Abstract
Purpose
Evaluation of a lozenge for targeted micronutrition (holo-BLG), a new invention based on the farm effect, in house dust mite (HDM) allergic rhinoconjunctivitis (ARC) patients in a standardized allergen exposure chamber (AEC).
Methods
Eligible HDM allergic patients were exposed to HDM raw material in an AEC for 120 min before (V1) and after (V3) 3 months of holo-BLG supplementation. Nasal, conjunctival, bronchial and other symptoms were rated by the patients every 10 min and, wellbeing, peak nasal inspiratory flow (PNIF), and lung function parameters every 30 min. Primary endpoint was the change in median Total Nasal Symptom Score (TNSS) at V3 compared to V1 at 120 min of exposure. Secondary endpoints consisted of the exploratory analysis of the temporal evolution of symptom scores using linear mixed effects models.
Results
A total of 32 patients were included in the analysis. A significant improvement of 60% (p = 0.0034) in the primary endpoint TNSS (V1 2.5 [interquartile range, IQR 1–4], V3 1.0 [IQR 1–3]) was observed. 40% improvement was seen for the Total Symptom Score (V1 5.0 [IQR 3–9], V3 3.0 [IQR 2–4]; [Wilcoxon test: confidence interval 1.5–4.0, p < 0.0003]). The analysis of the temporal evolution of all symptom scores and the personal wellbeing revealed clinically meaningful improvement over time, manifested in a lower symptom increase during the final HDM exposure. No relevant differences were observed for PNIF and lung function parameters. Safety and tolerability were rated as excellent.
Conclusions
The effect of holo-BLG resulting in immune resilience might help to fight the allergy epidemic on a new front based on targeted micronutrition of immune cells.
Trial registration
The study was retrospectively registered at clinicaltrials.gov (NCT04477382).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The development of diseases like allergy and asthma is on the rise since decades [1]. However, luckily already discovered and intensively discussed, there is a known protective phenomenon called the farm effect [2,3,4,5]. As shown in several earlier studies [6,7,8,9], childbearing and growing up on a traditional farm with dairy farming provides protection against allergic sensitization, asthma, and hay fever. These diseases have been shown to occur at a much lower frequency in farm children compared to children from urban regions [10,11,12]. Exposure to a highly diverse microbial community, which shapes the immunity of the people living on farms early on, is an important contributory factor to the farm effect [10]. Secondly, studies have also shown a clear relationship between this protective effect to farm air exposure and to the consumption of unprocessed milk, which could be modelled in mice [13]. Notably, it is not recommended to consume unprocessed milk due to potential bacterial contamination [14].
Several components in this so called raw milk [13] are under discussion as potential mediators of the protective farm effect such as components of the fat fraction [11] or proteins of the whey fraction in their native and undestroyed form [2, 8]. BLG (beta-lactoglobulin), the major whey protein in cow’s milk, belongs to the lipocalin (LCN) protein family. LCNs are proteins sharing a highly conserved structure, albeit not sharing a high sequence similarity and they are present in a range of species [15]. The key aspect of their shared structure is a central molecular pocket, which can be loaded with hydrophobic ligands [15]. If the central pocket of BLG is loaded with micronutrients, it is called holo-BLG (immunoBON®), in the case of an empty central pocket the form is called apo-BLG. Previous studies discovered an unrecognized role of holo-BLG to shuttle its ligands into immune cells, therefore providing an antigen-unspecific immune-regulatory effect resulting in resilience to immune activation—“immune resilience”—and protection against allergic sensitization and symptoms [16,17,18]. BLG-targeted delivery of ligands such as retinoic acid (RA) and iron-flavonoid complexes compensate intracellular nutritional deficiencies of immune cells in an allergic individual [13, 17, 19, 20]. This in a concerted action leads to immune resilience: (i) by increasing intracellular iron levels, which are reduced in allergic subjects [20], (ii) activating the immune regulatory aryl hydrocarbon receptor (AHR) [13, 17, 19], or RA receptor pathways [21], and (iii) zinc enforcing a Th1 immune response (Pali-Schöll et al., manuscript in review).
The holo-BLG lozenge therefore is based on an invention on spiked lipocalins, patented for allergy treatment and prevention [22], a discovery relevant in terms of the farm effect. Holo-BLG is manufactured as a lozenge and registered as FSMP (Foods for Special Medical Purposes) in Germany and Austria (more countries to follow) containing the patented formulation of the whey protein BLG, iron complexed with flavonoids, retinol-palmitate, and zinc. A human pilot study in birch pollen allergic patients with the holo-BLG lozenge suggests that the molecular principles and preclinical expectations from mouse models can be translated into the human setting [23]. To further investigate the antigen-unspecific and beneficial effects of holo-BLG, we set up the present study to demonstrate whether this holds true in house dust mite (HDM)-induced allergic rhinoconjunctivitis (ARC). A standardized and reproducible exposure of HDM allergic patients with HDM material was performed in an allergen exposure chamber (AEC), a highly standardised platform that reliably and reproducibly generates allergic symptoms [24,25,26,27,28]. Therefore, an AEC is an excellent choice for the first evaluation of this novel FSMP in HDM-induced ARC, designed to investigate the effects of the daily intake of holo-BLG for 3 months by a controlled challenge.
Methods
Study design and materials
Holo-BLG (immunoBON®) was provided by Biomedical International R + D GmbH, Vienna, Austria [22] for this study.
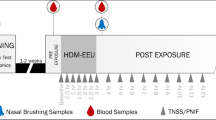
Between January and June 2020, a minimum of 30 HDM allergic patients with HDM ARC who met the eligibility criteria at screening (visit 0, V0) were exposed twice (V1, V3) to HDM raw material in the AEC of ECARF Institute, Berlin, Germany (Fig. 1). After V1 all patients were provided with the holo-BLG lozenge and were instructed to slowly suck one lozenge twice daily for 3 months.
Patient population
The eligibility criteria were the following: HDM allergy with ARC symptoms for ≥ 2 years according to the ARIA Guidelines, age 18–65, skin prick test (SPT) response (wheal diameter) to HDM extract (D. pt. and/or D. f.) ≥ 3 mm, positive response to nasal provocation test (NPT) to mite extract and/or a reaction to HDM allergen in an AEC of TNSS ≥ 3.
The main exclusion criteria were sublingual or subcutaneous allergen immunotherapy (SLIT/SCIT) during the last 2 years, clinically relevant hypersensitivity to ingredients of the holo-BLG lozenge, allergy to cow’s milk protein, clinically relevant sensitization to early flowering trees and/or cat allergen, severe or uncontrolled asthma during 3 months before screening, FEV1 < 80% predicted before allergen exposure, relevant infectious or severe chronic diseases or contraindication to adrenaline and/or other rescue medication, simultaneous intake of anti-allergic medication prior to screening process and exposure in the AEC. Wash-out time for different medications were as follows: 3 weeks for systemic corticosteroids, 2 weeks for topical nasal corticosteroids, 7 days for cromones, 72 h for anti-histamines, 3 months for antibiotics, 1 month for pro-, pre- and synbiotics, and anti-allergic medication during the whole study.
AEC
The GA2LEN AEC (ECARF) is a mobile flexible chamber made of two connected standard 24 feet (7.32 m) high-cube containers [24, 27, 28]. In the standardised and validated chamber, the exposure is performed with 250 μg/m3 HDM raw material (whole culture mite: D. pt. and D. f. body and faeces allergen 50:50; Allergon AB, Ängelholm, Sweden) for 120 min at 20 °C and 55% relative air moisture, after an acclimatisation period for 20 min [21,22,23]. The amount and composition of HDM allergens, as well as the exposure particle size is designed to mimic a high natural daily HDM exposure of a HDM-allergic patient [29].
Outcome parameter
Each symptom was evaluated by the patient on a scale from 0–3 (no, mild, moderate, or severe symptoms) and summed up to give a TNSS (runny, sneezing, itchy, and blocked nose), Total Eye Symptom Score (TESS: itchy, watery eyes, and gritty feeling), Total Bronchial Symptom Score (TBSS: breathlessness, wheezing, cough, and asthma) and Total Other Symptom Score (TOSS: itchy skin, and itchy palate). TSS is the sum of TNSS, TESS, TBSS, and TOSS, revealing a maximum score of 39. The primary endpoint was the change in TNSS at 120 min exposure to HDM raw material in the AEC at visit V3 compared to visit V1. Secondary endpoints were the exploratory analysis of the temporal evolution of TNSS, TESS, TBSS, TOSS and TSS during each 120 min exposure and the differences between these temporal trends between V1 and V3. Changes in personal wellbeing (VAS: 0 = very good to 10 = very bad), peak nasal inspiratory flow (PNIF, Peak Nasal Inspiratory Flow Meter, Clement Clarke International Ltd., Harlow, Essex, UK) and peak expiratory flow (PEF, Peak-Flow-Meter, Personal Best, Philips GmbH, Herrsching, Germany) were recorded before and every 30 min during the 120 min exposure. Forced expiratory volume in 1 second (FEV1), FEV1/FVC (forced vital capacity) (EasyOne™ Spirometer, ndd Medizintechnik AG, Zürich, Switzerland) was performed before and after exposure, analyzed and judged from a clinician’s point of view. Adverse events (AEs) related to the holo-BLG lozenge were monitored during the entire study. To record late phase reactions (LPRs) or AEs related to the exposure, participants received a safety call 24 h after each exposure.
Study oversight
The study protocol was approved by the Ethics Committee of the Charité, Berlin (EA1/314/19). The study, sponsored by Bencard Allergie GmbH, has been retrospectively registered at clinicaltrials.gov (NCT04477382). All participants received detailed information and gave their written informed consent to participate in the study as well as to the processing and storage of their data according to the General Data Protection Regulation. Pseudonymisation was applied in accordance with applicable laws and regulations. The study was conducted in accordance with the Declaration of Helsinki and in compliance with all federal, local, and regional requirements.
Statistical analysis
The confirmatory study was planned with 30 patients. The primary endpoint was analysed employing the paired Wilcoxon test (Wilcoxon signed rank test with continuity correction), several secondary endpoints were analysed in an exploratory way.
Percent changes between AEC visits were calculated by first calculating the median of values measured during V1 and V3 separately over all patients at 120 min. The following equation was employed to retrieve percentage changes: [(median V3—median V1)/median V1] × 100. Median, interquartile ranges (IQR) and percentage changes are given.
The linear evolution over time of the symptom scores and VAS over time were analysed by using linear mixed effects models with patients as random effects accounting for interindividual variability in baseline symptom scores and treatment, time and interaction between treatment and time as fixed effects. Model assumptions were analysed visually with quantile–quantile plots. All analyses were performed with R version 3.5.3 [30] using package “lme4” for mixed effects modelling [31] and package “multcomp” for estimating p-values of fixed effects [32, 33]. Together with 95% confidence intervals (CIs) for fixed effects in linear mixed effects models, p-values represent a descriptive summary measure not a result of confirmatory testing. Mean symptom scores over all patients for all 13 measurements were calculated and are presented with 95% CIs for comparison. The changes in PNIF and PEF were described using median and IQRs and judged from the point of clinical relevance.
Results
Baseline demographics and disease characteristics
In total, 38 patients suffering from HDM ARC and fulfilling the inclusion criteria started the study and 33 patients (14 with, 19 without asthma) completed it; 5 drop-outs were not related to the intake of the holo-BLG lozenge (2 patients failed to turn up for V3, 1 patient was sick and absent at V3, 1 patient was lactose-intolerant, but had not reported this exclusion criterion beforehand, and 1 patient had a gastroscopy). Mean age was 38.1 years (SD 11.5 years) and 27% were male (Table 1). All included patients rated at least two ARC symptoms (runny nose, blocked nose, itchy nose, sneezing, itchy eyes) as moderate or severe before study start. A total of 32 patients were included in the data analysis, one was excluded because of lack of symptoms during the exposure at V1 (TSS = 0).
Efficacy
Symptoms were recorded every 10 min for 120 min at both exposures (V1, V3). Primary endpoint was the difference between V3 and V1 for TNSS at 120 min exposure (Fig. 2a). At V1 after 120 min the median TNSS was 2.5 (IQR 1–4) compared to 1.0 (IQR 1–3) at V3, describing a significant difference of −60% (p = 0.0034) after 3‑months supplementation with holo-BLG.
a Total Nasal Symptom Score (TNSS), the primary endpoint of the study, showed a significant improvement at V3 (green) after 3 months of supplementation with holo-BLG compared to V1 (grey). At V1 after 120 min the median TNSS was 2.5 (IQR 1–4) compared to 1.0 (IQR: 1–3) at V3, describing a significant difference of −60% (p = 0.0034). b An important secondary outcome measure, the sum of all symptoms for all organs, depicted as median Total Symptom Score (TSS) at 120 min, was 5.0 (IQR 3–9) at V1 (grey) and 3.0 (IQR 2–4) at V3 (green, Wilcoxon test: confidence interval 1.5–4.0, p < 0.0003), describing a relevant improvement of 40%. (* p > 0.005)
As one secondary outcome measure, we analysed the sum of all symptoms for all organs, depicted as median TSS at 120 min (Fig. 2b), which was 5.0 (IQR 3–9) at V1 and 3.0 (IQR 2–4) at V3 (Wilcoxon test: CI: 1.5–4.0, p < 0.0003), describing a relevant improvement of −40%.
Further exploratory endpoints included the analysis of the temporal evolution during 120 min exposure for all four single symptom scores and the resulting TSS. We detected a relevant symptom improvement, which manifests as a lower rate of increase in symptoms over time (Fig. 3). The linear mixed effects models, adjusting for interindividual variability by estimating a random intercept per patient, identified relevant fixed effects for exposure time in the AEC and relevant interaction effects between the time spent in the AEC and the treatment with holo-BLG for all symptom scores. Fig. 3b summarises the results for all scores and as an example, the TNSS evolvement (Fig. 3a) over time is described in detail here: During 120 min of exposure in the AEC the TNSS increased at a rate of 0.018 per minute (95% CI 0.015–0.022, p < 2 × 10−16) on average during V1. After 3 months supplementation with holo-BLG, the slope was decreased by 0.011 per minute (95% CI 0.006–0.016, p = 5.16 × 10−5), which leaves an increase of 0.007 per minute during V3, identified by the interaction term in the linear mixed effects model. Mean TNSS over all measurements was reduced from 2.41 (95% CI 2.19–2.62) during V1 to 1.69 (95% CI 1.52–1.86) during V3, corresponding to a relevant reduction of 0.72 points (−30%) (Fig. 3b). We observed improvements in the mean values of other symptom scores ranging from −35 to −42% from V1 to V3 and a slope decrease (Fig. 3b) ranging from 0.005 (95% CI 0.002–0.007, p = 9.51 × 10−5) to 0.028 (95% CI 0.019–0.036, p = 6.79 × 10−10).
Symptom scores for nose (TNSS), eye (TESS), bronchial (TBSS), other (TOSS) and the sum of all symptoms (TSS) analysed for their linear evolution over time of HDM exposure in the AEC at baseline and after 3 months of supplementation with holo-BLG. a The analysis of the temporal evolution for all four single symptom scores, recorded every 10 min during 120 min exposure, and the resulting TSS showed improved symptoms over time after the intake of the holo-BLG lozenge (V3, green) compared to baseline (V1, grey) (smaller slope increase during V3 describes decreased symptoms during final exposure compared to baseline). b Summary of the results of the linear mixed effects model, analysing the symptom scores for their linear evolution over time. Mean scores (including 95% CI) during V1 and V3 and the difference (%) between baseline (V1) and final (V3) exposure are given. The slope per minute (including 95% CI) for V1, the corresponding slope decrease between V1 and V3 per minute (including 95% CI), and the related p-values as descriptive summary measures are given. V3 slope per minute is the difference between V1 slope/min and slope decrease between V1 and V3/min. a, b Detailed description about the TSS evolvement over time as example: Mean TSS over all measurements was reduced from 5.19 (95% CI 4.75–5.63) during V1 to 3.35 (95% CI 3.05–3.65) during V3, corresponding to a relevant reduction of 1.84 points (−35%). TSS increased by 0.043 per min (95% CI 0.037–0.049, p < 2 × 10−16) on average during V1. After holo-BLG intake, the slope was decreased by 0.028 per min (95% CI 0.019–0.036, p = 6.79 × 10−10) leaving a slope of 0.015 per min at V3
No relevant differences were observed for PNIF and PEF (not shown). Spirometry parameters (not shown) did not exhibit relevant differences between V1 and V3. No restrictions or obstructions were measured before and after baseline exposure (V1), and there were also no differences observed at V3.
At 120 min, median VAS was reduced from 32 at V1 (IQR 17.75–52) to 19 at V3 (IQR 12.25–35) (Wilcoxon test: CI 4.50–20.50, p = 0.0021), which represents a clinically meaningful increase of the patient’s personal wellbeing by 42% (Fig. 4).
Visual analog score (VAS) representing the personal wellbeing during 120 min of house dust mite (HDM) exposure in the allergen exposure chamber (AEC) at baseline (V1) and after 3 months (V3) of supplementation with holo-BLG. Evolution of the recorded wellbeing of the patients over time during HDM exposure, evaluated every 30 min. At the end of the exposure after 120 min, median VAS was reduced from 32 at V1 (IQR 17.75–52) (grey) to 19 at V3 (IQR 12.25–35) (green) (Wilcoxon test: CI 4.50–20.50, p = 0.0021), representing a clinically relevant increase of the patient’s personal wellbeing by 42%
During the safety call 24 h after baseline exposure with HDM (V2), 14 out of 31 patients (45%) reported LPRs due to the mite allergen exposure: itchiness and tearing eyes, irritated or sore throat, thirst, swallowing problems, dyspnoea, obstructed nose, headache, itchy skin and mild urticaria, cough and sneezing. After the end of the second exposure (V4) only 3 out of 25 patients (12%) reported LPRs: obstructed nose, mild cough or mild dyspnoea. This indicates an increase in tolerability to HDM allergens during exposure in the AEC after the intake of the holo-BLG lozenge.
Safety
One patient experienced mild head- and stomach-ache 24 h after the first exposure and the intake of at least one holo-BLG lozenge. No AEs were reported during the intake period of 3 months or at V4.
Discussion
The holo-BLG (beta-lactoglobulin) lozenge is based on an European patent [22] and distributed on the market as FSMP. This novel product mimics the farm effect (protection against allergies, asthma and eczema), being associated with exposure to farm air and consumption of unprocessed milk. It was demonstrated that holo-BLG, the major protein of the whey fraction, harbors allergy protective capacity as shown in cellular studies, mouse models and in a clinical DBPC pilot trial with birch pollen allergic patients [23]. The major whey protein beta-lactoglobulin (BLG), when properly loaded (= holo-BLG), counteracts sensitization and promotes tolerance. Holo-BLG delivers its ligands, micronutrients, directly to immune cells, thereby activating the immune regulatory AHR pathway [17, 19, 21, 23]. The immune modulatory effect results in a state of resilience to immune activation, protection against allergic sensitization and relief of allergic symptoms in an antigen-unspecific way [17, 19, 21, 23]. Interestingly, the secretory protein BLG cannot only be found in milk, but it could be detected in bovine urine and cattle stable dust [34]. BLG could therefore also represent the air-borne factor in the protective farm effect against allergies and asthma by inducing immune resilience and a favourable Th1-milieu [34].
The proof of concept was designed to demonstrate whether holo-BLG could, like the farm effect, protect from respiratory allergies in an antigen-unspecific way, using a model of double-sensitised mice. Mice treated with holo-BLG, but not with placebo, were protected from anaphylactic body temperature drop to the autologous allergen BLG, as well as in an antigen-unspecific manner to Bet v 1. IgE and IgG1 antibody production, antigen-presentation capacity of CD11c+ dendritic cells, and mast cell degranulation were impaired, while Tregs were induced [19].
Humans express a homologue to bovine BLG, lipocalin 2 (LCN2). This is an acute phase protein, elevated in bacterial infections and cancer. Surprisingly, serum levels of LCN2 were shown to be significantly reduced in allergic patients compared to nonallergic controls [29]. These levels were partially restored in HDM allergic patients who were treated with HDM SLIT for 9 months compared to the placebo group. An increased LCN2 level 9 months after HDM-SLIT was associated with a clinical benefit [35]. It is tempting to speculate that bovine BLG from whey in the holo-BLG lozenge may take over the role of LCN‑2 given their high structural homology. Accordingly, receptors for BLG on human and murine cells have been previously identified [36, 37]. Our data so far suggest that BLG targets its micronutrient ligands via such receptors into immune cells and thereby mitigates allergic inflammation, but in an allergen-unspecific manner.
After promising results from a human pilot study investigating the effects of holo-BLG in seasonal allergy (birch pollen) in a DBPC setting [23], another DBPC trial with more pollen-allergic patients is ongoing to broaden the clinical evaluation of holo-BLG.
Here we aimed to evaluate whether targeted micronutrition of immune cells with holo-BLG could also be effective in the field of perennial allergies. HDM allergens are the most relevant inducers of allergic diseases worldwide constituting a major public health problem that results in significant morbidity and an increased burden on health services [38]. HDMs are able to sensitize healthy individuals and induce allergic symptoms in sensitized and genetically predisposed individuals likely resulting in ARC, asthma, and atopic dermatitis [38].
It is very difficult for HDM allergic patients to realize changes in HDM exposure and the resulting difference in symptoms, because they are exposed year-round in a fluctuating manner to their allergen. Therefore, the effect of the daily supplementation with holo-BLG for 3 months in HDM allergic subjects was investigated in a standardised and validated AEC before and after intake. The major advantage of this method is its reproducibility; the same setting is used before and after the supplementation with holo-BLG, which increases the reliability of the results, especially in HDM allergy [28]. We did no sample size calculation during the planning phase for this first evaluation of holo-BLG in HDM allergic patients in an AEC, which is a limitation of our study. However, we chose the number of participating patients based on feasibility and our experience with the first human pilot DBPC study (n = 17), which showed a significant symptom reduction in seasonal allergy [23].
Supplementation with holo-BLG led to a robust and clinically relevant improvement, as indicated not only in the primary endpoint TNSS, but also in the symptom decrease exhibited by other organs. After 3 months of supplementation, the TNSS at 120 min of exposure was significantly reduced by 60% (p = 0.0034). The development of symptoms of all organs (nose, eyes, bronchi, others) during the 120 min session in the AEC was reduced to a clinically meaningful extent from 35–42% during the final exposure (V3). The patients’ wellbeing (VAS) was improved by 42% at the end of V3. The patient-reported LPRs induced by the AEC challenge were markedly reduced, from 45% of the patients at baseline to only 12% of patients after the final exposure. This points to increased tolerability to HDM exposure after intake of the holo-BLG lozenge for 3 months. The holo-BLG lozenge showed an excellent safety and tolerability profile; only 1 patient reported slight head- and stomach-ache after the intake of the first lozenge, but besides this no other AEs were reported during the duration of the study.
The placebo control plays a fundamental role when estimating the clinical effect of interventions in all diseases and several factors have an impact on this effect [39]. In DBPC clinical SLIT trials in HDM allergic patients using an AEC, the placebo effect ranged, e.g., from +0.5 to +11% after 6 months using the TNSS (−7% after 4 months and −9.4 after 2 months in 1 trial), and −4 to −17% after 6 months using the Total Ocular Symptom Score (−14% after 4 months and −8% after 2 months) [40]. Only a small improvement in symptoms was seen in the placebo groups, which were exposed to HDM in AECs, after 6 months of SLIT treatment. Shorter treatment periods were associated with worsening of symptoms in the placebo groups in different trials [40]. The supplementation with holo-BLG for 3 months resulted in a 60% improvement in the primary endpoint TNSS at 120 min exposure and up to 42% improvement of the symptoms in other organs during the whole exposure time. Furthermore, it is important that in addition to the improvement of all investigated symptoms, also the personal wellbeing of the patients improved. Although the lack of a placebo control is a clear limitation of our study and we cannot control for placebo effects, we are confident that our study demonstrates clinically relevant improvements for patients with HDM induced ARC following 3‑months supplementation with holo-BLG.
The management of HDM ARC is of utmost importance, especially because of the high risk of asthma development [38]. SLIT or SCIT, the only causal treatment available in this field, are often not used for a number of reasons [41]. At this point, the holo-BLG lozenge could provide a new option not only for adults with established HDM allergy as our trials suggests, but also for children from age 3 onwards.
Conclusion
After having shown efficacy in seasonal allergy in a DBPC trial, this investigation of the holo-BLG lozenge, a FSMP, revealed clinically relevant efficacy and an excellent safety and tolerability profile in perennial allergy in HDM ARC patients in the robust setting of an AEC. The antigen-independent mode-of-action of holo-BLG results in immune resilience, leading back to the regular state of a healthy immune system in homeostasis, by holo-BLG-targeted micronutrition of immune cells. This effect revolutionizes our understanding of allergy and the mechanisms of resilience to fight allergies. This novel approach based on the farm effect, might help to fight the allergy epidemic on a new front and evidence about mode-of-action is currently increasing and the collection of more clinical data is still ongoing.
Abbreviations
- AE:
-
Adverse event
- AEC:
-
Allergen exposure chamber
- AHR:
-
Aryl hydrocarbon receptor
- ARC:
-
Allergic rhinoconjunctivitis
- BLG:
-
Beta-lactoglobulin
- CI:
-
Confidence interval
- DBPC:
-
Double-blind, placebo controlled
- D. f.:
-
Dermatophagoides farinae
- D. pt:
-
Dermatophagoides pteronyssinus
- FEV1 :
-
Forced expiratory volume in 1 second
- FSMP:
-
Foods for special medical purposes
- FVC:
-
Forced vital capacity
- HDM:
-
House dust mite
- IQR:
-
Interquartile range
- LCN:
-
Lipocalin
- LPR:
-
Late phase reaction
- NPT:
-
Nasal provocation test
- PEF:
-
Peak expiratory flow
- PNIF:
-
Peak nasal inspiratory flow
- RA:
-
Retinoic acid
- SCIT:
-
Subcutaneous allergen immunotherapy
- SD:
-
Standard deviation
- SLIT:
-
Sublingual allergen immunotherapy
- SPT:
-
Skin prick test
- TBSS:
-
Total Bronchial Symptom Score
- TESS:
-
Total Eye Symptom Score
- TNSS:
-
Total Nasal Symptom Score
- TOSS:
-
Total Other Symptom Score
- TSS:
-
Total Symptom Score
- V:
-
Visit
- VAS:
-
Visual analogue scale
References
Agache I. EAACI guidelines on allergen immunotherapy-Out with the old and in with the new. Allergy. 2018;73(4):737–8.
Abbring S, Holsb G, Garssena J, van Esch BCAM. Raw cow’s milk consumption and allergic diseases—the potential role of bioactive whey proteins. Eur J Pharmacol. 2019;843:55–65.
Braun-Fahrländer C, von Mutius E. Can farm milk consumption prevent allergic diseases? Clin Exp Allergy. 2011;41:29–35.
Ege MJ, Frei R, Bieli C, Schram-Bijkerk D, Waser M, Benz MR, et al. Not all farming environments protect against the development of asthma and wheeze in children. J Allergy Clin Immunol. 2007;119(5):1140–7.
Perkin MR, Strachan DP. Which aspects of the farming lifestyle explain the inverse association with childhood allergy? J Allergy Clin Immunol. 2006;117(6):1374–81.
Alfven T, Braun-Fahrlander C, Brunekreef B, von Mutius E, Riedler J, Scheynius A, et al. Allergic diseases and atopic sensitization in children related to farming and anthroposophic lifestyle—the PARSIFAL study. Allergy. 2006;61(4):414–21.
Riedler J, Braun-Fahrlander C, Eder W, Schreuer M, Waser M, Maisch S, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001;358(9288):1129–33.
Loss G, Apprich S, Waser M, Kneifel W, Genuneit J, Büchele G, et al. The protective effect of farm milk consumption on childhood asthma and atopy: The GABRIELA study. J Allergy Clin Immunol. 2011;128:766–73.
Pfefferle PI, Buchele G, Blumer N, Roponen M, Ege MJ, Krauss-Etschmann S, et al. Cord blood cytokines are modulated by maternal farming activities and consumption of farm dairy products during pregnancy: the PASTURE Study. J Allergy Clin Immunol. 2010;125(1):108–115e1–3.
Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, et al. Innate immunity and asthma risk in amish and Hutterite farm children. N Engl J Med. 2016;375(5):411–21.
Brick T, Hettinga K, Kirchner B, Pfaffl M, Ege M. The beneficial effect of farm milk consumption on asthma, allergies, and infections: from meta-analysis of evidence to clinical trial. J Allergy Clin Immunol Pract. 2019;3:878–89.
Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WO, Braun-Fahrlander C, et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364(8):701–9.
Abbring S, Hols G, Baars T, Verheijden KAT, Garssen J, Diks MAP, et al. Raw cow’s milk prevents the development of airway inflammation in a murine house dust mite-induced asthma model. Front Immunol. 2017;8:1045.
Lucey J. Raw milk consumption—risks and benefits. J Nutr Food Sci. 2015;50(4):189–93.
Jensen-Jarolim E, Pacios LF, Bianchini R, Hofstetter G, Roth-Walter F. Structural similarities of human and mammalian lipocalins, and their function in innate immunity and allergy. Allergy. 2016;71(3):286–94.
Roth-Walter F, Pacios LF, Gomez-Casado C, Hofstetter G, Roth GA, Singer J, et al. The major cow milk allergen Bos d 5 manipulates T‑helper cells depending on its load with siderophore-bound iron. PLoS ONE. 2014;9(8):e104803.
Roth-Walter F, Afify SM, Pacios LF, Blokhuis BR, Redegeld F, Regner A, Petje LM, Fiocchi A, Untersmayr E, Dvorak Z, Hufnagl K, Pali-Schöll I, Jensen-Jarolim E. “Cow milk protein beta-lactoglobulin confers resilience against allergy by targeting complexed iron into immune cells.” J Allergy Clin Immunol. 2020. https://doi.org/10.1016/j.jaci.2020.05.023.
Hufnagl K, Ghosh D, Wagner S, Fiocchi A, Dahdah L, Bianchini R, et al. Retinoic acid prevents immunogenicity of milk lipocalin Bos d 5 through binding to its immunodominant T‑cell epitope. Sci Rep. 2018;8:1598.
Afify SM, Pali-Schöll I, Hufnagl K, Hofstetter G, El-Bassuoni MAR, Roth-Walter F, Jensen-Jarolim E. Bovine beta-lactoglobulin cross-protects against pollen allergies in an innate manner: mimicking the farm effect in a BALB/c mouse model. Frontiers in Immunology. In press.
Roth-Walter F, Pacios LF, Bianchini R, Jensen-Jarolim E. Linking iron-deficiency with allergy: role of molecular allergens and the microbiome. Metallomics. 2017;9(12):1676–92.
Hufnagl K, Afify SM, Braun N, Wagner S, Wallner M, Hauser M, et al. Retinoic acid-loading of the major birch pollen allergen Bet v 1 may improve specific allergen immunotherapy: In silico, in vitro and in vivo data in BALB/c mice. Allergy. 2020;75(8):2073–7.
Roth-Walter F, Jensen-Jarolim E, Gomez-Casado C, Diaz-Perales A, Pacios L, Singer J. Method and means for diagnosing and treating allergy EP14150965A·2014-01-13.
Bartosik T, Afify SM, Petje LM, Bastl M, Berger U, Hufnagl K, et al. Dietary supplementation with a new immune tablet ameliorates human symptom load during birch pollen season: lower B‑cell numbers yet with higher intracellular iron. EAACI Congress. 2020. p. Abstract 1213.
Pfaar O, Calderon M, Andrews C, Angjeli E, Bergmann K, Bonlokke J. Allergen exposure chambers: harmonizing current concepts and projecting the needs for the future—an EAACI Position Paper. Allergy. 2017;72(7):1035–42.
Pfaar O, Zieglmayer P. Allergen exposure chambers: implementation in clinical trials in allergen immunotherapy. Clin Transl Allergy. 2020;10:33.
Boelke G, Berger U, Bergmann K‑C, Bindslev-Jensen C, Bousquet J, Gildemeister J, et al. Peak nasal inspiratory flow as outcome for provocation studies in allergen exposure chambers: a GA2LEN study. Clin Transl Allergy. 2017;7:33.
Voegler T, Goergen F, Bergmann K, Boelke G, Salame J, Gildemeister J. Technical specification of the Global Allergy and Asthma European Network (GA2LEN) chamber: a novel mobile allergen exposure chamber. Allergo J Int. 2017;26:287–94.
Zuberbier T, Abelson MB, Akdis CA, Bachert C, Berger U, Bindslev-Jensen C, et al. Validation of the Global Allergy and Asthma European Network (GA(2)LEN) chamber for trials in allergy: innovation of a mobile allergen exposure chamber. J Allergy Clin Immunol. 2017;139(4):1158–66.
Tovey ER, Liu-Brennan D, Garden FL, Oliver BG, Perzanowski MS, Marks GB. Time-based measurement of personal mite allergen bioaerosol exposure over 24 hour periods. PLoS ONE. 2016;11(5):e153414.
Team RC. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2019.
Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme. J Stat Soft. 2015;67(1):1–48.
Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biometrical J. 2008;50(3):346–63.
Bergmann KC, Krause L, Hiller J, Becker S, Kugler S, Tapparo M, et al. First evaluation of a symbiotic food supplement in an allergen exposure chamber in birch pollen allergic patients. World Allergy Organ J. 2021;14(1).
Pali-Schöll I, Bianchini R, Afify SM, Hofstetter G, Winkler S, Ahlers S, et al. Completing the hygiene hypothesis: bovine beta-lactoglobulin contributes to the allergy protective farm effect. 2020. Manuscript in preparation.
Roth-Walter F, Schmutz R, Mothes-Luksch N, Lemell P, Zieglmayer P, Zieglmayer R, et al. Clinical efficacy of sublingual immunotherapy is associated with restoration of steady-state serum lipocalin 2 after SLIT: a pilot study. World Allergy Organ J. 2018;11:21.
Fluckinger M, Merschak P, Hermann M, Haertle T, Redl B. Lipocalin-interacting-membrane-receptor (LIMR) mediates cellular internalization of beta-lactoglobulin. Biochim Biophys Acta. 2008;1778(1):342–7.
Mansouri A, Gueant J, Capiaumont J, Pelosi P, Nabet P, Haertle T. Plasma membrane receptor for beta-lactoglobulin and retinol-binding protein in murine hybridomas. Biofactors. 1998;7:287–98.
Sanchez-Borges M, Fernandez-Caldas E, Thomas WR, Chapman MD, Lee BW, Caraballo L, et al. International consensus (ICON) on: clinical consequences of mite hypersensitivity, a global problem. World Allergy Organ J. 2017;10(1):14.
Frew AJ, Pfaar O. Placebo effects in allergen immunotherapy: an experts’ opinion. Allergo J Int. 2018;27(6):162–6.
Pfaar O, Agache I, Bergmann KC, Bindslev-Jensen C, Bousquet J, Creticos PS, et al. Placebo effects in allergen immunotherapy—an EAACI Task Force Position Paper. Allergy. 2020. https://doi.org/10.1111/all.14331.
Pfaar O, Bachert C, Bufe A, Buhl R, Ebner C, Eng P, et al. Guideline on allergen-specific immunotherapy in IgE-mediated allergic diseases: S2k Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Society for Pediatric Allergy and Environmental Medicine (GPA), the Medical Association of German Allergologists (AeDA), the Austrian Society for Allergy and Immunology (OGAI), the Swiss Society for Allergy and Immunology (SGAI), the German Society of Dermatology (DDG), the German Society of Oto- Rhino-Laryngology, Head and Neck Surgery (DGHNO-KHC), the German Society of Pediatrics and Adolescent Medicine (DGKJ), the Society for Pediatric Pneumology (GPP), the German Respiratory Society (DGP), the German Association of ENT Surgeons (BV-HNO), the Professional Federation of Paediatricians and Youth Doctors (BVKJ), the Federal Association of Pulmonologists (BDP) and the German Dermatologists Association (BVDD). Allergo J Int. 2014;23(8):282–319.
Acknowledgements
We thank Dr. A. Narkus, MC Narkus GmbH Medical Consulting & Services for medical writing service and Dr. Matthew Heath for critical review.
Funding
This study was funded by Bencard Allergie GmbH, Leopoldstr. 175, 80804 München, and in part by Biomedical International R + D GmbH.
Author information
Authors and Affiliations
Contributions
KCB, MFK, FRW, EJJ, and SG designed the study. Patient enrollment and follow-up were done by KCB, SB and SK. Study conduction and study quality control were done by KCB and TZ. Data acquisition was performed by KCB, SB and SK. LK performed the statistical analysis. KCB, AG, JR, WB, VBO and SG evaluated and interpreted the data. AG prepared the manuscript in collaboration with a medical writing service. All authors provided critical feedback, reviewed and approved the final version of the manuscript and its submission.
Corresponding author
Ethics declarations
Conflict of interest
A. Graessel, J. Raab, W. Banghard, V.B. Ott, M.F. Kramer and S. Guethoff are employees of Allergy Therapeutics/Bencard Allergie GmbH. K.-C. Bergmann reports personal fees for Lectures: ALK, AstraZeneca, Allergopharma, Almirall, Bencard, Chiesi, GSK, HAL, LETI, Lofarma, Mundipharma, Novartis, Sanofi. Non-financial support as Chair of German Pollen Information Service Foundation, personal fees and non-financial support from Consultant physician for ECARF, personal fees and non-financial support from Advisory Board member of AstraZeneca, ECARF, GSK, Robert-Koch-Institute Berlin (Vice chairman Public Health), Sanofi, outside the submitted work. E. Jensen-Jarolim and F. Roth-Walter are inventors on the immunoBON® patent Patent EP 2 894 478 B1, owned by Biomedical International R + D, Vienna, Austria, of which E. Jensen-Jarolim is shareholder. E. Jensen-Jarolim received honoraria for presentations from Allergy Therapeutics, Allergopharma, Bencard, Meda, Roxall, ThermoFisher, and Vifor, and is consultant or in Advisory Boards for Allergy Therapeutics, Vifor Pharma, Sanofi, previously for MediGene, Germany, Novartis, and Dr. Schär (ceased). T. Zuberbier reports personal fees from Bayer Health Care, FAES, Novartis, Henkel, and AstraZeneca. He received fees for talks and personal fees from AbbVie, ALK, Almirall, Astellas, Bayer Health Care Fee, Bencard Allergie GmbH, Berlin Chemie, HAL, Leti, Meda, Menarini, Merck, MSD, Novartis, Pfizer, Sanofi, Stallergenes, Takeda, Teva, UCB Henkel, Kryolan, L’Oréal outside the submitted work. S. Becker, L. Krause and S. Kugler declare that they have no competing interests.
Ethical standards
The study protocol was approved by the Ethics Committee of the Charité Berlin (EA1/314/19). All participants received detailed information from the supervising physician and provided their written informed consent to participate. They also agreed to the processing and storage of their data in accordance with the General Data Protection Regulation. The study was conducted in accordance with the Declaration of Helsinki and in compliance with all federal, regional and local requirements. Informed consent was obtained from all participants. All data provided were pseudonymised to protect the privacy of the patients who participated in the study as mandated by the applicable laws and regulations.
Additional information
Availability of data and material
The datasets generated during and/or analysed during the current study are not publicly available. Bencard Allergie GmbH is committed to sharing access to patient-level data, and supporting clinical documents from related studies with qualified external researchers. Requests for these data sets are reviewed and approved by an independent review panel on the basis of scientific merit.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bergmann, KC., Graessel, A., Raab, J. et al. Targeted micronutrition via holo-BLG based on the farm effect in house dust mite allergic rhinoconjunctivitis patients – first evaluation in a standardized allergen exposure chamber. Allergo J Int 30, 141–149 (2021). https://doi.org/10.1007/s40629-021-00163-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40629-021-00163-9