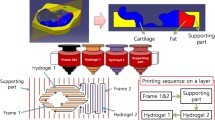
Abstract
Tissue engineering has played a very significant role in the medical field with an ever-growing demand for various tissue donations. One crucial factor is the fabrication of a desirable artificial three-dimensional (3D) tissue scaffold to act as the extracellular matrix (ECM), meeting the complex requirements for specific cell cultures. Existing scaffold fabrication techniques and systems used in constructing extracellular matrix are two-dimensionally limiting, expensive, and time-consuming. For instance, some simple fabrication methods cannot control fabricated structures with morphologies accurately, while others may introduce harmful organic solvents into scaffolds during the fabrication processes. To achieve an optimal scaffold for tissue engineering, we developed a novel 3D printing system capable of printing tissue scaffold structures with improved efficiency. The uniqueness of our system is the transparent diffractive optical elements (DOEs) of linear binary Fresnel lens fabricated to control the luminous intensity distribution. These DOEs of different patterns are arranged in series on a coverslip with each optical element designed to diffact and focus incident light at a particular plane within the device. Coupled with other optical components of the system, 3D woodpile scaffolds were printed in an effective and efficient one-step light exposure process to photo cross-link the polymer solution upon demand. The combination of photo cross-linking and diffractive optical technique incorporated within our system enables the patterning of polymer solutions within seconds, making large-scale fast production not only feasible, but also making printing of complex features simple. With this system, 3D two-layered woodpile structures were successfully fabricated within 3 seconds. While cell toxicity studies showed that the scaffold can be used for tissue engineering.








Similar content being viewed by others
References
Lanza, R., Langer, R., & Vacanti, J. P. (2011). Principles of tissue engineering. New York: Academic.
Langer, R., & Vacanti, J. (1993). Tissue engineering. Science, 260(5110), 920–926.
Boland, E. D., Espy, P. G., & Bowlin, G. L. (2004). Tissue engineering scaffolds. Encyclopedia of biomaterials and biomedical engineering (pp. 1630–8). Milton Park: Taylor and Francis.
Tsang, V. L., & Bhatia, S. N. (2004). Three-dimensional tissue fabrication. Advanced Drug Delivery Reviews, 56(11), 1635–1647.
Ho, C. M. B., Mishra, A., Lin, P. T. P., Ng, S. H., Yeong, W. Y., Kim, Y. J., et al. (2017). 3D printed polycaprolactone carbon nanotube composite scaffolds for cardiac tissue engineering. Macromolecular Bioscience, 17(4), 1600250.
Hutmacher, D. W. (2001). Scaffold design and fabrication technologies for engineering tissues—state of the art and future perspectives. Journal of Biomaterials Science, Polymer Edition, 12(1), 107–124.
Ikada, Y. (2006). Challenges in tissue engineering. Journal of the Royal Society Interface, 3(10), 589–601.
Hou, Q., Grijpma, D. W., & Feijen, J. (2003). Porous polymeric structures for tissue engineering prepared by a coagulation, compression moulding and salt leaching technique. Biomaterials, 24(11), 1937–1947.
Mishra, A., Ferhan, A. R., Ho, C. M. B., Lee, J., Kim, D.-H., Kim, Y.-J., et al. (2020). Fabrication of plasmon-active polymer-nanoparticle composites for biosensing applications. International Journal of Precision Engineering and Manufacturing-Green Technology., 210, 1–10.
Hollister, S. J. (2005). Porous scaffold design for tissue engineering. Nature Materials, 4(7), 518.
Kohane, D. S., & Langer, R. (2008). Polymeric biomaterials in tissue engineering. Pediatric Research, 63(5), 487.
Teo, A. J., Mishra, A., Park, I., Kim, Y.-J., Park, W.-T., Yoon, Y.-J. J. A. B. S., et al. (2016). Polymeric Biomaterials for Medical Implants and Devices, 2(4), 454–472.
Sun, Z.-B., Dong, X.-Z., Chen, W.-Q., Shoji, S., Duan, X.-M., & Kawata, S. (2007). Two-and three-dimensional micro/nanostructure patterning of CdS–polymer nanocomposites with a laser interference technique and in situ synthesis. Nanotechnology, 19(3), 035611.
Ho, C. M. B., Ng, S. H., & Yoon, Y.-J. (2015). A review on 3D printed bioimplants. International Journal of Precision Engineering and Manufacturing, 16(5), 1035–1046.
Ho, C. M. B., Ng, S. H., Li, K. H. H., & Yoon, Y.-J. (2015). 3D printed microfluidics for biological applications. Lab on a Chip, 15(18), 3627–3637.
Kwon, J., Park, H. W., Park, Y.-B., & Kim, N. (2017). Potentials of additive manufacturing with smart materials for chemical biomarkers in wearable applications. International Journal of Precision Engineering and Manufacturing-Green Technology, 4(3), 335–347.
Lee, J., Kim, H.-C., Choi, J.-W., & Lee, I. H. (2017). A review on 3D printed smart devices for 4D printing. International Journal of Precision Engineering and Manufacturing-Green Technology, 4(3), 373–383.
Shin, D.-G., Kim, T.-H., & Kim, D.-E. (2017). Review of 4D printing materials and their properties. International Journal of Precision Engineering and Manufacturing-Green Technology, 4(3), 349–357.
Jeon, S., Han, J., Jeong, W., Son, J., Kim, J. B., & Kang, H.-W. (2019). Flexibility Enhancement of poly(lactide-co-glycolide) for fused deposition modeling technology. International Journal of Precision Engineering and Manufacturing-Green Technology, 6(3), 465–475.
Sharma, A., Mondal, S., Mondal, A. K., Baksi, S., Patel, R. K., Chu, W.-S., et al. (2017). 3D printing: It’s microfluidic functions and environmental impacts. International Journal of Precision Engineering and Manufacturing-Green Technology, 4(3), 323–334.
Lutolf, M., & Hubbell, J. (2005). Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nature biotechnology, 23(1), 47.
Ma, T., Li, Y., Yang, S. T., & Kniss, D. A. (1999). Tissue engineering human placenta trophoblast cells in 3-D fibrous matrix: spatial effects on cell proliferation and function. Biotechnology Progress, 15(4), 715–724.
LaVan, D. A., George, P. M., Langer, R. Simple (2003). Three-Dimensional Microfabrication of Electrodeposited Structures. Angewandte Chemie International Edition, 42(11), 1262–1265.
Lee, M., Dunn, J. C., & Wu, B. M. (2005). Scaffold fabrication by indirect three-dimensional printing. Biomaterials, 26(20), 4281–4289.
Pattison, M. A., Wurster, S., Webster, T. J., & Haberstroh, K. M. (2005). Three-dimensional, nano-structured PLGA scaffolds for bladder tissue replacement applications. Biomaterials, 26(15), 2491–2500.
Wang, D., Williams, C. G., Li, Q., Sharma, B., & Elisseeff, J. H. (2003). Synthesis and characterization of a novel degradable phosphate-containing hydrogel. Biomaterials, 24(22), 3969–3980.
Jenness, N. J., Wu, Y., & Clark, R. L. (2012). Fabrication of three-dimensional electrospun microstructures using phase modulated femtosecond laser pulses. Materials Letters, 66(1), 360–363.
Doshi, J., & Reneker, D. H. (1995). Electrospinning process and applications of electrospun fibers. Journal of Electrostatics, 35(2–3), 151–160.
Yang, S., Leong, K.-F., Du, Z., & Chua, C.-K. (2001). The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Engineering, 7(6), 679–689.
Matsiko, A., Gleeson, J. P., & O’Brien, F. J. (2014). Scaffold mean pore size influences mesenchymal stem cell chondrogenic differentiation and matrix deposition. Tissue Engineering Part A, 21(3–4), 486–497.
Chen, A. A., Tsang, V. L., Albrecht, D. R., & Bhatia, S. N. (2006). 3-D fabrication technology for tissue engineering, BioMEMS (pp. 23–38). New York: Springer.
Wang, X., Yan, Y., & Zhang, R. (2007). Rapid prototyping as a tool for manufacturing bioartificial livers. Trends in Biotechnology, 25(11), 505–513.
van Wolferen, H., & Abelmann, L. (2011). Laser interference lithography. Lithography: Principles, Processes and Materials. 133–48.
Lasagni, A. F., Yuan, D., Shao, P., & Das, S. (2009). Periodic micropatterning of polyethylene glycol diacrylate hydrogel by laser interference lithography using nano-and femtosecond pulsed lasers. Advanced Engineering Materials, 11(3), B20–B24.
Kang, J. H., Moon, J. H., Lee, S. K., Park, S. G., Jang, S. G., Yang, S., et al. (2008). Thermoresponsive hydrogel photonic crystals by three-dimensional holographic lithography. Advanced Materials, 20(16), 3061–3065.
Yuan, L. L., & Herman, P. R. (2016). Laser scanning holographic lithography for flexible 3D fabrication of multi-scale integrated nano-structures and optical biosensors. Scientific Reports, 6, 22294.
Shusteff, M., Panas, R. M., Henriksson, J., Kelly, B. E., Browar, A. E., Fang, N. X., et al. (eds) (2016). Additive fabrication of 3d structures by holographic lithography. In: 27th Annual Solid Freeform Fabrication Symposium (SFF), Austin, TX, Aug; 2016.
Ho, C. M. B., Mishra, A., Hu, K., An, J., Kim, Y.-J., & Yoon, Y.-J. (2017). Femtosecond-laser-based 3D printing for tissue engineering and cell biology applications. ACS Biomaterials Science & Engineering, 3(10), 2198–2214.
Campbell, M., Sharp, D., Harrison, M., Denning, R., & Turberfield, A. (2000). Fabrication of photonic crystals for the visible spectrum by holographic lithography. Nature, 404(6773), 53.
Fischer, J., & Wegener, M. (2013). Three-dimensional optical laser lithography beyond the diffraction limit. Laser & Photonics Reviews, 7(1), 22–44.
Lin, Y., Rivera, D., & Chen, K. (2006). Woodpile-type photonic crystals with orthorhombic or tetragonal symmetry formed through phase mask techniques. Optics Express, 14(2), 887–892.
Vyas, S., Singh, R. K., Ghai, D. P., & Senthilkumaran, P. (2012). Fresnel lens with embedded vortices. International Journal of Optics
Xia, Y., & Whitesides, G. M. (1998). Soft Lithography. Angewandte Chemie International Edition, 37(5), 550–575.
Love, J. C., Wolfe, D. B., Jacobs, H. O., & Whitesides, G. M. (2001). Microscope projection photolithography for rapid prototyping of masters with micron-scale features for use in soft lithography. Langmuir, 17(19), 6005–6012.
Han, L.-H., Suri, S., Schmidt, C. E., & Chen, S. (2010). Fabrication of three-dimensional scaffolds for heterogeneous tissue engineering. Biomedical Microdevices, 12(4), 721–725.
Taylor, H., Boning, D., & Iliescu, C. (2011). A razor-blade test of the demolding energy in a thermoplastic embossing process. Journal of Micromechanics and Microengineering, 21(6), 067002.
Wu, J., Zhao, Z., Hamel, C. M., Mu, X., Kuang, X., Guo, Z., et al. (2018). Evolution of material properties during free radical photopolymerization. Journal of the Mechanics and Physics of Solids, 112, 25–49.
Schildknecht, C. E. (1977). Polymerization processes. Amsterdam: Wiley.
Cavin, L., Rouge, A., Meyer, T., & Renken, A. (2000). Kinetic modeling of free radical polymerization of styrene initiated by the bifunctional initiator 2, 5-dimethyl-2, 5-bis (2-ethyl hexanoyl peroxy) hexane. Polymer, 41(11), 3925–3935.
Kusuma, V. A., Roth, E. A., Clafshenkel, W. P., Klara, S. S., Zhou, X., Venna, S. R., et al. (2015). Crosslinked poly (ethylene oxide) containing siloxanes fabricated through thiol-ene photochemistry. Journal of Polymer Science Part A: Polymer Chemistry, 53(13), 1548–1557.
Lee, S., Tong, X., & Yang, F. (2014). The effects of varying poly(ethylene glycol) hydrogel crosslinking density and the crosslinking mechanism on protein accumulation in three-dimensional hydrogels. Acta Biomaterialia, 10(10), 4167–4174.
Drira, Z., & Yadavalli, V. K. (2013). Nanomechanical measurements of polyethylene glycol hydrogels using atomic force microscopy. Journal of the Mechanical Behavior of Biomedical Materials, 18, 20–28.
Biondi, M., Ungaro, F., Quaglia, F., & Netti, P. A. (2008). Controlled drug delivery in tissue engineering. Advanced Drug Delivery Reviews, 60(2), 229–242.
Benedek, I. (2004). Pressure-sensitive adhesives and applications. Boca Raton: CRC Press.
Dana, S. F., Nguyen, D.-V., Kochhar, J. S., Liu, X.-Y., & Kang, L. (2013). UV-curable pressure sensitive adhesive films: effects of biocompatible plasticizers on mechanical and adhesion properties. Soft Matter, 9(27), 6270–6281.
Tsige, M., & Stevens, M. J. (2004). Effect of cross-linker functionality on the adhesion of highly cross-linked polymer networks: A molecular dynamics study of epoxies. Macromolecules, 37(2), 630–637.
Williams, C. G., Malik, A. N., Kim, T. K., Manson, P. N., & Elisseeff, J. H. (2005). Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials, 26(11), 1211–1218.
Pahoff, S., Meinert, C., Bas, O., Nguyen, L., Klein, T. J., & Hutmacher, D. W. (2019). Effect of gelatin source and photoinitiator type on chondrocyte redifferentiation in gelatin methacryloyl-based tissue-engineered cartilage constructs. Journal of Materials Chemistry B, 7(10), 1761–1772.
Lin, C.-H., Lin, K.-F., Mar, K., Lee, S.-Y., & Lin, Y.-M. (2016). Antioxidant N-Acetylcysteine and glutathione increase the viability and proliferation of MG63 cells encapsulated in the gelatin methacrylate/VA-086/blue light hydrogel system. Tissue Engineering Part C: Methods, 22(8), 792–800.
Acknowledgements
Y.J.Y acknowledges the financial support supported from the Korea Institute for Advanced of Technology and funded by the Korea Ministry of Trade, Industry and Energy (P0011339, 2019 Establishment of a Customized Platform Utilizing Semiconductor Infrastructure). This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1A2C1011859). M.S.Y acknowledges the financial support by the Institute of Information & Communication Technology Planning & Evaluation (IITP) grant funded by the Korea government (MIST) (No. 2020-0-00954, Development of high efficiency high transparency HOE screen).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ho, C.M.B., Hu, K., Mishra, A. et al. Printing of Woodpile Scaffold Using Fresnel Lens for Tissue Engineering. Int. J. of Precis. Eng. and Manuf.-Green Tech. 9, 507–522 (2022). https://doi.org/10.1007/s40684-021-00322-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40684-021-00322-x