaturation during the isolation process, it has been suggested that the recovery of native PHA granules should be achieved using mechanical methods (French press) or enzymatic cell lysis, followed by density gradient centrifugation. This approach enables preserving the distinctive spherical structure and shell-core composition of native PHA granules, making them suitable for various applications in biotechnology and medicine, such as protein purification and drug delivery (Jendrossek and Pfeiffer 2014). While this technique expands the potential applications of PHA by extracting these unique PHA carbonosomes, the focus of the present study revolves around PHA as a low, medium-cost bulk material with the potential to play a significant position in the future bioplastic industry.
The successful recovery of PHA is directly linked to the extraction yield, purity of the extracted PHA, and the initial PHA content present within microbial cells. Notably, PHA losses primarily occur during cell disruption due to significant PHA degradation. Consequently, the two critical factors influencing the cellular lysis approach are the purity and quality of the extracted product.
3.2 Polymer Characterization
Elucidation of functional group, chemical structure, and melting temperature (Tm) of PHA polymer was carried out by FTIR, 1HNMR, and DSC, respectively. FTIR spectrum (Fig. 3), obtained for PHA film, showed a sharp peak at 1721 cm−1, representing C = O stretching as the signature peak of PHB, according to literature (Divyashree et al. 2009; Nandiyanto et al. 2019). Other absorption bands for CH3 bend, and C-O were observed at 2976, 2933, 1454, 1378, 1277, 1050, 1099, and 1130 cm−1, respectively. Also, peaks at 978 and 513 cm−1 were observed. These were characterized as PHB peaks following the literature (Nandiyanto et al. 2019; Madhusoodanan et al. 2022).
To further confirm PHB obtained by structural elucidation, 1HNMR Analysis was performed (Fig. 4).
The literature studied showed the appearance of protonic peaks for methyl (CH3), methylene (CH2), and methyne groups (CH). Peaks at 1.26, 1.28, 1.28 ppm and 0.84 (CH3), 2.44–2.63 ppm (CH2), and peaks at 5.23–5.28 ppm (CH) observed were found to be the characteristic peak of PHB from the literature (Divyashree et al. 2009; Balakrishna Pillai et al. 2017; Morya et al. 2021). Further, gas chromatography retention time was observed at 4.15 with an area % of 0.02. The area % and retention time of standard PHB were 0.20 and 4.17, respectively.
The melting temperature (Tm) of PHB polymer is determined by its crystal thickness (Tsuge et al. 2005). PHB recovered in the current study showed Tm (Fig. 5) at 173 °C confirming Tm of PHB, as the literature reports support the same (Tsuge et al. 2005; Ansari and Fatma 2016). A high thermal degradation temperature was observed at 271 °C in the literature (Balakrishna Pillai et al. 2017), confirming the PHB degradation temperature.
4 Conclusions
The objective of the present experimental work was to optimize extraction methods involving various physical parameters. Downstream processes for polymer extraction significantly influence the overall PHA production cost from cells. Solvent extraction, currently the most widely employed method on an industrial scale, is highly effective for pure culture. However, it is not considered the most economical and environmentally friendly method. Hence, this study utilizes glass beads in PHA extraction as it is reusable and feasible. The Design of Experiments (DOE) methodology was demonstrated to clarify complicated system behavior and determine the best conditions. The experimental yield of PHA, achieved by modifying the optimized values (glass beads: 8 g; incubation time: 60 min; water volume: 5 mL; incubation temperature: 55 °C; shaker speed: 160 rpm), was approximately 30.53% (w/v). The wet biomass of known dry weight was utilized in the experiment, resulting in a predicted yield of 32.70% (w/v) for 1 g/L biomass dry weight after three days of fermentation under optimum conditions. Therefore, the experimental outcome obtained was closer to the predicted value. PHA recovery was confirmed as PHB by characterization. Also, Bacillus sp. used in PHB production is an additional advantage of the method for being endotoxin-free, fast-growing, and possessing feasible growth conditions. Further optimization of glass bead size, pretreatment of biomass, and advanced green methods for maximum PHA recovery as a green downstream approach can undoubtedly promise a PHB to flourish soon.
Data Availability
The authors declare that the data supporting the findings of this study are available within the paper. Any raw data files needed are available from the corresponding author upon reasonable request.
References
Anil Kumar PK, Shamala TR, Kshama L, Prakash MH, Joshi GJ, Chandrashekar A, Latha Kumari KS, Divyashree MS (2007) Bacterial synthesis of poly(hydroxybutyrate- co-hydroxyvalerate) using carbohydrate-rich mahua (Madhuca sp.) flowers. J Appl Microbiol 103:204–209. https://doi.org/10.1111/j.1365-2672.2006.03221.x
Ansari S, Fatma T (2016) Cyanobacterial Polyhydroxybutyrate ( PHB ): Screening, Optimization and Characterization. PLoS ONE 11:1–20. https://doi.org/10.1371/journal.pone.0158168
Balakrishna Pillai A, Jaya Kumar A, Thulasi K, Kumarapillai H (2017) Evaluation of short-chain-length polyhydroxyalkanoate accumulation in Bacillus aryabhattai. Braz J Microbiol 48:451–460. https://doi.org/10.1016/j.bjm.2017.01.005
Braunegg G, Lefebvre G, Genser KF (1998) Polyhydroxyalkanoates, biopolyesters from renewable resources: Physiological and engineering aspects. J Biotechnol 65:127–161. https://doi.org/10.1016/S0168-1656(98)00126-6
Chaudhry WN, Jamil N, Ali I, Ayaz MH, Hasnain S (2011) Screening for polyhydroxyalkanoate ( PHA ) -producing bacterial strains and comparison of PHA production from various inexpensive carbon sources. 623–629. https://doi.org/10.1007/s13213-010-0181-6
Chen G (2010) Introduction of Bacterial Plastics PHA, PLA, PBS, PE, PTT, and PPP. Plastics from Bacteria. Microbiology Monographs. Springer, Berlin, Heidelberg, pp 1–16
Colombo B, Pereira J, Martins M, Torres-Acosta MA, Dias ACRV, Lemos PC, Ventura SPM, Eisele G, Alekseeva A, Adani F, Serafim LS (2020) Recovering PHA from mixed microbial biomass: Using non-ionic surfactants as a pretreatment step. Sep Purif Technol 253:1–12. https://doi.org/10.1016/j.seppur.2020.117521
Divyashree MS, Shamala TR (2009) Effect of gamma irradiation on cell lysis and polyhydroxyalkanoate produced by Bacillus flexus. Radiat Phys Chem 78:147–152. https://doi.org/10.1016/j.radphyschem.2008.08.010
Divyashree MS, Shamala TR, Rastogi NK (2009) Isolation of polyhydroxyalkanoate from hydrolyzed cells of Bacillus flexus using aqueous two-phase system containing polyethylene glycol and phosphate. Biotechnol Bioprocess Eng 14:482–489. https://doi.org/10.1007/s12257-008-0119-z
Furrer P, Panke S, Zinn M (2007) Efficient recovery of low endotoxin medium-chain-length poly([R]-3-hydroxyalkanoate) from bacterial biomass. J Microbiol Methods 69:206–213. https://doi.org/10.1016/j.mimet.2007.01.002
Geethu M, Vrundha R, Raja S, Raghu Chandrashekar H, Divyashree MS (2019) Improvement of the Production and Characterisation of Polyhydroxyalkanoate by Bacillus endophyticus Using Inexpensive Carbon Feedstock. J Polym Environ 27:917–928. https://doi.org/10.1007/s10924-019-01397-z
Haward M (2018) Plastic pollution of the world ’ s seas and oceans as a contemporary challenge in ocean governance. Nat Commun 667:1–3. https://doi.org/10.1038/s41467-018-03104-3
Jacquel N, Lo CW, Wei YH, Wu HS, Wang SS (2008) Isolation and purification of bacterial poly(3-hydroxyalkanoates). Biochem Eng J 39:15–27. https://doi.org/10.1016/j.bej.2007.11.029
Jan S, Roblot C, Courtois J, Courtois B, Barbotin JN, Séguin JP (1996) 1H NMR spectroscopic determination of poly 3-hydroxybutyrate extracted from microbial biomass. Enzyme Microb Technol 18:195–201. https://doi.org/10.1016/0141-0229(95)00096-8
Jendrossek D, Pfeiffer D (2014) New insights in the formation of polyhydroxyalkanoate granules (carbonosomes) and novel functions of poly(3-hydroxybutyrate). Environ Microbiol 16:2357–2373. https://doi.org/10.1111/1462-2920.12356
Jiang XR, Chen GQ (2016) Morphology engineering of bacteria for bio-production. Biotechnol Adv 34:435–440. https://doi.org/10.1016/j.biotechadv.2015.12.007
Koller M (2017) Advances in Polyhydroxyalkanoate (PHA) Production. Bioengineering 4:1–7. https://doi.org/10.3390/bioengineering4040088
Koller M, Niebelschütz H (2013) Strategies for recovery and purification of poly [( R ) -3-hydroxyalkanoates ] ( PHA ) biopolyesters from surrounding biomass. Eng Life Sci 1:549–562. https://doi.org/10.1002/elsc.201300021
Kosseva MR, Rusbandi E (2018) Trends in the biomanufacture of polyhydroxyalkanoates with focus on downstream processing. Int J Biol Macromol 107:762–778. https://doi.org/10.1016/j.ijbiomac.2017.09.054
Kuchta K, Chi L, Fuchs H, Pötter M, Steinbüchel A (2007) Studies on the influence of phasins on accumulation and degradation of PHB and nanostructure of PHB granules in Raistonia eutropha H16. Biomacromol 8:657–662. https://doi.org/10.1021/bm060912e
Kunasundari B, Sudesh K (2011) Isolation and recovery of microbial polyhydroxyalkanoates. Express Polym Lett 5:620–634. https://doi.org/10.3144/expresspolymlett.2011.60
Laura de Donno M, Moreno S, Rene ER (2021) Polyhydroxyalkanoate (PHA) production via resource recovery from industrial waste streams: A review of techniques and perspectives. Bioresour Technol 331:124985. https://doi.org/10.1016/j.biortech.2021.124985
Madhusoodanan G, Hariharapura RC, Somashekara D (2022) Dissolved oxygen as a propulsive parameter for polyhydroxyalkanoate production using Bacillus endophyticus cultures. Environ Dev Sustain 24:4641–4658. https://doi.org/10.1007/s10668-021-01626-3
Madkour MH, Heinrich D, Alghamdi MA, Shabbaj II, Steinbüchel A (2013) PHA recovery from biomass. Biomacromol 14:2963–2972. https://doi.org/10.1021/bm4010244
Mannina G, Presti D, Montiel-Jarillo G, Carrera J, Suárez-Ojeda ME (2020) Recovery of polyhydroxyalkanoates (PHAs) from wastewater: A review. Bioresour Technol 297:122478. https://doi.org/10.1016/j.biortech.2019.122478
Mayer F, Hoppert M (1997) Determination of the thickness of the boundary layer surrounding bacterial PHA inclusion bodies, and implications for models describing the molecular architecture of this layer. J Basic Microbiol 37:45–52. https://doi.org/10.1002/jobm.3620370108
Merrick JM, Doudoroff M (1964) Depolymerization of Poly– hydroxybutyrate by an intracellular enzyme system. J Bacteriol 88:60–71. https://doi.org/10.1128/jb.88.1.60-71.1964
Mohapatra S, Maity S, Dash HR, Das S, Pattnaik S, Rath CC, Samantaray D (2017) Bacillus and biopolymer: Prospects and challenges. Biochem Biophys Reports 12:206–213. https://doi.org/10.1016/j.bbrep.2017.10.001
Morya R, Sharma A, Kumar M, Tyagi B, Singh SS, Thakur IS (2021) Polyhydroxyalkanoate synthesis and characterization: A proteogenomic and process optimization study for biovalorization of industrial lignin. Bioresour Technol 320:124439. https://doi.org/10.1016/j.biortech.2020.124439
Nandiyanto ABD, Oktiani R, Ragadhita R (2019) How to read and interpret ftir spectroscope of organic material. Indones J Sci Technol 4:97–118. https://doi.org/10.17509/ijost.v4i1.15806
Pagliano G, Galletti P, Samorì C, Zaghini A, Torri C (2021) Recovery of Polyhydroxyalkanoates From Single and Mixed Microbial Cultures: A Review. Front Bioeng Biotechnol 9:1–28. https://doi.org/10.3389/fbioe.2021.624021
Palmeiro-Sánchez T, O’Flaherty V, Lens PNL (2022) Polyhydroxyalkanoate bio-production and its rise as biomaterial of the future. J Biotechnol 348:10–25. https://doi.org/10.1016/j.jbiotec.2022.03.001
Pérez-Rivero C, López-Gómez JP, Roy I (2019) A sustainable approach for the downstream processing of bacterial polyhydroxyalkanoates: State-of-the-art and latest developments. Biochem Eng J 150. https://doi.org/10.1016/j.bej.2019.107283
Prasad K, Sharma M (2019) Green solvents for the dissolution and processing of biopolymers. Curr Opin Green Sustain Chem 18:72–78. https://doi.org/10.1016/j.cogsc.2019.02.005
Ramanan RN, Ling TC, Ariff AB (2008) The performance of a glass bead shaking technique for the disruption of Escherichia coli cells. Biotechnol Bioprocess Eng 13:613–623. https://doi.org/10.1007/s12257-008-0047-y
Ren X, Yu D, Han S, Feng Y (2007) Thermolysis of recombinant Escherichia coli for recovering a thermostable enzyme. Biochem Eng J 33:94–98. https://doi.org/10.1016/j.bej.2006.09.017
Ryan S, Fathi A, Bahramian B, Manavitehrani I, Mcclure DD, Valtchev P, Schindeler A, Dehghani F, Kavanagh JM (2018) A green process for the purifi cation of biodegradable poly( β - hydroxybutyrate ). J Supercrit Fluids 135:84–90. https://doi.org/10.1016/j.supflu.2018.01.007
Saavedra del Oso M, Mauricio-Iglesias M, Hospido A (2021) Evaluation and optimization of the environmental performance of PHA downstream processing. Chem Eng J 412:127687. https://doi.org/10.1016/j.cej.2020.127687
Saavedra del Oso M, Mauricio-Iglesias M, Hospido A (2021b) Evaluation and optimization of the environmental performance of PHA downstream processing. Chem Eng J 412:127687. https://doi.org/10.1016/j.cej.2020.127687
Samorì C, Abbondanzi F, Galletti P, Giorgini L, Mazzocchetti L, Torri C, Tagliavini E (2015) Extraction of polyhydroxyalkanoates from mixed microbial cultures: Impact on polymer quality and recovery. Bioresour Technol 189:195–202. https://doi.org/10.1016/j.biortech.2015.03.062
Shamala TR, Divyashree MS, Davis R, Kumari KSL, Vijayendra SVN, Raj B (2009) Production and characterization of bacterial polyhydroxyalkanoate copolymers and evaluation of their blends by fourier transform infrared spectroscopy and scanning electron microscopy. Indian J Microbiol 49:251–258. https://doi.org/10.1007/s12088-009-0031-z
Shrivastav A, Mishra SK, Pancha I, Jain D, Bhattacharya S, Patel S, Mishra S (2011) Biodegradability studies of polyhydroxyalkanoate (PHA) film produced by a marine bacteria using Jatropha biodiesel byproduct as a substrate. World J Microbiol Biotechnol 27:1531–1541. https://doi.org/10.1007/s11274-010-0605-2
Torres-Acosta MA, Mayolo-Deloisa K, González-Valdez J, Rito-Palomares M (2019) Aqueous Two-Phase Systems at Large Scale: Challenges and Opportunities. Biotechnol J 14:1–31. https://doi.org/10.1002/biot.201800117
Tsuge T, Yano K, Imazu SI, Numata K, Kikkawa Y, Abe H, Taguchi S, Doi Y (2005) Biosynthesis of polyhydroxyalkanoate (PHA) copolymer from fructose using wild-type and laboratory-evolved PHA synthases. Macromol Biosci 5:112–117. https://doi.org/10.1002/mabi.200400152
Xanthos D, Walker TR (2017) International policies to reduce plastic marine pollution from single-use plastics (plastic bags and microbeads): A review. Mar Pollut Bull 118:17–26. https://doi.org/10.1016/j.marpolbul.2017.02.048
Yeh CY, Lan JCW (2014) Direct recovery of polyhydroxyalkanoates synthase from recombinant Escherichia coli feedstock by using aqueous two-phase systems. J Taiwan Inst Chem Eng 45:1119–1125. https://doi.org/10.1016/j.jtice.2013.10.020
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal Authors are grateful to The Department of Biotechnology (DBT), Government of India, for sponsoring the work (PR 18430/BIC/101/703/2016) and the Department of Biotechnology, Manipal Institute of Technology, Manipal University, India, for providing the facilities to carry out the research work.
Author information
Authors and Affiliations
Contributions
Sohani Bhat G: Performing the experiments, design analysis, and manuscript writing; Deekshitha B K: Model analysis; Selvaraj Raja: Supervision of experimental design and analysis, Thivaharan V: Manuscript review; Divyashree M S: Supervision of experiments and manuscript review.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bhat, S.G., Kodavoor, D.B., Raja, S. et al. Optimization of Physical Parameters Involved in Cell Lysis of Bacillus Sp. to Recover Bioplastic Polyhydroxyalkanoates. Environ. Process. 10, 47 (2023). https://doi.org/10.1007/s40710-023-00661-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40710-023-00661-8