Abstract
Background
Coronavirus disease 2019 (COVID-19) has been recognised as a risk factor for acute kidney injury (AKI). Our aim was to investigate the risk factors contributing to hospitalised and outpatient paediatric COVID-19-associated AKI.
Methods
A retrospective observational study was conducted on patients aged 1 month to 18 years with diagnosed COVID-19-associated AKI applied to a tertiary paediatric referral hospital between March 1, 2020 and March 1, 2022.
Results
A total of 6683 patients were evaluated and 486 patients were included in the study. Acute kidney injury was observed in 3.7% of outpatients and 23.9% of hospitalised patients. Multivariate logistic regression analysis showed that, on admission, a history of contact with a COVID-19 positive person (p < 0.001), age below 12 months (p = 0.004), presence of comorbidities (p < 0.001), abdominal pain (p = 0.008), anorexia (p = 0.003), dyspnoea (p = 0.005), higher lactate dehydrogenase values (p = 0.004), neutrophilia (p < 0.001), higher neutrophil-to-lymphocyte ratio (NLR) (p = 0.003), higher white blood cell counts (p = 0.006), elevated C-reactive protein (CRP) levels (p = 0.002), anaemia (p = 0.015), hypoalbuminaemia (p < 0.001), hyperglycaemia (p = 0.006), and presence of proteinuria (p = 0.003) were independent predictors of AKI. Higher rates of hospitalisation (p < 0.001) and admission to the paediatric intensive care unit (PICU) (p < 0.001), longer length of hospitalisation (p < 0.001), and greater need for mechanical ventilation (p < 0.001) were associated with AKI.
Conclusions
This study reveals that not only hospitalised children, but also paediatric patients are at risk for AKI. The presence of comorbidities, abdominal pain, anorexia, dyspnoea, anaemia, inflammation, hypoalbuminaemia, proteinuria and history of contact with a COVID-19 positive person were the main risk factors for AKI. COVID-19-associated AKI was associated with worse outcomes.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), known as the cause of coronavirus disease 2019 (COVID-19), was first identified in Wuhan, China in December 2019 [1]. The presentation of COVID-19 varies widely, ranging from an asymptomatic response, to isolated respiratory and/or gastrointestinal symptoms, or to the development of multiorgan systemic disease [2]. There is evidence that the kidney is a target organ for SARS-CoV-2, and that the highly expressed angiotensin-converting enzyme 2 receptor on renal tubular cells and podocytes are required for SARS [3]. Acute kidney injury (AKI) is associated with COVID-19 severity and outcome, and seems to be a risk factor for adverse outcomes among both outpatients and hospitalised children [1, 4]. The reported incidence of AKI in children with COVID-19 ranges from 0 to 70% [5]. There are limited data regarding AKI-associated risk factors and potential outcomes in children with COVID-19. It has been reported that exposure to nephrotoxic drugs, high C-reactive protein (CRP), increased white blood cell (WBC) counts, and low serum albumin levels may be risk factors for AKI in children with COVID-19 [2, 3, 6]. The inflammatory state itself may be a manifestation of COVİD-19 infection that predisposes to renal hypoperfusion and AKI. Despite these data, the risk factors and potential outcomes of AKI associated with COVID-19 in paediatric patients are not well defined. Estimation of the frequency of AKI and identification of risk factors in children with COVID-19 remain essential to guide the treatment of these patients. Although risk factors for AKI have been reported in hospitalised patients, the factors for identifying patients at high risk of AKI in outpatient settings are unknown and may differ from those recorded in hospitalised children.
We aimed to describe risk factors for AKI in both hospitalised and outpatient paediatric COVID-19 patients and determine if previously known risk factors contribute to AKI associated with COVID-19.
Methods
Patients and data collection
In this retrospective observational study, 6683 children with a polymerase chain reaction (PCR)-confirmed diagnosis of COVID-19 infection admitted to the COVID-19 polyclinic or emergency department of a tertiary paediatric referral hospital from March 1, 2020 to March 1, 2022 were selected. Among them, serum creatinine values were evaluated in 486 patients due to their clinical conditions, and they were included in the study. Data regarding demographics and clinical characteristics, laboratory findings, radiologic investigations, hospital stay, and medication use during hospitalisation were collected from the hospital electronic records. The patients were divided into two groups i.e., AKI and non-AKI.
Baseline and peak serum creatinine levels, baseline and the lowest estimated glomerular filtration rate (eGFR), kidney and liver function tests, electrolytes, serum levels of blood urea nitrogen (BUN), serum albumin, lactate dehydrogenase (LDH), complete blood count, the highest CRP, ferritin, creatine kinase (CK), d-dimer, troponin values, and urine dipstick tests, urine output (UOP), urine culture and blood culture were recorded (if available). Echocardiography (ECHO), chest X-ray and computed tomography were performed when indicated.
Inclusion criteria
The inclusion criteria were: (1) patients aged between 1 month to 18 years with a confirmed diagnosis of COVID-19 detected by real-time reverse transcriptase-polymerase chain reaction (RT-PCR) assay of oropharyngeal and nasopharyngeal swab samples; (2) patients whose serum creatinine levels and eGFRs were evaluated during the follow-up period.
Exclusion criteria
Patients older than 18 years of age and neonates (aged 0–28 days at presentation) were excluded from the study. We also excluded patients with a known pre-existing history of chronic kidney disease and end-stage kidney disease (ESKD), and patients whose serum creatinine levels and medical history were unknown.
Definitions
COVID-19 infection was defined as real-time RT-PCR-positive oropharyngeal and nasopharyngeal swabs. An infected person (testing positive for COVID-19 via PCR testing) was defined as being COVID-19 positive.
The modified Schwartz formula to determine eGFR was used [7].
Using the “Kidney Disease: Improving Global Outcomes” (KDIGO) guidelines, AKI was defined as an increase of serum creatinine by ≥ 0.3 mg/dl within 48 h; or known/presumed increase from the baseline creatinine by ≥ 50% within the prior 7 days; or a urine output < 0.5 mL/kg/hours for 6 h [8]. Staging of AKI was defined according to KDIGO guidelines. For outpatients whose urine output could not be reliably collected due to difficulty of data capture of urine output, only an increase in serum creatinine was used as the definition of AKI.
Baseline serum creatinine was defined as the lowest serum creatinine level within the previous 3 months before admission [9]. In addition, in patients without previous creatinine values, the lowest creatinine value obtained at COVID-19 disease presentation was defined as the baseline value.
Proteinuria was defined as protein/creatinine ratio greater than 0.2 mg/mg in spot urine test, or higher than 4 mg/m2/hour in 24-h urine, or ≥ 1 + protein on urine dipstick test [10].
The absolute neutrophil count was divided by the absolute lymphocyte count to calculate the neutrophil-to-lymphocyte ratio (NLR).
Outcomes
The influence of AKI on clinical course and outcomes was also taken into consideration. The outcomes included were hospitalisation, length of hospitalisation, need for kidney replacement therapy (KRT), mechanical ventilation, admission to the paediatric intensive care unit (PICU), and mortality.
Statistical analysis
All analyses were performed using the statistical software package (SPSS) for Windows (version 21.0). Continuous data are expressed as means ± standard deviations, and categorical data are given as frequencies and percentages. Non-normally distributed data were expressed as median with data range (minimum to maximum). Logistic regression analysis was performed to identify predictive factors for increased risk of AKI associated with COVID-19. Data were expressed as odds ratios (OR) with 95% confidence intervals (CI). Between-group comparisons for continuous and categorical variables were performed by the Mann–Whitney U test and the chi-square test, respectively. Multivariate analysis was performed with parameters found to be significant in the evaluation of univariate analysis, and predictive values of variables were calculated to assess the relationship between AKI and outcomes. A p-value less than 0.05 was accepted as statistically significant.
Results
Incidence of AKI
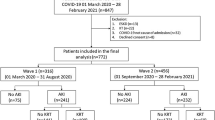
A total of 6683 patients admitted to our hospital with a PCR-confirmed diagnosis of COVID-19 infection during the study period were evaluated, and 486 children whose serum creatinine levels were assessed were included in the study (Fig. 1). Acute kidney injury was observed in 10 (3.7%) outpatients and in 51 (23.9%) hospitalised patients.
Clinical characteristics of patients
The median age of the patients was 31.7 (range 1.0–215.8) months. Patients with AKI were older than those without [55.2 (range 1.0–207.1) vs. 26.9 (range 1.0–215.8) months]. The majority of patients (63.6%) had had contact with a person who was COVID-19 positive. Fever (n = 316, 65.0%) was the most common presenting symptom of COVID-19-positive patients, while some patients (n = 49, 10.1%) were asymptomatic. Comorbidity was present in 28.6% of the patients, and patients with AKI had more comorbidities compared to those without AKI (p < 0.001) (Fig. 2; Table 1).
Clinical course and outcomes
Acute kidney injury was significantly associated with a greater likelihood of requiring hospitalisation (83.6% vs. 38.1%, p < 0.001). Acute kidney injury was significantly associated with admission to the PICU (26.2% vs. 2.4%, p < 0.001), need for mechanical ventilation (23.0% vs. 1.9%, p < 0.001), and mortality (9.8% vs. 0.2%, p < 0.001) in paediatric patients with COVID-19. Demographic data and admission characteristics of paediatric patients with COVID-19 with/without AKI diagnosis are presented in Table 1.
Patients with AKI had significantly higher levels of serum glucose, alkaline phosphatase, lactate dehydrogenase, ferritin, CRP, white blood cell and neutrophil count compared to patients without AKI. In addition, the levels of serum albumin, calcium and haemoglobin on admission were significantly lower in patients who developed AKI (Table 2). Patients with AKI had significantly more proteinuria when compared with patients without AKI (10.5% vs. 1.8%, p = 0.022), however, proteinuria was not associated with fever symptoms (p = 0.261). Furthermore, proteinuria was not detected in patients younger than 24 months of age.
Severity of AKI
Patients with AKI were staged according to the 2012 KDIGO guidelines and were stratified as follows: stage I, n = 47 (77.1%); stage II, n = 8 (13.1%); and stage III, n = 6 (9.8%). Three patients with AKI stage III required KRT. The medications administered to patients during hospitalisation included nephrotoxic drugs, bronchodilators, antihypertensive agents, antiepileptics, corticosteroids, intravenous immunoglobulin, vasopressors, and anticoagulant or antiplatelet drugs. The proportion of patients receiving vasopressor drugs in the AKI group was significantly higher than in the non-AKI group (9 versus 1, p < 0.001). Those treated with corticosteroids and antivirals were higher in the AKI group than in the non-AKI group (21 versus 16, and 11 versus 5, respectively [p > 0.05]). Also, there was no significant difference between AKI stage and use of medications during hospitalisation (p > 0.05), except for vasopressor drugs. In addition, no statistically significant relationship was found between AKI stage and the need for hospitalisation, length of hospital stay, and admission to the PICU and comorbidities (p > 0.05). However, a greater percentage of stage III AKI patients had higher mortality rates [stage I: 4.3% vs. stage II: 0.0% vs. stage III: 66.7%; p < 0.001] and need for mechanical ventilation [stage I: 19.1% vs. stage II: 12.5% vs. stage III: 66.7%; p = 0.025].
Risk factors for AKI
Multivariate logistic regression analysis revealed that on admission a history of contact with a COVID-19 positive person (p < 0.001), age younger than 12 months (p = 0.004), presence of underlying diseases (p < 0.001), abdominal pain (p = 0.008), anorexia (p = 0.003), and dyspnoea (p = 0.005) were significantly predictive risk factors for AKI. Also, multivariate analysis showed that neutrophilia (p < 0.001), anaemia (p = 0.015), hypoalbuminaemia (p < 0.001), hyperglycaemia (p = 0.006), and presence of proteinuria (p = 0.003) were independent predictors of AKI. The levels of white blood cells (p = 0.006), CRP (p = 0.002), NLR (p = 0.003), and lactate dehydrogenase (p = 0.004) were significantly higher in patients with AKI compared to those without AKI (Table 3).
Higher rates of hospitalisation (OR 7.37, 95% CI, 3.7–14.5, p < 0.001) and admission to the PICU (OR 14.72, 95% CI, 6.3–34.3, p < 0.001), longer length of hospitalisation (OR 1.20, 95% CI, 1.1–1.3, p < 0.001), use of more medications during hospitalisation (OR 18.94, 95% CI, 2.5–138.4, p = 0.004), and greater need for mechanical ventilation (OR 15.48, 95% CI, 6.1–38.8, p < 0.001) were associated with AKI (Table 3).
Discussion
There have been relatively few studies focusing on AKI in children [8, 11]. Although a high incidence of AKI has been reported as a prominent feature in adult COVID-19 infection [2, 12, 13], a lower incidence of AKI in children has been shown [8, 11, 14]. One of the studies reporting a lower rate of AKI was a retrospective study conducted on 238 paediatric hospitalised patients and outpatients in China, in whom AKI was present in only 3 patients (1.2%) [14], much lower than that reported in the United States (24%) [11], and in the United Kingdom (29%) [15]. A meta-analysis reported that 44% of critically ill children (a total of 106) developed AKI according to the KDIGO serum creatinine criteria [16]. The discrepancies in the frequency of COVID-19-associated AKI among countries may be explained by different inclusion criteria (age, severity of illness, hospitalised or outpatients, intensive care admissions, and different definitions of AKI), regional differences, and different demographic characteristics of the study populations [17].
Although conflicting results have been reported, comorbidities generally increase the severity of AKI in patients with COVID-19. The presence of comorbidities, such as respiratory diseases, cardiac diseases, haematological disorders, and malignancies are likewise important in children with COVID-19 who developed AKI [6, 19,20,21]. A systematic review reported the rate of underlying comorbid disease in children with AKI as 26.7% [21]. Our patients showed a similar prevalence of comorbidities (28.6%) and 52.5% of patients with AKI had underlying diseases (Table 1). A higher prevalence of comorbidities has been reported in adult patients with COVID-19-associated AKI [18]. By comparison with adults, paediatric patients had a lower prevalence of, and different comorbidities. COVID-19 and AKI may lead to worsening of comorbid disease [1]. However, we observed similar comorbidities in COVID-19 patients with and without AKI (p > 0.05). Providing early treatment may be an explanation for our positive results. A child with a comorbid disease is usually overprotected by parents and may have been brought to medical attention earlier than others, which might have reduced kidney injury by allowing timely medical interventions.
Various mechanisms are possibly involved in kidney injury during COVİD-19 infection, including direct infection of the kidney parenchyma by the virus, endothelial injury, ischaemic acute tubular necrosis, Renin–Angiotensin–Aldosterone System imbalance, microthrombosis, increased vascular permeability, and volume depletion, as well as the development of kidney damage secondary to haemodynamic instability, inflammatory cytokines, and therapeutic approaches (nephrotoxic medications, mechanical ventilation) [3, 6, 11, 22]. Studies have shown that the aetiologies of AKI in patients with COVID-19 seem to be multifactorial, which is also consistent with our results. A meta-analysis reported that fever and gastrointestinal symptoms, were more common in children with AKI associated with COVID-19 [3, 6, 11]. The present study showed that the main risk factors for the development of AKI were older age and the presence of abdominal pain, dyspnoea, fever, nasal congestion or anorexia as initial symptoms. All of these symptoms may lead to decreased fluid intake and increased fluid loss, which are triggering factors for the development of AKI in patients. Young children, and especially infants, with COVID-19 are more prone to develop gastrointestinal symptoms of COVID-19, and dehydration significantly increases the risk of AKI [14]. Haemodynamic monitoring with appropriate fluid management and vasopressor drugs are required to provide adequate renal perfusion in AKI patients. In our study, the need for vasopressors was higher in AKI patients (p < 0.001). We observed that AKI was associated with abdominal pain, but not nausea, vomiting and diarrhoea in our patients. These unexpected results can be explained by the fact that the amount of vomiting and diarrhoea in our patients was not high enough to cause fluid loss. Due to the retrospective nature of our study, the frequency and amount of both vomiting and diarrhoea are unknown.
It has been suggested that the systemic pro-inflammatory response may play an important role in the development of AKI in COVID-19 patients [6, 11, 17, 22]. Similarly, in our study, increased WBC and neutrophil count, increased NLR, increased serum levels of CRP and ferritin, and decreased serum albumin levels were found to be statistically significant for AKI patients compared to patients without AKI. The lower serum albumin and consequently lower serum calcium levels we detected in our patients with AKI may be explained by increased capillary permeability secondary to systemic inflammation.
Proteinuria was detected in 10.5% of our AKI patients by urinalysis and AKI was significantly associated with the presence of proteinuria (p = 0.022). Although it is known that febrile episodes can cause transient proteinuria in children, we could not show any difference in proteinuria between the patients with or without fever (p = 0.261). On the other hand, unlike other infections that cause transient proteinuria [10], COVID-19 may act directly on the kidney through multiple mechanisms [3, 22].
The present study confirmed that AKI was a risk factor for admission to hospital and the paediatric ICU, and that patients with AKI were more likely to require longer hospital stays. The association between AKI and admission to the ICU, and hospital length of stay has also been reported by other paediatric studies [6, 11, 19]. This association may be explained by haemodynamic instability and a diagnosis of AKI. In the present study, we did not find a statistically significant relationship between AKI stage and hospitalisation requirement, admission to the ICU and length of hospital stay. It may be speculated that the medical care or attention given to patients with COVID-19 could positively influence the outcomes of patients who receive an early diagnosis of AKI. It may also reflect the early management of transient renal ischaemia, which can cause an increase in serum creatinine. This may also be explained by our small sample size.
Mechanical ventilation and nephrotoxic medications were reported as risk factors for AKI development in children with COVİD-19 in a retrospective chart review of children [6, 11, 19].
In the present study, most AKI patients were critically ill and required mechanical ventilation and multiple medications. These results were also consistent with already reported AKI findings in seriously ill children [23]. In this study, there was no significant difference between AKI and use of medications, except for vasopressor drugs. Nephrotoxic medications (i.e., nonsteroidal anti-inflammatory drugs, aminoglycosides, trimethoprim/sulfamethoxazole, vancomycin and acyclovir) are commonly used in most cases. Early identification of AKI and supportive therapy may have helped in the rapid recovery of patients with COVID-19 and AKI, and may also have the potential to decrease the use of nephrotoxic medications. Efficient anti-inflammatory therapy makes prompt renal recovery possible. A beneficial effect of anticoagulant therapy on reducing COVID-19 mortality has been reported [24].
Acute kidney injury has a significant effect on mortality in COVID-19 patients, and higher AKI stage is associated with greater mortality. COVID-19 patients with AKI had a significantly higher risk of mortality compared to COVID-19 patients without AKI [11, 19, 24]. We found a mortality rate of 1.4% in COVID-19-positive patients. This was lower than the AKI mortality rate of 3.3% reported in the literature regarding patients with COVID-19 [25].
Our study has several limitations. Firstly, a baseline serum creatinine measurement was unavailable as most of our COVID-19 patients were previously healthy. Also, given the observational nature of this study, information on serum creatinine levels could not be retrieved as it was not usually evaluated in outpatients. Therefore, we cannot generalise our findings for AKI outpatients. The baseline serum creatinine level was unknown for some patients, which may have caused under-reporting of AKI diagnoses. The second limitation of our study is that only PCR-positive confirmed COVID-19 patients were included, and the sensitivity reported in clinical practice ranges from 42 to 83%, depending on the patient’s symptom duration and viral load, and the quality of the test sample. Thirdly, we may have missed asymptomatic or mildly symptomatic patients treated at home. The lack of examination on tubular proteinuria is a further limitation of this study. Despite these limitations, this study draws its strength from the inclusion of AKI patients in both outpatient and inpatient settings [26].
In conclusion, AKI was diagnosed in 3.7% of outpatients and in 23.9% of hospitalised paediatric patients with COVID-19. Our findings highlight that children with higher levels of inflammation markers, particularly a higher NLR and lower serum albumin levels on admission may be more prone to develop COVID-19-associated AKI. In the present study, COVID-19 patients complicated by AKI had more co-morbidities, lower haemoglobin levels, and higher serum glucose and lactate dehydrogenase levels on admission. We also showed that paediatric COVID-19 patients with AKI requiring hospitalisation had more admissions to the PICU, longer hospital stays, and greater need for mechanical ventilation compared to those without AKI. Patients with COVID-19 AKI had a higher risk of mortality than patients with COVID-19 without AKI. Given the link between COVID-19-associated AKI and both mortality and poor prognosis, the outcome of this study suggests that renal function in children should be closely monitored.
References
Nadim MK, Forni LG, Mehta RL, Connor MJ Jr, Liu KD, Ostermann M, Rimmelé T, Zarbock A, Bell S, Bihorac A, Cantaluppi V, Hoste E, Husain-Syed F, Germain MJ, Goldstein SL, Gupta S, Joannidis M, Kashani K, Koyner JL, Legrand M, Lumlertgul N, Mohan S, Pannu N, Peng Z, Perez-Fernandez XL, Pickkers P, Prowle J, Reis T, Srisawat N, Tolwani A, Vijayan A, Villa G, Yang L, Ronco C, Kellum JA (2020) COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat Rev Nephrol 16:747–764. https://doi.org/10.1038/s41581-020-00356-5
Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, Holden KA, Read JM, Dondelinger F, Carson G, Merson L, Lee J, Plotkin D, Halpin Sigfrid L, Jackson S, Gamble C, Horby C, Nguyen-Van-Tam PW, Ho JS, Russell A, Dunning CD, Openshaw J, Baillie PJ (2020) Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ 369:m1985. https://doi.org/10.1136/bmj.m1985
Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, Yi F, Yang HC, Fogo AB, Nie X, Zhang C (2020) Renal histopathological analysis of 26 post-mortem findings of patients with COVID-19 in China. Kidney Int 98:219–227. https://doi.org/10.1016/j.kint.2020.04.003
Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G (2020) Kidney disease is associated with in-hospital mortality of patients with COVID-19. Kidney Int. 97:829–838. https://doi.org/10.1016/j.kint.2020.03.005
Chadha V, Warady BA (2021) COVID-19 and the multisystem inflammatory syndrome in children: how vulnerable are the kidneys? Kidney Int 100:16–19. https://doi.org/10.1016/j.kint.2021.03.043
Basalely A, Gurusinghe S, Schneider J, Shah SS, Siegel LB, Pollack G, Singer P, Castellanos-Reyes LJ, Fishbane S, Jhaveri KD, Mitchell E, Merchant K, Capone C, Gefen AM, Steinberg J, Sethna CB (2021) Acute kidney injury in pediatric patients hospitalized with acute COVID-19 and multisystem inflammatory syndrome in children associated with COVID-19. Kidney Int 100:138–145. https://doi.org/10.1016/j.kint.2021.02.026
Schwartz GJ, Work DF (2009) Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol 4:1832–43. https://doi.org/10.2215/CJN.01640309
Khwaja A (2012) KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 120:c179-84. https://doi.org/10.1159/000339789
Ciccia E, Devarajan P (2017) Pediatric acute kidney injury: prevalence, impact and management challenges. Int J Nephrol Renovasc Dis 10:77–84. https://doi.org/10.2147/IJNRD.S103785
Yap K, Lau PYW (2016) Hematuria and proteinuria. In: Geary DF, Schaefer F (eds). Pediatr Kidney Dis, 2nd edn. Berlin, pp 391–418
Grewal MK, Gregory MJ, Jain A, Mohammad D, Cashen K, Ang JY, Thomas RL, Valentini RP (2021) Acute kidney injury in pediatric acute SARS-CoV-2 infection and Multisystem Inflammatory Syndrome in Children (MIS-C): is there a difference? Front Pediatr. 9:692256. https://doi.org/10.3389/fped.2021.692256
Kolhe NV, Fluck RJ, Selby NM, Taal MW (2020) Acute kidney injury associated with COVID-19: a retrospective cohort study. PLoS Med 17:e1003406. https://doi.org/10.1371/journal.pmed.1003406
Cheng Y, Luo R, Wang X, Wang K, Zhang N, Zhang M, Wang Z, Dong L, Li J, Zeng R, Yao Y, Ge S, Xu G (2020) The incidence, risk factors, and prognosis of acute kidney injury in adult patients with Coronavirus disease 2019. Clin J Am Soc Nephrol 15:1394–1402. https://doi.org/10.2215/CJN.04650420
Wang X, Chen X, Tang F, Luo W, Fang J, Chang Q et al (2021) Be aware of acute kidney injury in critically ill children with COVID19. Pediatr Nephrol 36:163–169. https://doi.org/10.1007/s00467-020-04715-z
Stewart DJ, Hartley JC, Johnson M, Marks SD, du Pré P, Stojanovic J (2020) Renal dysfunction in hospitalised children with COVID-19. Lancet Child Adolesc Health 4:e28–e29. https://doi.org/10.1016/S2352-4642(20)30178-4
Bjornstad EC, Krallman KA, Askenazi D, Zappitelli M, Goldstein SL, Basu RK (2021) SPARC investigators. preliminary assessment of acute kidney injury in critically Ill children associated with SARS-CoV-2 infection: a multicenter cross-sectional analysis. Clin J Am Soc Nephrol 16:446–448. https://doi.org/10.2215/CJN.11470720
Saygili S, Canpolat N, Cicek RY, Agbas A, Yilmaz EK, Sakalli AAK, Aygun D, Akkoc G, Demirbas KC, Konukoglu D, Cokugras H, Caliskan S, Sever L (2022) Clinical and subclinical acute kidney injury in children with mild-to-moderate COVID-19. Pediatr Res 9:1–7. https://doi.org/10.1038/s41390-022-02124-6
Arikan H, Ozturk S, Tokgoz B, Dursun B, Seyahi N, Trabulus S, Islam M, Ayar Y, Gorgulu N, Karadag S, Gok M, Akcali E, Bora F, Aydın Z, Altun E, Ahbap E, Polat M, Soypacacı Z, Oguz EG, Koyuncu S, Colak H, Sahin İ, Dolarslan ME, Helvacı O, Kurultak I, Eren Z, Dheir H, Ogutmen MB, Taymez DG, Genek DG, Ozkurt S, Bakır EA, Yuksel E, Sahutoglu T, Oto OA, Boz G, Sengul E, Kara E, Tuglular S (2021) Characteristics and outcomes of acute kidney injury in hospitalized COVID-19 patients: a multicenter study by the Turkish society of nephrology. PLoS ONE 16:e0256023. https://doi.org/10.1371/journal.pone.0256023
Kari JA, Shalaby MA, Albanna AS, Alahmadi TS, Alherbish A, Alhasan KA (2021) Acute kidney injury in children with COVID-19: a retrospective study. BMC Nephrol 22:202. https://doi.org/10.1186/s12882-021-02389-9
Patel NA (2020) Pediatric COVID-19: systematic review of the literature. Am J Otolaryngol 41:102573. https://doi.org/10.1016/j.amjoto.2020.102573
Raina R, Mawby I, Chakraborty R, Sethi SK, Mathur K, Mahesh S, Forbes M (2022) Acute kidney injury in COVID-19 pediatric patients in North America: Analysis of the virtual pediatric systems data. PLoS One 17:e0266737. https://doi.org/10.1371/journal.pone.0266737
Ng JH, Bijol V, Sparks MA, Sise ME, Izzedine H, Jhaveri KD (2020) Pathophysiology and pathology of acute kidney injury in patients with COVID-19. Adv Chronic Kidney Dis 27:365–376. https://doi.org/10.1053/j.ackd.2020.09.003
Raina R, Chakraborty R, Mawby I, Agarwal N, Sethi S, Forbes M (2021) Critical analysis of acute kidney injury in pediatric COVID-19 patients in the intensive care unit. Pediatr Nephrol 36:2627–2638. https://doi.org/10.1007/s00467-021-05084-x
Sivaloganathan H, Ladikou EE, Chevassut T (2020) COVID-19 mortality in patients on anticoagulants and antiplatelet agents. Br J Haematol 190:192–195. https://doi.org/10.1111/bjh.16968
Tai CW, Gibbons K, Schibler A, Schlapbach LJ, Raman S (2022) Acute kidney injury: epidemiology and course in critically ill children. J Nephrol 35:559–565. https://doi.org/10.1007/s40620-021-01071-5
Tang X, Chen D, Yu S, Yang L, Mei C, ISN AKF 0 by 25 China Consortium (2017) Acute kidney injury burden in different clinical units: Data from nationwide survey in China. PLoS One 12(2):e0171202. https://doi.org/10.1371/journal.pone.0171202
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Ethical approval
This study was conducted in accordance with the principles of the Declaration of Helsinki.
Informed consent
Informed consent was waived due to the retrospective observational nature of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yazılıtaş, F., Çakıcı, E.K., Güngör, T. et al. Retrospective evaluation of acute kidney injury in paediatric COVID-19 patients: a tertiary referral hospital experience. J Nephrol (2024). https://doi.org/10.1007/s40620-024-01986-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40620-024-01986-9