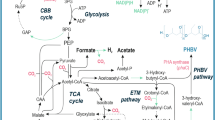
Abstract
Conventional biopolymers resembling synthetic polymers produced from microorganisms, polyhydroxyalkanoate (PHA) synthesized utilizing renewable resources have gained supreme attention recently. PHA accumulation within the microbial cell is an innate capability of bacteria to store carbon and energy when nutrient imbalance pertains. Gram positive Bacillus endophyticus capable of synthesizing PHA was focused in this study. Study focuses on the possibility of attaining high PHA yield in relation to the varying dissolved oxygen levels induced during production phase. There was a gradual increment in PHA production from 34.5 to 53.03% when cultivated in bioreactor that maintained least dissolved oxygen of 0.4 mg/L at 32 °C. The metabolic flux of organism was altered during oxygen stress brought by varying agitation rate and volume resulting in the accumulation of Nicotinamide Adenine Dinucleotide Hydrogen (NADH), which led to increase in the overall PHA production. PHA yield was found to be favored by decreasing the oxygen supply thereby inducing an oxygen stress environment. This report was the first one that was correlating the hypothesis that links PHA yield and oxygen stress condition during production phase. PHA produced was characterized by FTIR and 1HNMR spectra in which the presence of Polyhydroxybutyrate was confirmed.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Worldwide interest to use bio-based polymers has been accelerated as an eco-friendly alternative to plastic. Their physio-chemical properties can overcome the increased demands in the rapidly developing fields like biomedical and industrial sector (Ojumu et al., 2004). The use of biodegradable polymers forefront the problems associated with the petrochemical waste disposals and helps in effectively minimizing the energy consumption and greenhouse effect which has significantly elevated in recent years (Khatami et al., 2020). Conventional biopolymers resembling synthetic polymers produced from microorganisms have gained supreme attention as they synthesize the building blocks (monomer) utilizing the renewable resources. Among the microbial synthesized biopolymers, Polyhydroxyalkanoates (PHA) are gaining much attention due to their similar material properties toward plastic (Zheng et al., 2020; Chen and Jiang, 2018).
PHA are polymers synthesized entirely by a biological process within the cytoplasm as inclusion bodies (Sathya et al., 2018) and are naturally occurring aliphatic polyesters produced by more than 75 genera of gram positive and gram negative bacteria. PHAs are produced by microbial fermentation of carbon-rich substrates (sugars, lipids and their derivatives) in an environment of low or limited nutrients like nitrogen, phosphorus, sulphur, magnesium or oxygen concentration (Kundu et al., 2014; Blunt et al., 2018). Characteristic elevation of NAD(P)H, acetyl CoA and ATP induces Polyhydroxybutyrate (PHB) formation and are critical in regulating the energy flow intracellularly (Sathya et al., 2018). For a PHA producing organism, if the growth conditions are restricted approximately over a time period, like removal of nitrogen and phosphorus the biomass tends to get exposed to fluctuating electron donors and acceptors (Moralejo-Gárate et al., 2013). During this time, referred to as ‘feast and famine condition’ the organism tends to store a major portion of the substrate as biopolymer as a survival strategy (Moralejo-Gárate et al., 2011; Wang et al., 2017). The substrate storage in the form of polyhydroxyalkanoate is usually thought to serve as a mechanism to overcome adverse growth conditions (Narayanan et al., 2021; Third et al., 2003).
PHA production by microorganism mainly depends on the carbon source and other nutrients provided. A strategy can be introduced to induce pressure on PHA producers to convert available carbon source to biopolymer. This condition can be achieved by optimizing the ecosystem and by maintaining a feast and famine condition to organism. Oxygen is a requisite requirement of Bacillus spps for the growth and other metabolic activities. The possibility of attaining increased biopolymer production by aerobic bacterial species under low oxygen supply induced by stress condition has been scarcely reported. Most of the studies have been focused on limiting nutrients like nitrogen, phosphorus to increase PHA yield in aerobic strains, but oxygen limitation and its impact on the survival of aerobic strains are rarely reported. Few studies were focused on how dissolved oxygen, and substrate competition was linked in enhancing PHA production (Wang et al., , 2017, 2019). This aspect need to be studied in detail to link the hypothesis that correlates decreased oxygen concentration on increased PHA yield (Kulpreecha et al., 2009; Mohammed et al., 2019). Oxygen limitation is also reported to play a role in enhancing PHA production; contradictorily aeration is prerequisite for the increase in biomass density especially for aerobic organism (Maheshwari et al., 2018). So evaluation of the role of oxygen and its influence on PHA production is a necessary parameter in considering maximum PHA production (Mohanrasu et al., 2020). It was reported that excessive agitation and aeration improved cell density as more ATP is required to increase biomass, whereas the PHA conversion rate seems to be improved at low dissolved oxygen levels in few organisms (Vikström et al., 2019; Wang et al., 2017; Koller et al., 2017; Carter and Dawes, 1979).
The impact of the dissolved oxygen on PHA production process and its linkage has been scarcely reported. This work mainly focuses in providing experimental insight toward hypothesis linking PHA accumulation and dissolved oxygen in Bacillus endophyticus which is an aerobic organism. The impact of oxygen limitation and the metabolic stress brought about resulting in triggering PHA biosynthesis is a different line of study that has been focused here. An optimized nutrient limiting medium with an excess of sucrose concentration was used to study the PHA production profile of organism under induced oxygen stress. The effect of low dissolved oxygen (DO) during the production phase along with the consequent influence of the aeration on biopolymer production was studied. This study highlights the novel approach in introducing Wrinkler’s titration method to estimate the DO concentration in the shake flask studies. Also different bioreactor set up studies so as to induce oxygen stress was focused in this study as a novel experimental approach to correlate the oxygen limitation in an aerobic strain for PHA biosynthesis. The novelty of the work is this correlated the linkage of dissolved oxygen stress on aerobic PHA producer in both shake flask and bioreactor. The oxygen stress as an input to determine PHA production is related to production cost since large amount of energy is required to carry out large scale production. This study determines different strategies selected to induce oxygen stress thereby to enhance PHA production.
2 Materials and methods
2.1 Microorganism and culture maintenance
Bacillus endophyticus was used for the biosynthesis of PHA and was characterized earlier in lab. The culture was maintained and sub-cultured on nutrient agar medium (Hi-media, Mumbai, India) once in a month and stored at 4 °C for further use.
2.2 Shake flask cultivation
Impact of oxygen stress on test strain was studied in shake flask (i) by changing the volume of the medium and (ii) by varying the agitation rate. Biopolymer production was conducted in a statistically optimized production medium (PM), composition (g/L): Na2HPO4 2H2O 2.2, KH2PO4 1.5, (NH4)2SO4 1.5, MgSO47H2O 0.2, Sucrose 20–40 g/L: PM I: 20 g/L, PM II: 40 g/L. in cotton plugged 250 mL Erlenmeyer flask with three different volumes (50, 100 and 150 ml) and to study the role of agitation and aeration on PHA production, Bacillus endophyticus was cultivated in production medium with a fixed volume of 50 mL in a 250 mL. Erlenmeyer flask on shaker with varying agitation rate of 100, 150 and 200 rpm was maintained, and the schematic is represented in Fig. 1. The concentration of sucrose was varied in order to study the influence of sugar content on PHA accumulation. The pH of the PM was maintained at 7 before inoculation. 10% of the inoculum was added to both PM (I and II) which was later incubated at 32 °C for 72 h in duplicates. Samples were drawn at regular intervals for DO analysis and estimation of overall PHA yield.
2.3 Dissolved oxygen control process in Batch cultivation
PHA production was carried out in batch in a 3 L bioreactor with a working volume of 1L and was equipped with pH, dissolved oxygen and temperature probe. External water supply helped in maintaining 31 °C throughout the process, and the DO % was recorded from the display provided by DO sensor (Mettler-Toledo) attached to bioreactor to monitor the variation in DO during PHA cultivation stage. Airflow was controlled with rotameter, and the culture medium was maintained as same as in shake flask cultivation. To study the influence of oxygen limitation on PHA production during batch cultivation, alterations were made in the agitation and airflow rate during the course of PHA production (late log phase 36 h of cultivation in case of B. endophyticus). The initial DO % were measured during PHA production phase at constant temperature of 31 °C, and samples were drawn from bioreactor during 0, 24, 36, 48 and 72 h and analyzed for biomass, PHA accumulation, sucrose uptake and DO %. Agitation rates were varied accordingly to obtain lesser DO %, and aeration rates were switched in the range of 0.5–2 vvm. Oxygen stress condition was maintained by altering DO levels by varying the agitation rate and restricting oxygen supply to the reactor.
Four different experimental strategies were used to compare the DO limitation on biopolymer production in Bacillus spp. (i) Initially 100% and 65% after 36 h of cultivation, (ii) 65% initially and 25% after 36 h (iii) 100% initial DO and 50% after 36 h and (iv) 100% initial DO and 0.4% after 36 h of PHA cultivation. At the end of the batch cultivation, broth was centrifuged, and cells were harvested to analyse overall PHA production. In Control experiment (Expt 1) organism was grown without altering the airflow (1vvm) and agitation rate (250 rpm). These trials were to observe how DO levels influence the growth and PHA biosynthesis. In second strategy, the initial DO itself was maintained at 65% by controlling the air flow rate by 1 vvm and maintained agitation at 150 rpm till 36 h of cultivation. Later DO was altered to 25% by reducing agitation rate by 100 rpm and 0.5 vvm of air flow rate at 36 h which was maintained till 72 h. In the third strategy (Expt 3), initial DO was 100%, and the agitation rate was reduced from 250 to 100 rpm at 36 h. The airflow rate was reduced to 1.5 vvm to maintain DO rate as 50% at 36 h of cultivation. To maintain detectable dissolved oxygen limitation state, finally in expt 4, 100% DO was maintained during the initial phase (lag and log phase) till 36 h, and airflow and agitation were stopped to maintain very low DO rate by maintaining complete absence of external air supply till 72 h which resulted in 0.4% of DO inside bioreactor set up.
The schematic of four different experimental set up using bioreactor is represented in Fig. 2. All batch cultivation was carried out in duplicates under same experimental conditions by altering oxygen supply in bioreactor. To confirm the DO % provided by the bioreactor and to cross check, Wrinkler’s titration method was periodically performed during 0, 24, 36, 48 and 72 h which gave DO in mg/L. The results were compared with that of the DO % provided by DO sensors. The pH was maintained at 7 by automatic addition of 1 M NaOH/HCl.
2.4 Estimation of cell dry mass
The culture broth was collected and centrifuged at 10,000 rpm (Plastocraft SSR-V/FM) for 10 min, and the supernatant was analyzed for residual sugar by dinitrosalicylic acid method (Miller, 1959) through spectrophotometric analysis at 540 nm. The harvested cell pellet was washed with distilled water and then dried at 80 °C to a constant weight.
2.5 Extraction of PHA
The PHA content of the cells was estimated by subjecting dry cells into hydrolysis for 1 h using 4% Sodium hypochlorite solution at 40 °C (Slepecky and Law, 1960). The hydrolysate was centrifuged followed by water and acetone wash and dissolved in chloroform. PHA obtained was quantified gravimetrically (Lakshman and Shamala, 2006).
2.6 Determination of dissolved oxygen (DO) by Wrinkler’s titration method
The cultures maintained at different agitation rate by shake flask cultivation were subjected to DO estimation by Wrinkler’s titration. 25 mL of the culture was drawn at 0, 24, 48 and 72 h aseptically in a 25 mL centrifuge tube and tightly closed with care to exclude air bubble. The remaining culture was used to extract PHA and calculated PHA % based on cell dry weight. The culture was centrifuged at 10,000 rpm for 8 min and 10 mL of the supernatant was subjected to titration against 0.01 N sodium thiosulfate, prior to titration 1 mL of Manganese sulfate was added followed by the addition of Alkaline Potassium iodide azide solution. Addition of solutions resulted in precipitation of the supernatant, which later gets settled above manganese hydroxide floc. Addition of 0.5 ml of sulfuric acid lead to dissolution of precipitate. 10 mL of this solution was taken for titration followed by addition of 3 drops of freshly prepared starch indicator was added that imparted deep blue color. The disappearance of blue color was indicated as end point (Gouda et al., 2001). The volume of the end point and normality of sodium thiosulfate divided by the volume of supernatant used for titration gave normality of sample and from this DO in mg/L was calculated (Hargreaves & Tucker, 2002; Vikström et al., 2019).
2.7 FTIR and 1 HNMR analysis of PHB standard
The PHA film obtained after dissolving in chloroform was thoroughly mixed with potassium bromide (KBr) and pelletized. This pellet was placed in the sample chamber in FTIR spectrophotometer and exposed to infrared radiation to obtain a spectrum using Shimadzu 8400 S with spectral range of 4000–400 cm−1.1H NMR spectrum was obtained by dissolving 5 mg of PHA film in deuterated chloroform and analyzed in Bruker Ascend 400 NMR spectrometer at 22 °C.
3 Result and discussion
3.1 Effect of dissolved oxygen (DO) on PHA accumulation in shake flask
3.1.1 Impact of dissolved oxygen on PHA accumulation based on agitation rate
With an objective to study the effect of aeration on the growth and PHA accumulation rate. Two different medium, PM I and PM II with 2 and 4% of sucrose concentration, respectively, was used to identify the maximum yield of biopolymer with course of time and aeration. From the results obtained, aeration played a significant role in increasing biopolymer with high cell density. At low aeration, provided by maintaining agitation rate of 100 rpm with minimum sugar concentration (2%), the organism showed reduced PHA production (0.26 g/L) with low biomass accumulation (0.367 g/L). The maximum PHA yield (1.047 g/L) from 2.101 g/L of biomass (PHA % 49.83) was reported at 200 rpm with maximum sugar conentration of 4%.
The relationship between the growth and overall PHA yield with DO (mg/L) levels was analyzed by taking the sample at 0, 24, 48 and 72 h. From the batch fermentation studies of organism carried out in Erlenmeyer flask, it was found that PHA production was initiated in the late stationary phase (36–48 h) which was in agreement with the DO estimation results at 48 h which showed a decrease in DO. The initial DO of the medium before inoculation (0 h) was estimated (control) and considered as maximum which was observed to decrease in the due course of time during growth and the polymer production (Fig. 3a). The maximum PHA percentage and its correlation with the DO are represented in Fig. 3a, b and c at different agitation rates. From the result, it was clear that when DO mg/L was less than 10, PHA production seems to increase before which the production was slow. This observation links stress brought about by reduced DO concentration during the late exponential phase favors PHA production (Moralejo-Gárate et al., 2013; Ward et al., 1977). DO and bacterial growth are linked and as the organism grows, the DO levels of the medium get reduced unless aerating the medium frequently. This principle is used for the study and less DO levels can cause stress in aerobic organism. PHA is produced under stress condition, when NADH level within the organism increases the acetyl CoA will not get oxidized in the absence of oxygen leading organism to switch to PHA pathway liberating more intermediates for PHA biosynthesis blocking Krebs cycle. In present study, attempt was made to induce the PHA production by maintaining relatively low oxygen for the strain to push the strain under stress. In general, PHA synthesis is often induced by nutrient stress. To carry out the above analysis, various conditions are maintained as mentioned in the methodology, and dissolved oxygen levels were measured periodically. It has been reported that variation in DO lead to oxygen stress, and this may also play an important role in increasing the overall polymer turnover (Hargreaves & Tucker, 2002; Lefebvre et al., 1997).
3.1.2 Impact of dissolved oxygen on PHA accumulation based on varying volume
Production medium with varying sucrose concentration (2 and 4%) was maintained in three different volume (50, 100 and 150 mL) in 250 mL Erlenmeyer flask at 200 rpm to study the impact of oxygen available in the medium and to its correlation with varying volume of the medium. DO (mg/L) was evaluated at 0, 24, 36, 48 and 72 h by Wrinkler’s titration method to investigate the DO levels of two different production medium (PM I and PM II) with varying volume (50,,100 and 150 mL). The DO level of the production medium (PM I and PM II) at three different volume level is shown as a line graph in Fig. 4 (a and b) whereas the biomass and PHA with PHA % is given in Table 1. Lesser the volume of the medium than the available capacity or vice versa, higher or lower the oxygen supply which can be enhanced or reduced by providing suitable agitation rate at different rpm. To bring out oxygen limitation, during the stationary phase where PHA production initiates, the agitation rate was made constant with increment in the volume of production medium so as to create lesser air space with respect to the flask mouth. Bacillus endophyticus was allowed to grow by providing optimal conditions, and PHA was estimated gravimetrically.
Bacillus endophyticus being aerobic organism largely depends on the aeration provided for growth and cell maintenance. Initially during the first phase of study, to investigate PHA production by test organism under different fermentation parameters, influence of varying agitation rate and volume-based studies were carried out. PHA production largely depends on the biomass of the organism as the storage polymer is synthesized within cytoplasm (Jacquel et al., 2008). Experiments were performed to find relationship between PHA accumulation rate and dissolved oxygen to assess the hypothesis that relates PHA biosynthesis with stress induced by the reduction in the oxygen supply. The experimental results for variation in the DO level are shown in Fig. 4a and b with respect to varying volume of PM (PM I and PM II) is represented. It was found that lesser the volume of production medium (50 ml in 250 ml Erlenmeyer flask) greater the PHA accumulation (48.2% of PHA from 2.53 g/L of biomass in PM II) shown in Table 1. This observation suggests that increase in the biomass yield, due to decreased volume maintained in shake flask is directly proportional to the biopolymer production.
The oxygen supply conditions within the shake flask may greatly affect the growth and PHA production by test organism. In Erlenmeyer, flasks used as shake culture vessel provide relatively low oxygen condition for the organism that requires high oxygen demand (McDaniel et al., 1965). PHA being produced under stress, the availability and limitation of oxygen may play a crucial role in inducing stress along with nutrient limitation which can possibly enhance or reduce PHA biosynthesis (Wang et al., 2017). This hypothesis was considered and studied by varying the volume of the production medium within the flask to produce variation in the oxygen availability. Regulating DO within the medium is easier than optimizing the media components to develop oxygen stress which in turn improve PHA yield. Previous reports have suggested that PHA synthesis is known to be influenced by environmental stress like nitrogen, phosphorous and oxygen limitation (Moralejo-Gárate et al., 2013; Ward et al., 1977). In 2005, Quilaguaman et al. reported the significant influence on increase PHA production brought about by oxygen limiting conditions (Quillaguaman et al., 2005). Third et al., in 2003 reported excessive aeration rate was required for biomass accumulation and low DO level for biomass accumulation in the presence of acetate as substrate for mixed microbial culture (Third et al., 2003). This study as well highlighted the relationship between DO and PHA accumulation, whereas in this study highlights the presence of sucrose as substrate. Similar results were reported by Moralejo-Garate et al., in 2013 highlighted that polyglucose production under limited oxygen supply was more. The least DO % in feast phase contributed more toward PHA production (Moralejo-Gárate et al., 2013). Also Coats et al., in 2016 investigated the role of aeration on PHA accumulation and found no significant impact on PHA production (Coats et al., 2016). This study mainly focused on the DO level at the point where PHA production was found to rapidly increase and the results justified the hypothesis in linking lower DO level and increased PHA production.
3.2 Impact of dissolved oxygen on PHA accumulation based on bioreactor studies
Dissolved oxygen limitation was reported to influence PHA production positively in many organism (Kshirsagar et al., 2013; Kaur and Roy, 2015; Borah et al., 2002). It was reported that 0–1% of DO (Oxygen limited condition) had increased PHA production in A.vinelandii ATCC 9046, and 5–10% of DO was used by same organism to grow and increase biomass (García et al., 2014). The influence of DO in B.endophyticus was initially determined in experiments at flask scale, by altering the agitation rate and volumes. From those results, it was clearly observed that PHA production was initiated when there was a reduction in DO (during late log phase 36 h). So it is important to extrapolate shake flask study to batch cultivation in bioreactor. To study the impact of dissolved oxygen on growth and PHA production on large scale, it is essential to confirm the relationship between these two parameters at different time intervals as batch cultivation in bioreactor. From volume-based shake flask study, it was observed that minimum volume of the production medium with maximum agitation rate gave maximum PHA production. Considering this study, we maintained minimum voilume of 1L of production medium in 3 L capacity bioreactor in all the experimental conditions. The dissolved oxygen was maintained at 100% initally (control experiment) during the fermentation process and noted the fluctuation in the dissolved oxygen levels at 0, 24, 36, 48 and 72 h. DO in percentage provided by the batch fermentor system was compared by Wrinklers titration as mg/L.
At bioreactor scale with optimized production medium (Sucrose 4%), the biomass, PHA production pattern, DO and sugar utilization by the organism was studied. The time course study of the essential parameters was peformed at different time intervals, and the results are depicted as line graph (Fig. 5) as four differet experimental trials. In batch cultivation, oxygen excess or complete absence can be programmed to study the dissolved oxygen induced stress. But initially, an exponential increase in the oxygen supply need to be provided to study the pace of growth, to accumulate sufficient biomass as the organism especially in Bacillus endophyticus which is an aerobic organism. Attempts were gradually introduced later during the stationary phase which induced an additional stress element during batch cultivation which was observed to increase PHA accumulation. Lately, three different bioreactor run was carried out to study the average production and to study the variation from control experiment in producing biomass and PHA by organism. In all the four batch cultivation Fig. 5a,b,c and d no PHA was produced during the first 24 h, and the nutrients were predominantly used for biomass production.
3.2.1 Control batch cultivation without inducing oxygen limitation
The Bacillus endophyticus strain produces PHA under optimized production medium (previously optimized statistically) in shake flask. Initial bioreactor batch cultivation was maintained as control experiment without altering the DO percentage. The agitation and air flow rate was maintained as 250 rpm and 2 vvm, respectively, for 72 h. The conditions provided was not altered throughout 72 h, biomass and PHA accumulation pattern along with sugar utilization was monitered as gravimetric analysis and by DNS, respectively. The PHA percentage observed was 34.54 from the control experiment (Fig. 5a) without altering the initial DO % which was eventually reduced during the course of bacterial growth. Biomass was produced on higher rates in the initial growth phase (Log phase 18–28 h) in the presence of 100% DO content, and the PHA production was noted to initiate during 36 h with a drop in DO level (65%) which was in well accordance with the shake flask cultivation results. At 24 h, the biomass was 1.8 g/L with residual sugar concentration of about 3.8 g/L indicating that sucrose is not being utilized, whereas at 36 h the residual sugar concentration also dropped to 2.8 g/L indicating that the organism started utilizing carbon source for initiating PHA production (Fig. 5a).
The organism was provided with a balanced nutrient medium which lately got exhausted during the growth, nevertheless, the biopolymer concentration seems to increase after 34 h in all condition. This experiment was maintained as control experiment to compare the variation of DO and the stress thus induced on aerobic PHA producer by altering the agitation and air flow rate thereby maintaining reduced DO level.
3.2.2 Batch cultivation with inducing oxygen limitation during initial lag and log phase
In the second batch cultivation with same media conditions, DO alteration was brought about in the initial lag and log phase by reducing the DO % to 65 which was again reduced to 25% at 36 h. This was maintained by reducing the agitaton rate to 150 rpm and airflow rate as 1 vvm. PHA production profile was observed by drawing samples at 24, 36, 48 and 72 h. The biomass yield (1.24 ± 0.02 g/L at 72 h) was observed to be less in this batch due to restricted air supply during the initial hours giving minimum PHA production of 25.87%. Figure 5b clearly depicts the decrease in biomass production with restricted air supply to organism during initial hours (DO 65%) which gave 0.41 ± 0.01 g/L of biomass at 24 h which was low when compared to other three batch run with 100% of initial DO concentration. This clearly indiate that aerobic organism B. endophyticus requires abundant oxygen supply for growth and establishment in the initial phase and later when famine condition pertains, in the excess supply of carbon source initiates PHA production. At 36 h, the DO % was at 25, still this stress element did not have a role in incresasing PHA production with reduced biomass. It is been proved that biomass and PHA production is directly proportional, so the necessity of maintaining increased DO level is important to obtain maximum PHA production even if stress condition pertains.
3.2.3 Batch cultivation with inducing oxygen limitation during stationary phase
In the third batch cultivation, the DO % was reduced to 50% at 36 h by reducing the agitation and airflow rate by 100 rpm and 1.5 vvm, respectively. Initial DO was maintained at 100% inorder to accelerate biomass production during the log phase and thus increase PHA production in presence of abundant cell density. As expected the biomass concentration was 1.2 ± 0.06 g/L at 24 h, and it went upto 2.7 ± 0.01 g/L at 72 h (Fig. 5c). The increament in the biomass during the stationary phase (48–68 h) with least oxygen supply can be explained with the availability of nitrogen source which helps in continuous growth of organism (Maheshwari et al., 2018). The production of PHA was 48.46% in this condition which was higher compared to control experiment without giving oxygen stress, which indicates that DO stress play a major role in increasing PHA accumulation. From the results, it is clearly observed that introduction of DO stress in the stationary phase had positive influence on PHA production. At 36 h where PHA production initiates, the nutrients available will be reduced due to continuous consumption by organsim and also the oxygen in the medium gets reduced which add on an additional stress element helping in increasing PHA production.
3.2.4 Inducing oxygen limitation by halting the agitation and airflow rate during stationary phase
In this batch cultivation, the agitation and airflow rate was halted during 36 h in order to observe the impact of DO stress on PHA accumulation. To bring maximum stress, compared to former batch cultivation which maintained (50% at 36 h) in this batch, we have reduced the airflow rate to 0.4% with zero agitation at stationary phase. Initial DO % was maintained as 100% which stipulated increament in cell density which was maximum till late log phase. At 36 h, the batch cultivation didnt show much increase in cell density whereas the overall PHA production was increased by giving maximum production by organism. The maximum PHA production (0.77 ± 0.01 g/L from 1.45 ± 0.02 g/L of biomass) was observed in fourth condition with least air supply and agitation Fig. 5d stipulated that increament in PHA production was supported by reduced oxygen levels in B.endophyticus. this was the first study to report that complete halting of agitation helped in increasing PHA by occupying 90% of the cell cytoplasm. This represented a opposite production profile indicating that organism tend to produce PHA with least oxygen supply. Maximum glucose consumption was also observed in same condition with least oxygen supply stipulating the available carbon source to be completely converted to its storage form, PHA within cell.
Comparison of the dissolved oxygen %, airflow rate and agitation condition and respective PHA production profile is represented in Table 1. Experimental results with oxygen stress showed positive profile by increasing PHA content from 34.5% (control experiment) to 53.03% (Fig. 5d) with varying oxygen concentration. Low aeration and agitation rate influenced PHA production in B.endophyticus significantly which inturn reduced the overall production cost. Similar reports were observed by Quillaguaman et al., in Halomonas bolieviensis (Quillaguamán et al., 2007). In our study, it was observed that stopping the air flow and reducing agitation rate didn’t influence organism to stop producing PHA, instead increased production by two fold. Low dissolved oxygen level increases. PHA production was reported by Quillaguaman in 2005 and Lefebre et al., in 1997 supported the same hypothesis by their study which resulted increased overall productivity of HA monomer when DO level was maintained at 1–4% (Lefebvre et al., 1997; Quillaguaman et al., 2005). Similarly in our study when the air saturation level was at 49% and 0.4%, maximum PHA production of 48.4% and 53.03% was observed, respectively, Fig. 5c and d. In second experiment, eventhough the DO % was at 24%, the PHA production was less (25. 87%), but in this trial the initial aeration was less compared all the three trial which resulted in less biomass inturn reducing PHA. So from the study, it is possible to conclude that B. endophyticus requires eminent air flow during the exponential phase for its growth and cell division, on inducing stress it produces PHA abunduntly during the later stages of fermentation.
During the lag and log phase, organism harboring PHA operon, usually gets accustomed to new environmental conditions and starts to divide exponentially. The energy used to be derived from carbohydrate pathway within the cell, which inturn blocks PHA metabolic pathway initially. The biosynthetic role of oxygen can be explained in terms of the fate of Acetyl CoA which initiates PHA biosynthesis or gets oxidized by entering Tricarboxylic Acid (TCA) cycle. During oxygen stress condition, the metabolic response of the organism is altered and NAD/NADH within the microbial system increases. The functional enzymes in the TCA cycle get inhibited and acetyl CoA acts as an alternate electron acceptor. Acetyl CoA doesn’t enter TCA cycle but initiates PHA biosynthetic cycle to store excess of carbon available (Kulpreecha et al., 2009; Sathya et al., 2018; Tripathi et al., 2013). According to our experimental observation, an imbalance in the nutrients or oxygen supplied, increases PHA production during batch fermentation profile. To asseverate the actual pathway functional during the biosynthetic yield of PHA, deeper molecular level study should be done. Activation of genes functional in PHA production was reported to be due to environmental stimuli like less concentration of nitrogen and oxygen (Ahmed et al.). In Bacillus myocoides, reduced dissolved oxygen had a negative influence on PHA accumulation, and this was reported by Borah et al., in 2002 (Borah et al., 2002). In this study, from the observed results it is concluded that B. endophyticus requires oxygen during the growth stage and for enhanced PHA production, nutrient and oxygen limitation plays a vital role. Similar study and results was reported in Halomonas campisalis demanded high oxygen supply during growth phase and least during PHA accumulation phase (Kshirsagar et al., 2013).
3.3 FTIR and 1 HNMR analysis of the obtained polymer
The chemical nature of the extracted PHA was confirmed by Fourier transform Infrared (FTIR) and Nuclear Magnetic resonance (NMR) spectroscopy. Characterization of PHA obtained from B. endophyticus is represented in Fig. 6b which showed characteristic peak at 1726 cm−1 corresponding to C=O stretch of the ester group. Observation of strong adsorption band at 1379, 1458, 2929, 1649 and 3749 cm−1 corresponds to –CH3, –CH2, CH, C–O and O–H groups, respectively, which was reported to be similar with pure PHB (Fig. 6a).
1H NMR was another analysis technique used to determine PHA produced by B. endophyticus using sucrose. The obtained spectra (Fig. 7b) was found to be similar to the standard PHB spectra (Fig. 7a) (Chaijamrus & Udpuay, 2008). The entire spectrum showed a doublet at 1.29 ppm which attributed to methyl group coupled with single proton. Doublet of quadruplet at 2.57 ppm corresponding to methylene group adjacent to an asymmetric carbon bearing single atom was observed. Multiplet at 5.27 ppm characteristic of methylene group was determined from the NMR spectra.
4 Conclusion
The process development strategy for Bacillus endophyticus, an aerobic PHA producer mainly rely on two basic principles like oxygen utilization behavior and its capability to cope up with the fermentation parameters economically to synthesize PHA. Biopolymer production in the optimized fermentative conditions could enhance the quality and quantity of PHA. Experimental studies was conducted to identify and optimize factors that significantly influence PHA production in bioreactor. Effects of fermentative factors like aeration, impact of dissolved oxygen content and sucrose as carbon sources was carried out in shake flask cultivation. Role of oxygen and its influence on the biosynthetic process is important in determining PHA biosynthesis. Optimal PHA production by aerobic B. endophyticus and the relationship between oxygen consumption at different production stages was studied by Wrinklers titration and DO probes. Experimental results validated the significant role of oxygen stress during the course of PHA production by B.endophyticus. The direct correlation between reduced DO (0.4 vvm) and increased PHA % 53.03 clearly justified the hypothesis put forward by this research work during bioreactor studies. It was worth to observe that organisms divided exponentially in the abundance of nutrient and oxygen where as the PHA production was found to be increased drasticaly in the lesser concentration or in absence of oxygen. The blessing in disguise profile of the PHA accumulation during stress condition helps to reduce overall expenses of production phase. Future studies can be focused on optimizing alternative media components and cheaper carbon source by limiting oxygen concentration to extract maximum PHA yield from test organsim.
Abbreviations
- DO:
-
Dissolved Oxygen
- PHA:
-
Polyhydroxyalkanoates
- PM:
-
Production medium
References
Ahmed, F. E., Hussein, L., Gouda, M. M., Vos, P. W., & Ahmed, N. C. (2018). Melt curve analysis for interpretation of MicroRNAs’ gene expression data in foods. Trends in Research, 1(1), 1–2.
Blunt, W., Dartiailh, C., Sparling, R., Gapes, D., Levin, D. B., & Cicek, N. (2018). Carbon flux to growth or polyhydroxyalkanoate synthesis under microaerophilic conditions is affected by fatty acid chain-length in Pseudomonas putida LS46. Applied Microbiology and Biotechnology, 102(15), 6437–6449.
Borah, B., Thakur, P., & Nigam, J. (2002). The influence of nutritional and environmental conditions on the accumulation of poly-β-hydroxybutyrate in Bacillus mycoides RLJ B-017. Journal of Applied Microbiology, 92(4), 776–783.
Carter, I. S., & Dawes, E. A. (1979). Effect of oxygen concentration and growth rate on glucose metabolism, poly-β-hydroxybutyrate biosynthesis and respiration of Azotobacter beijerinckii. Microbiology, 110(2), 393–400.
Chaijamrus, S., & Udpuay, N. (2008). Production and characterization of polyhydroxybutyrate from molasses and corn steep liquor produced by Bacillus megaterium ATCC 6748. Agricultural Engineering International: the CIGR EJournal, 10, 1–12.
Chen, G.-Q., & Jiang, X.-R. (2018). Engineering microorganisms for improving polyhydroxyalkanoate biosynthesis. Current Opinion in Biotechnology, 53, 20–25.
Coats, E. R., Watson, B. S., & Brinkman, C. K. (2016). Polyhydroxyalkanoate synthesis by mixed microbial consortia cultured on fermented dairy manure: Effect of aeration on process rates/yields and the associated microbial ecology. Water Research, 106, 26–40.
García, A., Segura, D., Espín, G., Galindo, E., Castillo, T., & Pena, C. (2014). High production of poly-β-hydroxybutyrate (PHB) by an Azotobacter vinelandii mutant altered in PHB regulation using a fed-batch fermentation process. Biochemical Engineering Journal, 82, 117–123.
Gouda, M. K., Swellam, A. E., & Omar, S. H. (2001). Production of PHB by a Bacillus megaterium strain using sugarcane molasses and corn steep liquor as sole carbon and nitrogen sources. Microbiological Research, 156(3), 201–207.
Hargreaves, J. A., & Tucker, C. S. (2002). Measuring dissolved oxygen concentration in aquaculture: Southern Regional Aquaculture Center. Publication no. 4601, 1–6.
Jacquel, N., Lo, C.-W., Wei, Y.-H., Wu, H.-S., & Wang, S. S. (2008). Isolation and purification of bacterial poly (3-hydroxyalkanoates). Biochemical Engineering Journal, 39(1), 15–27.
Kaur, G., & Roy, I. (2015). Strategies for large-scale production of polyhydroxyalkanoates. Chemical and Biochemical Engineering Quarterly, 29(2), 157–172.
Khatami, K., Perez-Zabaleta, M., Owusu-Agyeman, I., & Cetecioglu, Z. (2020). Waste to bioplastics: How close are we to sustainable polyhydroxyalkanoates production? Waste Management, 119, 374–388.
Koller, M., Maršálek, L., de Sousa Dias, M. M., & Braunegg, G. (2017). Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnology, 37, 24–38.
Kshirsagar, P. R., Suttar, R., Nilegaonkar, S. S., Pradhan, S., & Kanekar, P. P. (2013). Scale up production of polyhydroxyalkanoate (PHA) at different aeration, agitation and controlled dissolved oxygen levels in fermenter using Halomonas campisalis MCM B-1027. Journal of Biochemical Technology, 4(1), 512–517.
Kulpreecha, S., Boonruangthavorn, A., Meksiriporn, B., & Thongchul, N. (2009). Inexpensive fed-batch cultivation for high poly (3-hydroxybutyrate) production by a new isolate of Bacillus megaterium. Journal of Bioscience and Bioengineering, 107(3), 240–245.
Kundu, P., Nandy, A., Mukherjee, A., & Pramanik, N. (2014). Polyhydroxyalkanoates: Microbial synthesis and applications. In Encyclopedia of biomedical polymers and polymeric biomaterials polyhydroxyalkanoates: Microbial synthesis and applications (pp. 1–21).
Lakshman, K., & Shamala, T. R. (2006). Extraction of polyhydroxyalkanoate from Sinorhizobium meliloti cells using Microbispora sp. culture and its enzymes. Enzyme and Microbial Technology, 39(7), 1471–1475. https://doi.org/10.1016/j.enzmictec.2006.03.037
Lefebvre, G., Rocher, M., & Braunegg, G. (1997). Effects of low dissolved-oxygen concentrations on Poly-(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Production by Alcaligenes eutrophus. Applied and Environmental Microbiology, 63(3), 827–833.
Maheshwari, N., Kumar, M., Thakur, I. S., & Srivastava, S. (2018). Production, Process optimization and Molecular characterization of Polyhydroxyalkanoate (PHA) by CO2 sequestering B. cereus SS105. Bioresource Technology, 254, 75–82.
McDaniel, L., Bailey, E., & Zimmerli, A. (1965). Effect of Oxygen-Supply Rates on Growth of Escherichia coli I. Studies in Unbaffled and Baffled Shake Flasks. Applied Microbiology, 13(1), 109–114.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31(3), 426–428.
Mohammed, S., Behera, H. T., Dekebo, A., & Ray, L. (2019). Optimization of the culture conditions for production of Polyhydroxyalkanoate and its characterization from a new Bacillus cereus sp. BNPI-92 strain, isolated from plastic waste dumping yard. International Journal of Biological Macromolecules, 156, 1064–1080.
Mohanrasu, K., Rao, R. G. R., Dinesh, G., Zhang, K., Prakash, G. S., Song, D.-P., et al. (2020). Optimization of media components and culture conditions for polyhydroxyalkanoates production by Bacillus megaterium. Fuel, 271, 117522.
Moralejo-Gárate, H., Kleerebezem, R., Mosquera-Corral, A., & van Loosdrecht, M. C. (2013). Impact of oxygen limitation on glycerol-based biopolymer production by bacterial enrichments. Water Research, 47(3), 1209–1217.
Moralejo-Gárate, H., Maratusalihat, E., Kleerebezem, R., & van Loosdrecht, M. C. (2011). Microbial community engineering for biopolymer production from glycerol. Applied Microbiology and Biotechnology, 92(3), 631–639.
Narayanan, M., Kandasamy, G., Murali, P., Kandasamy, S., Ashokkumar, V., Nasif, O., et al. (2021). Optimization and production of polyhydroxybutyrate from sludge by Bacillus cereus categorized through FT-IR and NMR analyses. Journal of Environmental Chemical Engineering, 9(1), 104908.
Ojumu, T., Yu, J., & Solomon, B. (2004). Production of Polyhydroxyalkanoates, a bacterial biodegradable polymer. African Journal of Biotechnology, 3(1), 18.
Quillaguaman, J., Hashim, S., Bento, F., Mattiasson, B., & Hatti-Kaul, R. (2005). Poly (β-hydroxybutyrate) production by a moderate halophile, Halomonas boliviensis LC1 using starch hydrolysate as substrate. Journal of Applied Microbiology, 99(1), 151–157.
Quillaguamán, J., Muñoz, M., Mattiasson, B., & Hatti-Kaul, R. (2007). Optimizing conditions for poly (β-hydroxybutyrate) production by Halomonas boliviensis LC1 in batch culture with sucrose as carbon source. Applied Microbiology and Biotechnology, 74(5), 981.
Sathya, A., Sivasubramanian, V., Santhiagu, A., Sebastian, C., & Sivashankar, R. (2018). Production of Polyhydroxyalkanoates from Renewable Sources Using Bacteria. Journal of Polymers and the Environment, 26, 3995–4012.
Slepecky, R. A., & Law, J. H. (1960). A rapid spectrophotometric assay of alpha, beta-unsaturated acids and beta-hydroxy acids. Analytical Chemistry, 32(12), 1697–1699.
Third, K. A., Newland, M., & Cord-Ruwisch, R. (2003). The effect of dissolved oxygen on PHB accumulation in activated sludge cultures. Biotechnology and Bioengineering, 82(2), 238–250.
Tripathi, A. D., Srivastava, S. K., & Singh, R. P. (2013). Statistical optimization of physical process variables for bio-plastic (PHB) production by Alcaligenes sp. Biomass and Bioenergy, 55, 243–250.
Vikström, K., Tengberg, A., & Wikner, J. (2019). Improved accuracy of optode-based oxygen consumption measurements by removal of system drift and nonlinear derivation. Limnology and Oceanography: Methods, 17(3), 179–189.
Wang, X., Bengtsson, S., Oehmen, A., Carvalho, G., Werker, A., & Reis, M. A. (2019). Application of dissolved oxygen (DO) level control for polyhydroxyalkanoate (PHA) accumulation with concurrent nitrification in surplus municipal activated sludge. New Biotechnology, 50, 37–43.
Wang, X., Oehmen, A., Freitas, E. B., Carvalho, G., & Reis, M. A. (2017). The link of feast-phase dissolved oxygen (DO) with substrate competition and microbial selection in PHA production. Water Research, 112, 269–278.
Ward, A. C., Rowley, B. I., & Dawes, E. A. (1977). Effect of oxygen and nitrogen limitation on poly-β-hydroxybutyrate biosynthesis in ammonium-grown Azotobacter beijerinckii. Microbiology, 102(1), 61–68.
Zheng, Y., Chen, J.-C., Ma, Y.-M., & Chen, G.-Q. (2020). Engineering biosynthesis of polyhydroxyalkanoates (PHA) for diversity and cost reduction. Metabolic Engineering, 58, 82–93.
Acknowledgements
This work was ostensibly supported by The Department of Biotechnology (DBT), Government of India, (PR 18430/BIC/101/703/2016) and grateful to The Department of Biotechnology, Manipal Institute of Technology, Manipal Academy of Higher Education (MAHE), India; for providing the facilities to carry out the research work.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest involved in this publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Madhusoodanan, G., Hariharapura, R.C. & Somashekara, D. Dissolved oxygen as a propulsive parameter for polyhydroxyalkanoate production using Bacillus endophyticus cultures. Environ Dev Sustain 24, 4641–4658 (2022). https://doi.org/10.1007/s10668-021-01626-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10668-021-01626-3