Summary
Purpose
The long-term effects of targeted micronutrition with the holoBLG lozenge in house dust mite (HDM) allergic rhinoconjunctivitis (ARC) patients were evaluated at a follow-up visit in an allergen exposure chamber (AEC).
Methods
Patients who were supplemented for 3‑months with the holoBLG lozenge in a previous study with two controlled HDM-AEC challenges [visits: V1, V3] were recruited for a third AEC challenge (V5) 7–8 months after cessation of supplementation. Symptoms (nose, conjunctival, bronchial, others), well-being, and lung function parameters were recorded exactly as in the previous study. Primary endpoint was change in median Total Nasal Symptom Score (TNSS) at V5 compared to V1. Secondary endpoints included e.g. change in median Total Symptom Score (TSS) and the exploratory analysis of temporal evolution of symptom scores using linear mixed effects models.
Results
Of the 32 patients included in the original study, 27 could be recruited for the follow-up visit with a third AEC challenge. An improvement of 20% (p = 0.15) in the primary endpoint TNSS [V1: 2.5 (interquartile range [IQR]: 1–4), V5: 2.0 (IQR: 1–3)] was observed; 40% (p = 0.04) improvement was seen for the TSS [V1: 5.0 (IQR: 3–9), V5: 3.0 (IQR: 2–5.5)]. Analysis of temporal evolution of all symptom scores, and the personal well-being revealed sustained, clinically meaningful improvement at V5 compared to V1. No relevant lung function parameter differences were observed.
Conclusions
Sustained long-term reduction of TNSS (primary endpoint) and sustained long-term improvement of secondary endpoints (temporal evolution of all symptom scores and well-being) were demonstrated 7–8 months after cessation of holoBLG supplementation, indicative of a long-lasting nature of immune resilience induced by holoBLG.
Trial registration
The study was registered at clinicaltrials.gov (NCT04872868).
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Background
The concept of targeted micronutrition to correct micronutritional deficiencies in immune cells of allergic patients, the main attribute of mode-of-action of loaded beta-lactoglobulin (holoBLG), has been unravelled only recently [3,4,5,6]. There are multiple studies showing that atopy is associated with iron deficiencies [7,8,9,10,11] and/or vitamin deficiencies [12,13,14,15]. These deficiencies turn the immune system into a hyperreactive state, contributing to the atopic state [8, 15]. The holoBLG lozenge has been shown to correct these micronutritional deficiencies by shuttling micronutrients such as iron, retinoic acid and zinc to immune cells of the myeloid lineage [3,4,5,6, 16].
To date, the only causative treatment for allergic diseases is allergen-specific immunotherapy (AIT) with disease-modifying potential when administered compliantly and for at least 3 years. The concept of classical AIT is desensitization, administering the allergy-eliciting allergen in a repeated fashion in order to induce tolerance and mount a long-lasting effect based on cellular and humoral immunity [17, 18]. However, one of the major principal limitations of AIT is allergen-specificity. Consequently, one AIT formulation is needed for each allergy or combinations thereof. Furthermore, there are patients who are simply not suitable or motivated to receive an AIT course of 3 years. A unique opportunity for allergic patients presents itself, based on correcting micronutritional deficiencies which provide an antigen-unspecific immune-regulatory effect resulting in resilience to immune activation—“immune resilience”—and protection against allergic sensitization and symptoms [2, 3, 16].
In contrast to AIT, the described approach herein is tailored to tackle atopy by targeted micronutrition using the holoBLG lozenge. Here, induction of immune resilience via innate immunity in an allergen-unspecific way provides symptom alleviation after a short supplementation phase [2, 16]. The holoBLG lozenge, a safe and well-tolerated FSMP (food for special medical purpose) [2, 19], which is a novel invention [1] in the field of dietary products (sold over the counter [OTC] in pharmacies) currently available in Germany and Austria [20], is breaking new grounds in the fight against allergic diseases.
Two proof-of-concept studies with allergic patients have been completed so far. A double-blind placebo controlled (DBPC) study (NCT03816800) in 51 birch and/or grass pollen allergic women combined nasal provocation before and after supplementation with a field study assessing combined symptom medication scores (CSMS) during the respective pollen seasons via an eHealth application [16]. This DBPC study showed a significant reduction in CSMS of 41% in the holoBLG-supplemented group for the birch pollen seasons of 2019/2020 and of 26% for the grass pollen seasons 2019/2020. Congruently, the total nasal symptom score measured via a controlled nasal provocation test (NPT) was reduced by 42% in the holoBLG group compared to 13% in the placebo group [16]. In addition, an improved iron status in the allergic patients after the supplementation was reported.
The second proof-of-concept study investigated the effects of the holoBLG lozenge on symptoms of house dust mite (HDM) allergic patients by a controlled and standardised provocation in a validated allergen exposure chamber (AEC) [2, 21]. Thirty-two HDM-allergic patients were included in this study (NCT04477382), which was conducted at the AEC of the ECARF Institute in Berlin (Germany). The patients recorded their nasal, conjunctival, bronchial and other symptoms, measured peak nasal inspiratory flow (PNIF), peak expiratory flow (PEF), and documented their well-being during the exposure; lung function was recorded before and after the challenge in the AEC. After 3 months of daily supplementation with the holoBLG lozenge (2 lozenges/day), patients were again challenged in the AEC. The median TNSS at 120 min of exposure (primary outcome) was significantly reduced by 60% (p = 0.0034), the median TSS (sum of all symptoms), a secondary outcome, was reduced by 40% (p < 0.0003), and these results were mirrored by the improvement of 42% in the well-being of the patients recorded via visual analogue scale (VAS). The number of patients reporting late phase reactions (LPRs) due to the allergen challenge in the chamber was markedly reduced from 45% of the patients at baseline to only 12% of patients after the final exposure, also pointing to an increased tolerability to HDM exposure after supplementation with the holoBLG lozenge [2].
Both trials demonstrated symptom improvement in allergic patients in an allergen-unspecific way in a magnitude which is comparable to allergen immunotherapy [2, 16] with the caveat of being proof-of-concept studies. Both trials however focused on immediate effects observed at end of supplementation and did not investigate potential long-term benefits.
Immune memory was considered in the past to only exist in adaptive immunity. However, “immune resilience” [3] is challenging this dogma like trained immunity [22], as it describes a way of memory formation for the innate branch of the immune system being not based on antigen-specificity in comparison to antigen-specific memory formation in adaptive immunity. Previous studies (preclinical and clinical) demonstrated that the holoBLG mechanism of action is not antigen-specific and holoBLG is influencing the reactivity of innate immune cells [2,3,4, 6, 16], both pointing towards a regulation via innate mechanisms as first line. Knowing about this kind of trained immunity, we were interested whether supplementation with holoBLG is able to induce long-term effects. Therefore, we decided to recruit the HDM allergic patients from our previous study in the allergen exposure chamber [2] for a third controlled allergen challenge in the AEC 7–8 months after the end of supplementation with holoBLG.
Methods
Study design and materials
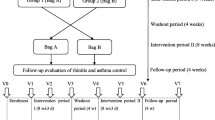
In January and February 2021, those 32 HDM allergic patients with HDM ARC (allergic rhinoconjunctivitis) who participated in the intervention phase (NCT04477382) [2], and underwent two controlled HDM-exposures (V1 and V3) as well as a 3-month course with the holoBLG lozenge, were asked to participate in a follow-up visit. They were once again challenged with the same amount of HDM allergen (V5) in the AEC of ECARF Institute, Berlin, Germany (Fig. 1).
Study design. This study is an extension from the previous study [2]. The scheme shows the study periods (intervention phase, follow-up phase) including the visits (V0–V6). V0 Screening and patient selection; V1, V3 and V5 HDM exposure in AEC; V2, V4 and V6 telephone calls 24 h after exposures
Patient population
The eligible population were HDM allergic patients who already participated in the intervention phase [2] (V1–V4 including 3 months of holoBLG supplementation) and were included in the final analysis. Exclusion criteria remained as in the previous study [2]. Wash-out time for different medications before the AEC challenge (V5) to prevent any symptom-reducing influence of these on the challenge were as follows: 3 weeks for systemic corticosteroids, 2 weeks for topical nasal corticosteroids, 7 days for cromones, 72 h for antihistamines, 3 months for antibiotics, 1 month for pro-, pre- and synbiotics.
In addition, we collected information whether patients started an AIT against HDM after V4 or prolonged or started supplementation with the holoBLG lozenge between V4 and V5 again, as it is commercially available.
AEC
The exposure was carried out under exactly the same protocol as in the previous study in the same allergen exposure chamber [2]. In brief, exposure was done in the mobile, standardised and validated GA2LEN AEC (ECARF) [21, 23,24,25]. After an acclimatisation period (20 min), the exposure was performed with 250 μg/m3 HDM raw material (whole culture mite: Dermatophagoides pteronyssinus and Dermatophagoides farinae body and faeces allergen 50:50; Allergon AB, Ängelholm, Sweden) for 120 min at 20 °C and 55% relative air moisture. Amount and composition of HDM allergens, and exposure particle size was designed to reflect a high natural daily HDM exposure of a HDM-allergic patient [26].
Outcome parameter
In order to achieve a high degree of comparability of the results, outcome parameters were assessed exactly as in the previous study [2]. Symptoms were evaluated by the patient on a scale from 0–3 (no-, mild-, moderate-, or severe symptoms). TNSS (Total Nasal Symptom Score) symptoms: runny, sneezing, itchy, and blocked nose, TESS (Total Eye Symptom Score) symptoms: itchy, watery eyes, and gritty feeling, TBSS (Total Bronchial Symptom Score) symptoms: breathlessness, wheezing, cough, and asthma, and TOSS (Total Other Symptom Score): itchy skin, and itchy palate. TSS reaching a maximum score of 39 is the sum of TNSS, TESS, TBSS, and TOSS. Primary endpoint was the change in median TNSS at 120 min at V5 compared to V1. Secondary endpoints were the change in median TSS at 120 min at V5 compared to V1, an exploratory analysis of the temporal evolution of TNSS, TESS, TBSS, TOSS and TSS during exposure and the differences between these temporal trends between V1, V3 and V5. Changes in personal well-being (VAS: 0 = very good to 10 = very bad, measured in millimeter 0–100), peak nasal inspiratory flow (PNIF, Peak Nasal Inspiratory Flow Meter, Clement Clarke International Ltd., Harlow, Essex, UK) and peak expiratory flow (PEF, Peak-Flow-Meter, Personal Best, Philips GmbH, Herrsching, Germany) were recorded before and every 30 min during the exposure. Spirometry (forced expiratory volume in 1 second (FEV1), FEV1/FVC (forced vital capacity); EasyOne™ Spirometer, ndd Medizintechnik AG, Zurich, Switzerland) was performed before and after exposure, analysed and judged from a clinician’s point of view. Late phase reactions (LPRs) or adverse events related to the exposure were recorded in a telephone call 24 h after V5.
Study oversight
The Ethics Committee of the Charité, Berlin approved the study protocol (EA1/412/20). Bencard Allergie GmbH sponsored the study and it was registered at clinicaltrials.gov (NCT04872868). Participants received detailed information and gave written informed consent to participate in the follow-up phase of the new study as well as to processing and storage of their data according to the General Data Protection Regulation. Same pseudonymisation and patient-identification as in the study before [2] was employed in accordance with applicable laws and regulations, and the study was conducted in accordance with the Declaration of Helsinki, as well as in compliance with all federal, local, and regional requirements.
Statistical analysis
The study consisting of the follow-up phase in the AEC was planned with 32 patients. The data used for all analyses is based on the 32 patients evaluated for V1 and V3 [2] and the 27 patients who participated in V5. Analysis was carried out as before [2], primary endpoint was analysed employing the paired Wilcoxon test (Wilcoxon signed rank test with continuity correction) and several secondary endpoints were analysed in an exploratory way. Percent changes between AEC visits were calculated by first calculating the median of values measured during V1 and V5 separately (over all patients at 120 min). To retrieve percentage changes, the following equation was employed: [(median V5 − median V1) / median V1] × 100. Median, interquartile ranges (IQR) and percentage changes are given.
The linear evolution over time of the symptom scores at V1, V3 and V5 was analysed by using linear mixed effects models [2, 27]. Model response, also called the dependent variable, were the different symptom scores. Patients were used as random effects accounting for interindividual variability in baseline symptom scores and treatment, time and interaction between treatment and time as fixed effects. Quantile–quantile plots were employed to visually confirm model assumptions. All analyses were performed with R version 3.5.3 [28] using package “lme4” for mixed effects modelling [29] and package “multcomp” for estimating p-values of fixed effects [2, 27, 30]. The 95% confidence intervals (CIs) for fixed effects in linear mixed effects models and p-values represent a descriptive summary measure, not a result of confirmatory testing. Results of the mixed effects models are presented (Fig. 3) as expected marginal means which are predictions for the response where certain variables are held constant, in our case the intervention (V1, V3, V5), and others are varied, in our case the time. PNIF and PEF were described using median and IQRs and judged from the point of clinical relevance (data not shown).
Results
Baseline demographics
Twenty-seven out of the pool of 32 participating patients from the intervention phase [2] could be recruited to sign up for a third exposure; the pandemic prevented the recruitment of the other five patients. Mean age was 40.7 years (SD 11.9 years) and 22% were male (details shown in Table 1). Before start of the intervention phase [2], all included patients rated at least two allergic rhinoconjunctivitis (ARC) symptoms (runny nose, blocked nose, itchy nose, sneezing, itchy eyes) as moderate or severe. It should be emphasised that none of the participating patients took any further holoBLG lozenges during the 7–8 months between V4 and V5.
Efficacy
Patients recorded their symptoms every 10 min for 120 min during the exposure at V5, as previously performed at V3 and V1 (intervention phase) [2].
Primary endpoint
Primary endpoint was the difference between V5 and V1 for TNSS at 120 min exposure (Fig. 2a). At V1 after 120 min the median TNSS was 2.5 (IQR: 1–4) compared to 2.0 (IQR: 1–3) [2] at V5, describing a difference of −20% (p = 0.1516) 7–8 months after the end of supplementation.
TNSS (primary endpoint) and TSS at baseline (V1), after supplementation (V3), and 7–8 months after cessation of the supplementation (V5). Shown are box plots describing median and interquartile ranges with an overlaid dot plot representing the single patients. Median TNSS at 120 min a was significantly reduced (p = 0.0034) by 60% at V3 compared to V1, at V5 the score was reduced (p = 0.1516) by 20% compared to V1 [median TNSS, V1: 2.5 (IQR: 1–4), V3: 1.0 (IQR: 1–3), V5: 2.0 (IQR: 1.0–3.0)]. Median TSS at 120 min b, a secondary outcome measure, was 5.0 (IQR: 3–9) at V1, 3.0 (IQR: 2–4) at V3, and 3.0 (IQR: 2.0–5.5) at V5, describing a relevant improvement of 40% between V3 and V1 (Wilcoxon test: CI: 1.5–4.0, p < 0.0003), which was also true for V5 compared to V1 (Wilcoxon test: CI: 0.00001–4.5, p = 0.04). CI confidence interval, IQR interquartile range, V visit, TNSS Total Nasal Symptom Score, TSS Total Symptom Score
Secondary endpoints
A secondary outcome measure was the analysis of the sum of all symptoms for all organs, shown as median TSS at 120 min (Fig. 2b), which was 5.0 (IQR: 3–9) at V1 [2] and 3.0 (IQR: 2.0–5.5) at V5 (Wilcoxon test: CI: 0.00001–4.5, p = 0.04), describing a relevant improvement of −40%. Median and IQR of all symptom scores at 120 min are listed in Table 2. In the previous study [2], the temporal evolution (during 120 min exposure) for all four single symptom scores and TSS at V3 compared to V1 were analysed in an exploratory way. Now, the data from V5 is included in the linear mixed effects models to compare the three visits (V1, V3 and V5). This model adjusted for interindividual variability by estimating a random intercept for each patient, identified relevant fixed effects for exposure time in the AEC and relevant interaction effects between the time spent in the AEC and the supplementation with the holoBLG lozenge, respectively 7–8 months after supplementation-end, for all symptom scores. At V5, there was a relevant symptom improvement detected as compared to V1, manifesting as a lower rate of increase in symptoms over time compared to V1 (Fig. 3). The slope decrease per minute for all scores between V5 and V1 was not as high as between V3 and V1, but the slope of all symptom scores at V5 was decreased to a relevant extent. Fig. 3b summarises the results of the linear mixed effects models, identified by the interaction term, for all scores, p-values reflecting descriptive summary measures. The evolvement of the TSS over time is explained as follows: During 120 min of exposure in the AEC, the TSS increased at a rate of 0.043 per minute (95% CI: 0.037–0.049, p < 2 × 10−16) on average during V1 [2]. At V3, directly after 3 months supplementation with holoBLG, the slope was decreased by 0.028 per minute (95% CI: 0.019–0.016, p = 4.86 × 10−10), leaving an increase of 0.015 per minute during V3 [2]. 7–8 months after the end of supplementation, the slope was still decreased by 0.021 per minute (95% CI: 0.001–0.003, p = 4.73 × 10−6) at V5 compared to V1, leaving an increase of 0.022 per minute during V5.
Linear evolution of symptom scores for nose (TNSS), eye (TESS), bronchial (TBSS), other (TOSS) and the sum of all symptoms (TSS) over time of HDM exposure in the AEC at V1, V3 and V5. a Analysis of the temporal evolution for all single symptom scores (recorded every 10 min during 120 min exposure), and the resulting TSS showed improved symptoms over time for V5 compared to V1 as also seen for V3 compared to V1 (smaller slope increase during V3 and V5 describes decreased symptoms during these exposures compared to baseline V1). Shown are predicted marginal means including their 95% confidence intervals. b Summary table of linear mixed effects model results, which analysed the symptom scores for their linear evolution over time. Slope per minute (including 95% CI) for V1, V3 and V5 as well as corresponding slope decreases between V1 and V3, and V1 and V5 per minute (including 95% CI), and the related p-values as descriptive summary measures are given. V3 slope per minute is the difference between V1 slope/min and slope decrease between V1 and V3/min. V5 slope per minute is the difference between V1 slope/min and slope decrease between V1 and V5/min. CI confidence interval, HDM house dust mite, V visit, TNSS Total Nasal Symptom Score, TESS Total Eye Symptom Score, TBSS Total Bronchial Symptom Score, TOSS Total Other Sympotom Score, TSS Total Symptom Score
We did not observe relevant differences for PNIF and PEF (not shown) between V1 [2] and V5, as also seen between V1 and V3 [2] before. No restrictions or obstructions were measured during spirometry before and after all exposures (V1, V3 and V5).
At 120 min, median VAS was reduced from 32 at V1 (IQR: 17.75–52) [2] to 14 at V5 (IQR: 6.5–34) (Wilcoxon test: CI: 8.00–24.00, p = 0.001), which represents a clinically meaningful increase of the patient’s personal well-being by 56% (Fig. 4).
Personal well-being (represented by VAS) during 120 min of HDM exposure in the AEC at V1, V3 and V5. At the end of the exposures after 120 min, median VAS was reduced from 32 at V1 (IQR: 17.75–52) to 19 at V3 (IQR: 12.25–35) (Wilcoxon test: CI: 4.50–20.50, p = 0.0021), and 14 (IQR: 6.5–34) at V5 (Wilcoxon test: CI: 8.00–24.00, p = 0.001). This represents a clinically relevant increase of the patient’s personal well-being by 42% between V3 and V1, and 56% between V1 and V5. Higher values represent lower well-being. CI confidence interval, IQR interquartile range, V visit, VAS visual analogue scale
During the telephone call V2 (24 h after baseline exposure with HDM), 14 out of 31 patients (45%) reported LPRs due to the mite allergen exposure: itchiness and tearing eyes, irritated or sore throat, thirst, swallowing problems, dyspnoea, obstructed nose, headache, itchy skin and mild urticaria, cough and sneezing [2]. At V6 (telephone call 24 h after V5), 9 out of 27 patients (33%) reported LPRs: itchiness, sneezing, and obstructed nose (Fig. 5).
Discussion
Allergies to airborne allergen sources such as pollen, HDM, moulds or animal epithelia are on the rise, as seen in food allergies [31]. AIT is still the only causative treatment to date, and this disease-modifying treatment option has been shown to be efficacious with favourable safety profiles in many clinical trials [32]. It is recommended by societies such as the European Academy for Allergy and Clinical Immunology for the treatment of for example seasonal and perennial allergic rhinitis and well-controlled allergic asthma [33, 34], but this unfortunately does not cover all patients with allergic diseases. Many patients do suffer from multiple allergies simultaneously and even when starting an AIT against one or two allergen sources, they often do not achieve satisfaction regarding full symptom control. Rare allergens as elicitors of allergic symptoms are another challenge as suitable AIT preparations are not available in every case. Another important group are patients not suitable to receive AIT because of contraindications and patients rejecting AIT because of various reasons such as lack of willingness to commit to an AIT course of 3 years with either daily or monthly therapeutic doses. In general, although AIT is the only disease-modifying option to date, clear disadvantages for certain patient populations are given such as allergen-specificity, compliance and adherence to treatment schedules and a residual risk of anaphylactic reactions. In addition to these important reasons, there are many patients who never reach an allergy specialist because they might not even go to a general practitioner (GP) for an initial diagnostic workup, or are not referred to an allergy centre.
A novel approach to ameliorate allergy symptoms was introduced harnessing the potential of targeted micronutrition. Immune resilience, as a phenomenon of activation of innate immune mechanisms, may be key here and help explain the allergen unspecific nature of this approach [3, 4, 6, 35]. This novel scientific concept has been translated into a Food for Special Medical Purpose (FSMP)—available throughout German and Austrian pharmacies (“over the counter”) addressing those allergy sufferers who never reach an allergy centre, or are not suited/unwilling to undergo AIT [20]. The allergen-unspecific mode-of-action of this FSMP is documented by a large body of preclinical data and first clinical trials [2,3,4, 6, 16]. HoloBLG mimics [2, 6, 16] the well-known protective “farm effect”, documenting that early-life and long-term exposure to stables and raw milk consumption do have a strong protective effect on the development of hay fever, asthma and atopic sensitisation [36, 37], and this concept [38, 39] was materialized in a lozenge. In a previous study, a 3-month course with the holoBLG lozenge demonstrated clinically meaningful benefits for HDM-allergic patients in a highly standardised HDM-challenge setting elicited in a state-of-the-art validated AEC [2]. The trial described here is a continuation of this previous AEC study with the aim to generate first data on long-term effectiveness of holoBLG supplementation. A beneficial sustained effect seen 7–8 months after cessation of supplementation was unravelled. The observed effects (V5) were slightly reduced compared to immediately after end of supplementation (V3) but remained remarkably consistent over the vast majority of endpoints and organs studied: in a holistic overview of both trials the previous study demonstrated directly after cessation of the 3 months supplementation (V3) significant reduction of 60% (p = 0.0034) in TNSS and a 40% reduction in TSS (p < 0.0003) compared to baseline (V1) [2]; 7–8 months afterwards there was still a reduction of 20% (p = 0.1516) in TNSS. In addition the study documented an ongoing clinically meaningful reduction of 40% (p = 0.004) in TSS, comparable to at the end of supplementation (V3). Although the primary endpoint TNSS was not significantly reduced at V5 compared to V1, the patients still benefited noticeable and consistently over all organ systems affected and additional endpoints measured. Occurrence of late-phase-allergic reactions after provocation are another powerful tool to study the allergic reaction. Consistently to the observations above, the study confirmed a reduced occurrence of LPRs 24 h after AEC exposure from 45% prior supplementation (V1) to 12% straight after 3 month supplementation (V3), which still remained on a reduced level 7–8 months later (33% at V5).
Standardized and validated allergen exposures in state-of-the-art AECs are an impressive tool to study allergic diseases [21, 23]. As a limitation of the first study we discussed in the previous paper [2] the missing control group, habituation effects of undergoing a chamber exposure for the third time, and the relatively low number of patients. Still, the highly standardized exposure of the same patients in the same chamber with identical conditions enables a high-quality comparison of before-and-after effects, which could also be seen as a control. Moreover, in this follow-up trial, not all of those 32 initial patients could be motivated to sign up for a third chamber session. However, almost 85% of those patients could be recruited, which is a meaningful achievement given the unpleasant and time-consuming nature of a chamber exposure.
The body of scientific evidence is growing for supplementation with the holoBLG lozenge. Data of a randomized DBPC clinical proof-of-concept trial combining allergen provocation with efficacy measures such as combined symptom medication scores in a hybrid design were recently presented [16]. The outcome of the study described here adds to the understanding of the advantages of holoBLG supplementation. Highlighting for the first time long-term immune resilience induced by targeted micronutrition. The exact underlying mechanisms remain open for speculation for the time being. “Trained immunity” appears to be an attractive hypothesis, since the mechanism of the lozenge is antigen-nonspecific and mainly leverages on the innate immune system. For instance, preclinical studies showed that specifically myeloid immune cells are supplemented with the nutrients by holoBLG [3, 4, 6, 16]; therefore the early steps of mechanism of action seems to be based on sensitization and effector cells of the allergic immune cascade. Trained immunity, alias trained innate immunity or innate immune memory, is a concept which was introduced in 2011 [22], postulating that also the innate branch of the immune system can build long-term memory, not only the adaptive immune system as thought before. Since then, there have been several publications showing the mid-term metabolic and epigenetic reprogramming of innate immune cells after the initial challenges, followed by altered responses on secondary challenge of these cells [40]. To name just one example, these altered innate responses could lead to a change in cytokine production and with this to a different microenvironment than upon the first challenge. This could then influence the cells of the adaptive immune system like B and T cells in a different way than before when they interact with each other [40]. We would like to hypothesize that the observed long-term efficacy 7–8 months after cessation of 3 months of holoBLG supplementation is linked to trained immunity. Micronutrients such as iron are shuttled into myeloid cells, nourishing them with the aim to balance their homeostasis, which in turn dampens their effector responses like antigen presentation or histamine release [3, 4, 6, 16]. This ameliorated function on the innate side would then create a more favourable microenvironment, which would also have an influence on the polarisation of T and B cells in a less Th2-prone manner as initially.
Conclusion
After having shown that 3 months of supplementation with the holoBLG lozenge led to a substantial level of immune resilience, characterized by a significant symptom reduction in HDM-induced ARC via HDM challenges in a state-of-the-art validated allergen exposure chamber [2], this study investigated potential long-term effects 7–8 months after cessation of supplementation and patients were recruited for a third challenge in the AEC. At the end of the supplementation (V3) a significant reduction of 60% (p = 0.0034) in the TNSS and 40% reduction in the TSS (p < 0.0003) compared to baseline (V1) were measured. 7–8 months afterwards, there was still a 20% (p = 0.1516) reduction in TNSS (V5). In addition a sustained clinically meaningful reduction of 40% (p = 0.004) in TSS (V5), comparable to immediately after cessation of supplementation (V3), also reflected consistently in other secondary endpoints, was documented. In conclusion this study could for the first time demonstrate sustained long-term efficacy of supplementation with the holoBLG lozenge. Phenomena like trained immunity or innate immune memory might account for the observed effects. Further work is required to unravel those exact mechanisms.
Abbreviations
- AEC:
-
Allergen exposure chamber
- AIT:
-
Allergen-specific immunotherapy
- ARC:
-
Allergic rhinoconjunctivitis
- BLG:
-
Beta-lactoglobulin
- CI:
-
Confidence interval
- DBPC:
-
Double-blind, placebo controlled
- FEV1 :
-
Forced expiratory volume in 1 second
- FSMP:
-
Food for special medical purposes
- FVC:
-
Forced vital capacity
- GP:
-
General practitioner
- HDM:
-
House dust mite
- IQR:
-
Interquartile range
- LPR:
-
Late phase reaction
- NPT:
-
Nasal provocation test
- OTC:
-
Over the counter (prescription-free products sold in pharmacies)
- PEF:
-
Peak expiratory flow
- PNIF:
-
Peak nasal inspiratory flow
- SD:
-
Standard deviation
- TBSS:
-
Total Bronchial Symptom Score
- TESS:
-
Total Eye Symptom Score
- TNSS:
-
Total Nasal Symptom Score
- TOSS:
-
Total Other Symptom Score
- TSS:
-
Total Symptom Score
- V:
-
Visit
- VAS:
-
Visual analogue scale
References
Roth-Walter F, Jensen-Jarolim E, Gomez-Casado C, Diaz-Perales A, Pacios L, Singer J. Method and means for diagnosing and treating allergy. Vienna, Austria: Biomedical Int. R+D GmbH; 2014.
Bergmann K‑C, Graessel A, Raab J, Banghard W, Krause L, Becker S, et al. Targeted micronutrition via holo-BLG based on the farm effect in house dust mite allergic rhinoconjunctivitis patients—first evaluation in a standardized allergen exposure chamber. Allergo J Int. 2021;30(4):141–9. https://doi.org/10.1007/s40629-021-00163-9.
Roth-Walter F, Afify SM, Pacios LF, Blokhuis BR, Redegeld F, Regner A, et al. Cow’s milk protein beta-lactoglobulin confers resilience against allergy by targeting complexed iron into immune cells. J Allergy Clin Immunol. 2021;147(1):321–34.e4. https://doi.org/10.1016/j.jaci.2020.05.023.
Afify SM, Pali-Scholl I, Hufnagl K, Hofstetter G, El-Bassuoni MA, Roth-Walter F, et al. Bovine holo-beta-lactoglobulin cross-protects against pollen allergies in an innate manner in BALB/c mice: potential model for the farm effect. Front Immunol. 2021;12:611474. https://doi.org/10.3389/fimmu.2021.611474.
Roth-Walter F. Compensating functional iron deficiency in patients with allergies with targeted micronutrition. Allergo J Int. 2021;30(4):130–4. https://doi.org/10.1007/s40629-021-00171-9.
Afify SM, Regner A, Pacios LF, Blokhuis BR, Jensen SA, Redegeld FA, et al. Micronutritional supplementation with a holoBLG based FSMP (food for specific medical purposes)-lozenge alleviates allergic symptoms in BALB/c mice: Imitating the protective farm effect. Clin Exp Allergy. 2021; https://doi.org/10.1111/cea.14050.
Drury KE, Schaeffer M, Silverberg JI. Association between atopic disease and anemia in US children. JAMA Pediatr. 2016;170(1):29–34. https://doi.org/10.1001/jamapediatrics.2015.3065.
Roth-Walter F, Pacios LF, Bianchini R, Jensen-Jarolim E. Linking iron-deficiency with allergy: role of molecular allergens and the microbiome. Metallomics. 2017;9(12):1676–92. https://doi.org/10.1039/c7mt00241f.
Rhew K, Oh JM. Association between atopic disease and anemia in pediatrics: a cross-sectional study. BMC Pediatr. 2019;19(1):455. https://doi.org/10.1186/s12887-019-1836-5.
Rhew K, Brown JD, Oh JM. Atopic disease and anemia in Korean patients: cross-sectional study with propensity score analysis. Int J Environ Res Public Health. 2020; https://doi.org/10.3390/ijerph17061978.
Petje LM, Jensen SA, Szikora S, Sulzbacher M, Bartosik T, Pjevac P, et al. Functional iron-deficiency in women with allergic rhinitis is associated with symptoms after nasal provocation and lack of iron-sequestering microbes. Allergy. 2021;76(9):2882–6. https://doi.org/10.1111/all.14960.
Hufnagl K, Jensen-Jarolim E. Does a carrot a day keep the allergy away? Immunol Lett. 2019;206:54–8. https://doi.org/10.1016/j.imlet.2018.10.009.
Julia V, Macia L, Dombrowicz D. The impact of diet on asthma and allergic diseases. Nat Rev Immunol. 2015;15(5):308–22. https://doi.org/10.1038/nri3830.
Devereux G, Craig L, Seaton A, Turner S. Maternal vitamin D and E intakes in pregnancy and asthma to age 15 years: a cohort study. Pediatr Pulmonol. 2019;54(1):11–9. https://doi.org/10.1002/ppul.24184.
Hufnagl K, Jensen-Jarolim E. Vitamin A and D in allergy: from experimental animal models and cellular studies to human disease. Allergo J Int. 2018;27(3):72–8. https://doi.org/10.1007/s40629-018-0054-2.
Bartosik TJS, Afify SM, Bianchini R, Hufnagl K, Hofstetter G, Berger M, et al. Abstract: Ameliorating allergic symptoms by supplementing micronutritional deficiencies in immune cells with a holoBLG-based FSMP (food for specific medical purposes)- lozenge in a double-blind placebo-controlled trial. Allergy. 2021;76(S110):424–582. https://doi.org/10.1111/all.15096.
Durham SR, Emminger W, Kapp A, de Monchy JG, Rak S, Scadding GK, et al. SQ-standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol. 2012;129(3):717–25.e5. https://doi.org/10.1016/j.jaci.2011.12.973.
Scadding GW, Calderon MA, Shamji MH, Eifan AO, Penagos M, Dumitru F, et al. Effect of 2 years of treatment with sublingual grass pollen immunotherapy on nasal response to allergen challenge at 3 years among patients with moderate to severe seasonal allergic rhinitis: the GRASS randomized clinical trial. JAMA. 2017;317(6):615–25. https://doi.org/10.1001/jama.2016.21040.
Bruhn C. Umfrage bestätigt große Akzeptanz von holo-BLG bei Patienten. HNO. 2021;71(4):220.
Bergmann C, Ehmann R, Jordakieva G, Koehler H‑J, Straub D, Untersmayr E, et al. Targeted micronutrition for allergic patients—possible applications of a food for special medical purposes. Allergo J Int. 2021;30(4):150–3. https://doi.org/10.1007/s40629-021-00172-8.
Pfaar O, Bergmann KC, Bonini S, Compalati E, Domis N, de Blay F, et al. echnical standards in allergen exposure chambers worldwide—an EAACI task force report. Allergy. 2021; https://doi.org/10.1111/all.14957.
Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9(5):355–61. https://doi.org/10.1016/j.chom.2011.04.006.
Pfaar O, Calderon MA, Andrews CP, Angjeli E, Bergmann KC, Bonlokke JH, et al. EAACI_allergen exposure chambers: harmonizing current concepts and projecting the needs for the future—an EAACI position paper. Allergy. 2017;72(7):1035–42. https://doi.org/10.1111/all.13133.
Voegler T, Goergen F, Bergmann K‑C, Boelke G, Salame J, Gildemeister J, et al. Technical specifications of the global allergy and asthma European network (GA2LEN) chamber: a novel mobile allergen exposure chamber. Allergo J Int. 2017;26(8):287–94. https://doi.org/10.1007/s40629-017-0040-0.
Zuberbier T, Abelson MB, Akdis CA, Bachert C, Berger U, Bindslev-Jensen C, et al. Validation of the global allergy and asthma European network (GA(2)LEN) chamber for trials in allergy: innovation of a mobile allergen exposure chamber. J Allergy Clin Immunol. 2017;139(4):1158–66. https://doi.org/10.1016/j.jaci.2016.08.025.
Tovey ER, Liu-Brennan D, Garden FL, Oliver BG, Perzanowski MS, Marks GB. Time-based measurement of personal mite allergen bioaerosol exposure over 24 hour periods. PLoS ONE. 2016;11(5):e153414. https://doi.org/10.1371/journal.pone.0153414.
Bergmann KC, Krause L, Hiller J, Becker S, Kugler S, Tapparo M, et al. First evaluation of a symbiotic food supplement in an allergen exposure chamber in birch pollen allergic patients. World Allergy Organ J. 2021;14(1):100494. https://doi.org/10.1016/j.waojou.2020.100494.
R Core Team. R: a language and environment for statistical computing. Austria: R Foundation for Statistical Computing; 2021.
Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Soft. 2015; https://doi.org/10.18637/jss.v067.i01.
Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008;50(3):346–63. https://doi.org/10.1002/bimj.200810425.
Genuneit J, Standl M. Epidemiology of allergy: natural course and risk factors of allergic diseases. Handb Exp Pharmacol. 2021; https://doi.org/10.1007/164_2021_507.
Dhami S, Nurmatov U, Arasi S, Khan T, Asaria M, Zaman H, et al. Allergen immunotherapy for allergic rhinoconjunctivitis: a systematic review and meta-analysis. Allergy. 2017;72(11):1597–631. https://doi.org/10.1111/all.13201.
Roberts G, Pfaar O, Akdis CA, Ansotegui IJ, Durham SR, Gerth van Wijk R, et al. EAACI guidelines on allergen immunotherapy: allergic rhinoconjunctivitis. Allergy. 2018;73(4):765–98. https://doi.org/10.1111/all.13317.
Agache I, Lau S, Akdis CA, Smolinska S, Bonini M, Cavkaytar O, et al. EAACI guidelines on allergen immunotherapy: house dust mite-driven allergic asthma. Allergy. 2019;74(5):855–73. https://doi.org/10.1111/all.13749.
Jensen-Jarolim E, Roth-Walter F, Jordakieva G, Pali-Scholl I. Allergens and adjuvants in allergen immunotherapy for immune activation, tolerance, and resilience. J Allergy Clin Immunol Pract. 2021;9(5):1780–9. https://doi.org/10.1016/j.jaip.2020.12.008.
Riedler J, Braun-Fahrländer C, Eder W, Schreuer M, Waser M, Maisch S, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001;358(9288):1129–33. https://doi.org/10.1016/s0140-6736(01)06252-3.
Loss G, Apprich S, Waser M, Kneifel W, Genuneit J, Buchele G, et al. The protective effect of farm milk consumption on childhood asthma and atopy: the GABRIELA study. J Allergy Clin Immunol. 2011;128(4):766–73.e4. https://doi.org/10.1016/j.jaci.2011.07.048.
Pali-Schöll IBR, Hofstetter G, Winkler S, Ahlers S, Altemeier T, Afify SM, et al. Secretory beta-lactoglobulin associated with zinc in stable dust causes a Th1 immune bias in vitro and may contribute to the allergy protective effect of cattle farms. Allergy. 2020;75(S109):120–274. https://doi.org/10.1111/all.14506.
Mayerhofer H, Pali-Schöll I. The farm effect revisited: from β‑lactoglobulin with zinc in cowshed dust to its application. Allergo J Int. 2021;30(4):135–40. https://doi.org/10.1007/s40629-021-00174-6.
Bekkering S, Dominguez-Andres J, Joosten LAB, Riksen NP, Netea MG. Trained immunity: reprogramming innate immunity in health and disease. Annu Rev Immunol. 2021;39(1):667–93. https://doi.org/10.1146/annurev-immunol-102119-073855.
Author information
Authors and Affiliations
Contributions
KCB, JR, MFK, SB and AG designed the study. Patient enrollment, study conduct and study quality control, patient follow-up were done by KCB, TZ, SB and SK. Data acquisition was performed by KCB, SB and SK. LK performed the statistical analysis. KCB, LK, MFK and AG evaluated and interpreted the data. FRW and EJJ provided support and contributed in manuscript revision. AG wrote the manuscript. All authors provided critical feedback, reviewed and approved the final version of the manuscript and its submission.
Corresponding author
Ethics declarations
Conflict of interest
K.-C. Bergmann reports personal fees for lectures: ALK, AstraZeneca, Allergopharma, Bencard, Chiesi, GSK, HAL, LETI, Lofarma, Mundipharma, Novartis, Sanofi. Non-financial support as Chair of German Pollen Information Service Foundation, personal fees and non-financial support from Consultant physician for ECARF, personal fees and non-financial support from Advisory Board member of AstraZeneca, ECARF, GSK, Robert-Koch-Institute Berlin (Vice chairman Public Health), Sanofi, outside the submitted work. J. Raab, M.F. Kramer, and A. Graessel are employees of Allergy Therapeutics/Bencard Allergie GmbH. S. Becker, L. Krause and S. Kugler declare that they have no competing interests. F. Roth-Walter received honoraria for presentations from Allergy Therapeutics and Bencard Allergie GmbH. E. Jensen-Jarolim and F. Roth-Walter are inventors on the immunoBON® patent (Patent EP 2 894 478 B1), owned by Biomedical International R + D, Vienna, Austria, of which EJJ E. Jensen-Jarolim is shareholder. E. Jensen-Jarolim received honoraria for presentations from Allergy Therapeutics, Allergopharma, Bencard, Meda, Roxall, ThermoFisher, and Vifor, and is consultant or in Advisory Boards for Allergy Therapeutics, Vifor Pharma, Sanofi, previously for MediGene, Germany, Novartis, and Dr. Schär (ceased). Prof. T. Zuberbier reports personal fees from Bayer Health Care, FAES, Novartis, Henkel, and AstraZeneca. He received fees for talks and personal fees from AbbVie, ALK, Almirall, Astellas, Bayer Health Care, Bencard Allergie GmbH, Berlin Chemie, HAL, Leti, Meda, Menarini, Merck, MSD, Novartis, Pfizer, Sanofi, Stallergenes, Takeda, Teva, UCB Henkel, Kryolan, L’Oréal outside the submitted work.
Ethical standards
Study protocol was approved by the Ethics Committee of the Charité Berlin (EA1/412/20). All participants received detailed information from the supervising physician and provided their written informed consent to participate. Participants also agreed to the processing and storage of their data in accordance with the General Data Protection Regulation. Study was conducted in accordance with the Declaration of Helsinki and in compliance with all federal, regional and local requirements. All data provided were pseudonymised to protect the privacy of the study participants as mandated by the applicable laws and regulations.
Additional information
Availability of data and material
The study datasets generated during and/or analysed are not publicly available. Bencard Allergie GmbH is committed to share access to patient-level data, and supporting clinical documents from related studies with qualified external researchers. Requests are reviewed and approved by an independent review panel on the basis of scientific merit.
Code availability
Not applicable
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bergmann, KC., Raab, J., Krause, L. et al. Long-term benefits of targeted micronutrition with the holoBLG lozenge in house dust mite allergic patients. Allergo J Int 31, 161–171 (2022). https://doi.org/10.1007/s40629-021-00197-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40629-021-00197-z