Abstract
Effective vaccination strategies are of crucial importance to protecting patients who are vulnerable to infections, such as patients with chronic kidney disease. This is because the decreased efficiency of the immune system in chronic kidney disease impairs vaccine-induced immunisation. COVID-19 has prompted investigation of the immune response to SARS-CoV-2 vaccines in chronic kidney disease and in kidney transplant recipients in an effort to improve efficacy. The seroconversion rate after two vaccine doses is reduced, especially in kidney transplant recipients. Furthermore, although the seroconversion rate in chronic kidney disease patients is as high as in healthy subjects, anti-spike antibody titres are lower than in healthy vaccinated individuals, and these titres decrease rapidly. Although the vaccine-induced anti-spike antibody titre correlates with neutralising antibody levels and with protection against COVID-19, the protective prognostic significance of their titre is decreased due to the emergence of SARS-CoV-2 variants other than the Wuhan index virus against which the original vaccines were produced. Cellular immunity is also relevant, and because of cross-reactivity to the spike protein, epitopes of different viral variants confer protection against newly emerging variants of SARS-CoV-2. A multi-dose vaccination strategy is the most effective way to obtain a sufficient serological response. In kidney transplant recipients, a 5-week discontinuation period from antimetabolite drugs in concomitance with vaccine administration may also increase the vaccine’s efficacy. The newly acquired knowledge obtained from COVID-19 vaccination is of general interest for the success of other vaccinations in chronic kidney disease patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rapid circulation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the ensuing coronavirus disease 2019 (COVID-19) has posed extraordinary challenges for healthcare. The COVID-19 pandemic represented a challenge for nephrologists and their immunodeficient patients as well [1].
The absolute incidence of COVID-19 infection at the onset of the pandemic was fourfold higher in end-stage kidney disease patients undergoing haemodialysis (HD) compared with healthy people. Mortality in these patients at ages < 75 and > 75 years was 5.5- and 11-fold higher, respectively, than in the general population [2, 3]. In kidney transplant recipients (KTRs) compared with healthy people, the risk of contracting COVID-19 infection was 80-fold higher, and the risk of hospitalisation and death was 485-fold higher [4].
The risk of severe COVID-19 disease has now decreased thanks to the vaccination campaign, the development of effective antiviral drugs, anti-spike monoclonal antibodies, and also with the emergence of less severe variants of the virus. Indeed, the now dominant Omicron variant, while being more transmissible, is less virulent than previous SARS-CoV-2 variants [5].
Despite this, the risk in immunodeficient patients is still higher than in the general population. For instance, in a British cohort of patients with a miscellanea of secondary immunodeficiency forms, the hospitalisation rate, which was 75.8% before the introduction of vaccinations, decreased between January 2021 and March 2022 to 18.4%, but is still much higher than in the general population (2.95%) [6].
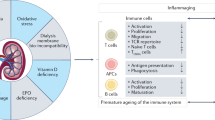
Although infections are the second most common cause of mortality in patients with chronic kidney disease (CKD), little is known about the mechanisms of immunodeficiency related to kidney failure. The uremic milieu, malnutrition, chronic inflammation, and specific medications contribute to altered T lymphocytes and antigen-presenting cells, with subsequently weakened humoral response. Haemodialysis has immunomodulatory effects [7]. Therefore, immunisation after vaccination may be impaired in patients with kidney failure, as observed for hepatitis B (HBV), influenza, and Streptococcus pneumoniae vaccinations [8]. Interestingly, a significantly lower response was detected in SARS-CoV-2-vaccinated patients who were HBV-poor responders [9] together with a correlation between anti-HBs and SARS-CoV-2 antibody titres in patients undergoing HD [10].
Because of the increased vulnerability to infection of patients with kidney failure, effective vaccination strategies are of the utmost importance to protect this population.
The generation of antibodies following exposure to an antigen is a key mechanism of vaccine-induced protection. They are in fact necessary to prevent viral entry into host cells. T cells also play an important role in humoral immunity by triggering B cell maturation and antibody production. However, their role is broader as they also have direct cytotoxic effects on infected cells, clearing the virus and reducing the viral load [11].
Modern vaccine platforms, based on mRNA carried by lipid nanoparticles, or on DNA delivered by a non-replicating recombinant adenoviral vector, have allowed the rapid launch -in less than a year- of the vaccination campaign to get the COVID-19 pandemic under control, a result never before achieved in the history of vaccinations. The mRNA/DNA contains the coding sequence of the target protein (the spike protein for COVID-19 vaccines). Additionally, mRNA vaccines are ideal for repeat boosters with formulations adapted to emerging virus variants. Like the adjuvant in vaccines that deliver protein subunits or attenuated viruses, mRNA- and DNA-based vaccines also stimulate innate immunity by providing the second signal for T-cell-driven adaptive immunity (see ref. 11 for a thorough description).
In COVID-19 vaccinations, the primary target of protective (neutralising) antibodies is the SARS-CoV-2 spike protein which is essential for viral entry into human cells. The gold standard for determining humoral immunization is serum neutralizing activity assays, but this is a cumbersome test that poses biosafety concerns and is available only in few laboratories. Since the serum IgG titre directed against the spike protein receptor binding domain correlates with the neutralizing capacity [12], its determination is routinely used to monitor the humoral response after vaccination assuming a specific threshold as an indicator of seroconversion. The SARS-CoV-2 specific T-cell response is usually measured by the commercially available interferon-gamma (IFN-γ) release assay, incubating post-vaccination whole blood with viral antigens that stimulate CD4 + and CD8 + T cells. After incubation, the production of IFN-γ in response to antigens is a measure of spike-specific T-cell response.
The COVID-19 pandemic has encouraged investigation of immune responses in kidney patients and evaluation of the most suitable vaccination schedules. Results may have general applications for immune response and vaccination strategies for kidney patients, not limited to COVID-19. This brief narrative review outlines the results obtained in this field (Table 1).
SARS-CoV-2 vaccination in CKD patients
Vaccination protects dialysis patients from severe COVID-19. In a real-world observational study in the United States, the use of mRNA SARS-CoV-2 vaccines (BNT162b2 and mRNA-1273) was associated with a greater than 70% decreased risk of contracting COVID-19 and lowered the risk of hospitalisation and death. Nearly all vaccinated patients generated antibodies [13]. In patients on chronic dialysis in Canada, two doses of the vaccine decreased the risk of COVID-19 infection and severe outcomes by 69% and 83%, respectively [14]. A French study reported that the vaccination coverage in HD patients was independently associated with protection from severe infection [15].
SARS-CoV-2 vaccination is also effective in KTRs; it decreases the risk of symptomatic COVID-19 infection by 80% [16].
Humoral immunity after SARS-CoV-2 vaccination
Studies have reported a high rate of immunisation in CKD patients who received SARS-CoV-2 vaccination, as evidenced by seroconversion and the presence of S-protein-reactive T cells, in line with individuals with normal kidney function. However, the antibody levels in this population were lower than in healthy people [17, 18].
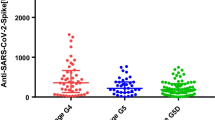
The SENCOVAC (Sociedad Espanola de Nefrologia COvid Vacunación) was a prospective, multicentre Spanish study that evaluated the kinetics of humoral response in four cohorts of patients with CKD: KTRs, HD patients, peritoneal dialysis patients, and non-dialysis CKD patients. Patients received an initial vaccination schedule (two injections); some received a third dose [19,20,21]. The study revealed that the anti-spike antibody titre decreased rapidly from month 3 to month 6 after the second injection, confirming the results of other studies [22, 23]. Furthermore, the data indicated that KTRs had lower response rates and anti-spike antibody titres than the other groups. A meta-analysis on the immunogenicity of SARS-CoV-2 vaccination in CKD patients reported that after two doses, only 26% of KTRs were seropositive compared with 84% of HD patients and 92% of peritoneal dialysis patients [24].
The SENCOVAC trial also reported that after immunisation, the loss of anti-spike antibodies in KTRs was more rapid than in other CKD patients. A third dose increased anti-spike antibody titres in each CKD group; however, 38% of the KTRs and 24% of the HD patients who had very low titres at month 3 (after the second dose) did not achieve an adequate humoral immune response. Conditions associated with a favourable response at 6 months included initial vaccination with the mRNA 1273 vaccine, a positive humoral response at month 3, having received a third dose, and not being a KTR. The ability of the mRNA 1273 vaccine to induce stronger seroconversion than BNT162b2 was confirmed by other studies in HD patients [18, 25] and in a meta-analysis of solid organ transplant patients (8 studies, 877 and 956 patients vaccinated with mRNA 1273 and BNT162b2, respectively) [26].
Similar results were reported in the Netherlands by the REnal patients COvid-19 VACcination Immune Response (RECOVAC IR) study [27], which also included a control group of healthy subjects. Humoral response 28 days after the initial vaccination schedule with the mRNA-1273 vaccine in CKD stage 4–5 and HD patients was not lower than in controls, whereas it was significantly lower in KTRs. This is a general problem in solid organ transplant recipients, as confirmed by a number of smaller single-centre studies, in which the seroconversion rate after two injections of an mRNA vaccine varied from 22 to 48% [28,29,30,31,32], and by a meta-analysis (29 studies; 11,713 KTRs) [33].
In summary, in all CKD patients and especially in KTRs, despite seroconversion after vaccination, anti-spike antibody titres are significantly lower than in healthy subjects, indicating a reduced level of protection against SARS-CoV-2.
Kinetics of humoral and cellular immunity after SARS-CoV-2 vaccination
Humoral immunity is only one aspect of the immunisation induced by vaccination. Protection against infections involves both humoral and cellular immunity; therefore, only the evaluation of both arms can provide an accurate understanding of the protection induced by vaccination.
The prospective observational study, Response of Hemodialyzed Patients to COVID-19 Vaccination (ROMANOV-II) [34], evaluated both arms of immunisation after second and third vaccine doses in patients on HD. After the second dose, the level of humoral response to vaccination in HD patients was lower than in healthy volunteers, except in those with prior history of COVID-19 infection, confirming the results of other studies [10, 28, 35]. The correlation between cellular and humoral immunity was strict; there was no cellular response in HD patients who did not develop humoral immunity, while there was cellular immunity in those who did.
After the third dose, anti-spike antibody titres increased, except in 46% of poor responders after the second dose, who continued to be poor responders. Multivariate analysis identified that seroconversion after the third dose was associated with the presence of spike-specific CD4 + T cells and detectable (although low) anti-spike antibodies after the second dose [34].
Other studies on HD patients obtained similar results regarding lower humoral response despite a high rate of immunisation, as evidenced by seroconversion and the presence of S-protein-reactive T cells that was comparable to patients with normal kidney function [17, 18].
The defective humoral response of HD patients is likely related to the adverse impact of uraemic toxins on the generation of spike-specific T follicular helper cells [36] and on memory B-cell differentiation [37]. The effect of uraemia can be inferred by the direct correlation in HD patients of Kt/V and anti-HBV surface antibody titres following HBV vaccination [38]. A positive correlation between Kt/V and the antibody response to SARS-CoV-2 vaccination has also been observed [10, 34].
As previously described in the SENCOVAC and RECOVAC IR studies, unlike the satisfactory rate of seroconversion in HD patients, the efficacy of SARS-COV-2 vaccination is suboptimal in KTRs. However, despite a low antibody response, the prevalence of KTRs with detectable spike-reactive CD4 + T cells is similar to that of non-CKD subjects [39]. This was confirmed by Cucchiari et al. in a study that investigated the kinetics of cellular and humoral immunisation after administering a three-dose vaccination schedule in KTRs [40]. They reported that the group with spike-reactive CD4+ T cells contributed to increasing humoral immunity from 61 to 90% after the third dose. IgG titres were higher in patients who maintained cellular immunity throughout the study [40]. Therefore, cellular immunity plays a key role in the development of a strong humoral response, which has also been reported in other studies [41, 42]. However, there is a small subset of KTRs who develop a cellular response, but in whom a humoral response is not detected [40].
The extent to which T cells offer protection is a matter of investigation [43]. Virus-specific T cells may play an important direct role in protection in addition to their indirect role in the development of antibody-producing cells [44]. In an animal model, CD4 + and CD8 + T cells were shown to play a key role in protection against COVID-19 [45]. T lymphocytes have a protective role in SARS-CoV-2 infections as is also evident from case studies of patients with X-linked or autosomal-recessive agammaglobulinaemia who recovered from infections while patients with T cell deficiency developed uncontrolled and fatal infections [46].
The pivotal role of T-cell immunity in COVID-19 infection was first underlined by Le Bert et al. [47]. They demonstrated that patients who had recovered from SARS in 2003 still had T-cells that were specific to epitopes in different SARS-CoV proteins 10 years after infection. These T cells exhibited strong cross-reactivity with the nucleocapsid protein of SARS-CoV-2. The presence of T-cell cross-reactivity in patients with a previous coronavirus infection (also reported by others [48]) could confer some immunoprotection against SARS‐CoV‐2, and thus explain the variable severity of COVID-19 clinical syndromes, which ranges from asymptomatic or mild influenza-like disease to severe pneumonia and acute respiratory distress syndrome. Moreover, they hypothesised that patients with SARS-CoV-2 infection could develop long-term T-cell immunity (Table 1).
Cross-reactivity of T cells induced by SARS-CoV-2 vaccination to the alpha and beta variant of SARS‐CoV‐2 has been demonstrated [49, 50], and this phenomenon may be crucial in preventing severe COVID-19 infection in patients with low antibody titres and with antibodies against the ‘index virus’. Brunelli et al. conducted a real-life study that supported the role of cellular immunity in protection against COVID-19 [51]. In their study, the clinical efficacy against COVID-19 of adenovirus vector-based Ad26.COV2.S and mRNA-based BNT162b2 vaccines in HD patients was identical, although the former induced an erratic humoral response.
How can response to vaccination against COVID-19 be improved in CKD patients?
As data suggest that a primary vaccine course of two doses does not offer adequate protection for HD patients, particularly KTRs, there is a need for more efficient vaccination strategies to decrease the risk of SARS-CoV-2 infection and its complications in CKD patients [52].
The addition of further doses was an obvious solution. Results from representative observational prospective studies and a double-blind randomised controlled trial show that extra-dose vaccination strategies are capable of increasing the seroconversion rate and the anti-spike protein antibody titre [21, 34, 40, 53], as well as the SARS-CoV-2–specific T-cell count [53]. However, a certain number of subjects still fail to respond, especially among KTRs [54].
Osmanodja et al. [55] evaluated the serological response in up to five vaccine doses in KTRs. They showed that the rate of humoral immunity increased with the number of vaccine administrations. In parallel, the cumulative humoral response increased from 19.1%, 42.0%, 74.2%, and 88.7% after two to five vaccine administrations, respectively. Their data support a multi-dose vaccination strategy in non-responders to achieve a sufficient serological response without relevant side effects.
However, the multi-dose vaccination strategy takes time to confer sufficient immunoprotection. More rapid methods to achieve protection for KTRs are necessary in view of emerging novel SARS-CoV-2 variants.
Interestingly, when a third dose was investigated in the general population in the COV-BOOST trial, one of the schedules among seven different vaccine regimens that produced the strongest reactivity after the initial two doses of ChAdOx1 or BNT162b2 was the heterologous schedule administering viral-vectored vaccines (ChAdOx1 or Ad26.COV2.S) after BNT162b2 [56]. With this background, studies on heterologous schedules of re-vaccination have been conducted in HD patients and KTRs.
Meijers et al. conducted a prospective study on HD patients investigating the humoral response to different SARS‐CoV‐2 vaccination schedules: homologous triple vaccination with BNT162b2 or mRNA-1272 versus heterologous triple vaccination (twice AZD1221 plus BNT162b2) [57]. While the anti-spike antibody titre significantly increased in all arms, there was no significant difference between the three regimens.
The RECOVAC consortium [58] investigated vaccination response in KTRs who did not seroconvert after two or even three doses of mRNA-1273 by comparing a control single-dose mRNA-1273 vaccination to a schedule of double vaccine doses, and one with heterologous vaccination with an adenovirus vector-based vaccine. All schedules increased the anti-spike antibody titre; however, there were no significant differences between the schedules.
Similar results were reported by Reindl-Schwaighofer et al. in a study investigating a heterologous schedule with an adenovirus vector-based vaccine in KTRs who were not immunoresponsive after the administration of two doses of mRNA vaccines [59].
However, the interpretation of the results obtained with heterologous vaccination schemes could in the future lead to different conclusions in the light of recent discoveries. Two studies have shown that in healthy people, repeated administrations of mRNA vaccines induce a long-term switch of anti-spike antibodies from the proinflammatory IgG1 and IgG3 subclasses to the non-inflammatory IgG4 subclass which has a weaker effect against SARS-CoV-2. In contrast, naïve individuals vaccinated with a heterologous schedule do not experience such long-term switch [60, 61].
In the cited RECOVAC study [58], the withdrawal of mycophenolic-based agents one week before and one week after a further dose of mRNA-1273 was investigated. The rationale for this type of strategy was based on the effect of immunosuppressive antimetabolites, such as mycophenolic drugs, on the maturation, differentiation, and proliferation of B cells and their inhibitory effect on T cells [62], which may impair immunisation after vaccines [63]. The negative impact on seroconversion of mycophenolic-based drugs in KTRs was observed after both SARS-CoV-2 [64, 65] and flu vaccinations [66]. However, in the RECOVAC trial, the temporary cessation of mycophenolic-based agents was not associated with any improvement in the rate of immunisation. It is possible that this was because of the short withdrawal period. In an observational study on KTRs who were seronegative and in whom mycophenolic acid was discontinued for 5 weeks (1 week before and 4 weeks after a fourth mRNA vaccine dose), humoral and cellular immunity was stimulated [67]. Other case series and small single-centre investigations [68, 69] support such a strategy, although concerns with this approach have been raised due to the risk of rejection. Belatacept has also been reported to impair the immune response to vaccination [70]. In contrast, triple immunosuppression with mTOR inhibitors improves humoral and cellular immunogenicity of the BNT162b2 mRNA vaccine in KTRs compared with triple immunosuppression with mycophenolate mofetil [71].
Another problem is related to the lower degree of protection afforded by the original vaccines due to the emergence of new viral variants whose spike proteins may differ in some epitope sequences used for vaccine preparation. It may be necessary to create new vaccine formulations containing mRNAs of emerging variants for annual administration of SARS-CoV-2 vaccines against persistent strains and/or seasonal variants.
Monitoring protective immune responses after SARS-CoV-2 vaccination: is it possible?
Studies have shown that the neutralising capacity correlates with protection against COVID-19 [72, 73] and that anti-spike protein IgG correlates with neutralising capacity [74]. The importance of the humoral response level is underlined by the association between low anti-spike protein antibody titre and breakthrough SARS-CoV-2 infections in vaccinated HD patients and KTRs [75,76,77]. Therefore, determination of the anti-spike protein antibody titre might be a reliable and simple tool for monitoring immunisation against SARS-CoV-2.
However, due to the emergence of novel SARS-CoV-2 variants, the neutralising antibody titre is decreased compared with the titre against the index virus (25.7–58.1-fold reduction for the Omicron variant in healthy vaccinated subjects) [78]. This may lead to waning of immunity post-vaccination and the occurrence of breakthrough infections, even in subjects who have developed a vaccine response against the index virus and the Omicron BA.4/BA.5 lineages of SARS-CoV-2 used for the formulation of the new adapted vaccine. Therefore, detection of anti-spike antibodies and titres are not sufficient to assess the overall protection against COVID-19. Furthermore, as previously observed, the cellular immune response contributes to viral clearance.
As discussed above, cross-reactivity of T cells induced by SARS-CoV-2 vaccination to the spike protein epitopes of the index virus SARS‐CoV‐2 and different variants [49, 50] likely contributes to protection against many COVID-19 variants in a broader way than with neutralising antibodies against the spike protein. However, the detection of antigen-specific T and B cells cannot be proposed for routine diagnostics because of the complexity and costs of the procedure.
Strategies for more robust protection against COVID-19 for CKD patients
On the basis of the presented results, the most efficient modality to improve vaccine response in CKD patients, especially those with poor response, would appear to be a multi-dose vaccination strategy. However, the uncertainty of this strategy is related to the hitherto unclear effect on SARS-CoV-2 neutralization of the multi-dose-induced switch of the anti-spike antibodies to the IgG4 subclass [60, 61]. It is not clear whether this switch has negative consequences on protection [79], especially in light of clinical experience demonstrating that repeated boosters with mRNA vaccines still confer protection [80].
The weakening of neutralising antibody titres against the index virus indicates the use of additional SARS-CoV-2 vaccinations against reformulated antigens to address novel variant lineages.
However, because of the broader protection offered by T-cell immunity against SARS-CoV-2, boosting with the present vaccines may also offer adequate protection [44].
Seroconversion rates in KTRs may increase with the temporary discontinuation of immunosuppressive antimetabolites, particularly mycophenolic-based agents, or, in KTRs treated with belatacept, with the momentary switch to tacrolimus or to an mTOR inhibitor. However, this measure should be conducted with caution because of the potential risk of anti-HLA antibody formation and rejection. Protection of KTRs from COVID-19 may also be obtained by immunisation of household contacts (the so-called ring vaccination) [81].
In non-responders, or in the immediate period following vaccination but before a sufficient anti-spike protein antibody titre is produced, monoclonal antibodies could improve outcomes if administered early in the course of COVID-19 infection; however, their effectiveness will change over time as novel virus variants emerge [82, 83]. Decreased efficacy of the monoclonal antibodies bamlanivimab with or without etesevimab, and casirivimab-imdevimab was observed with the emergence of SARS-CoV-2 beta, gamma and omicron. Currently, sotrovimab and bebtelovimab, and tixagevimab–cilgavimab can be used for the treatment or preexposure prophylaxis, respectively, of the predominant circulating SARS-CoV-2 Omicron infection [84].
As immunosuppressive agents can inhibit transcription/translation in viral vectored or mRNA vaccines, the use of spike protein subunit or inactivated virus vaccines could lead to some benefits. Unfortunately, initial results do not support this hypothesis. Indeed, a two-dose program of inactivated virus did not induce greater seroconversion than the schedule with BNT162b2 mRNA in KTRs under triple immunosuppression [85]. The use of adjuvants and the route of administration are known to influence the immune response to vaccination [86]. With reference to the latter, the intradermal administration of the influenza vaccine in KTRs has been shown to improve immunisation [86], most likely due to the greater number of antigen-presenting cells in the skin than in muscle [87, 88].
Lastly, some of these strategies may aid in the success of vaccinations other than SARS-CoV-2 in CKD patients.
Recommendations for the practicing nephrologist
Stratifying CKD patients according to immunologic risk would help tailor preventive measures [89]. This can be done by considering comorbidities, concomitant immunosuppressive treatments and by evaluating the presence of anti-spike antibodies and their titres. Identifying patients whose immune system has not responded to vaccines following the use of passive measures (ring vaccination, mask use, reduction of social contact, etc.) will be an indication for the use of long-acting monoclonal antibody therapy for pre-exposure prophylaxis and/or early administration of antiviral drugs (paying attention to drug interactions with immunosuppressants in KTRs). Conversely, identifying those who have had an immune response to vaccines will strengthen the use of repeat injections. In KTRs with a low risk of rejection, temporary withdrawal of mycophenolic derivatives or replacement of belatacept with an mTOR inhibitor could be considered [71, 81].
Data availability
This is not applicable for this type of review manuscript as it does not involve any data.
References
Noordzij M, Duivenvoorden R, Pena MJ, de Vries H, Kieneker LM, collaborative ERACODA authors, (2020) ERACODA: the European database collecting clinical information of patients on kidney replacement therapy with COVID-19. Nephrol Dial Transplant 35:2023–2025. https://doi.org/10.1093/ndt/gfaa179
Gansevoort RT, Hilbrands LB (2020) CKD is a key risk factor for COVID-19 mortality. Nat Rev Nephrol 16:705–706. https://doi.org/10.1038/s41581-020-00349-4
Puchalska-Reglińska E, Dębska-Ślizień A, Biedunkiewicz B et al (2021) Extremely high mortality rates among hemodialysis patients with COVID-19 before the era of SARS-CoV-2 vaccination: results from a large database from the North of Poland. Pol Arch Intern Med 131(7–8):643–648. https://doi.org/10.20452/pamw.16028
Qin CX, Moore LW, Anjan S et al (2021) Risk of Breakthrough SARS-CoV-2 Infections in Adult Transplant Recipients. Transplantation 105(11):e265–e266. https://doi.org/10.1097/TP.0000000000003907
Nyberg T, Ferguson NM, Nash SG, et al (2022) Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet 399(10332):1303–1312.https://doi.org/10.1016/S0140-6736(22)00462-7
Shields AM, Tadros S, Al-Hakim A, et al (2022) Impact of vaccination on hospitalization and mortality from COVID-19 in patients with primary and secondary immunodeficiency: The United Kingdom experience. Front Immunol 13:984376. https://doi.org/10.3389/fimmu.2022.984376
Babel N, Hugo C, Westhoff TH (2022) Vaccination in patients with kidney failure: lessons from COVID-19. Nat Rev Nephrol 18(11):708–723. https://doi.org/10.1038/s41581-022-00617-5
Reddy S, Chitturi C, Yee J (2019) Vaccination in Chronic Kidney Disease. Adv Chronic Kidney Dis 26(1):72–78. https://doi.org/10.1053/j.ackd.2018.10.002
Kolland M, Riedl R, Bachler B et al (2022) Decreased response to the mRNA anti-SARS-CoV-2 vaccine in hepatitis B vaccine non-responders and frail patients treated with peritoneal dialysis. Nephrol Dial Transplant 37(6):1188–1190. https://doi.org/10.1093/ndt/gfac031
Danthu C, Hantz S, Dahlem A et al (2021) Humoral Response after SARS-CoV-2 mRNA Vaccination in a Cohort of Hemodialysis Patients and Kidney Transplant Recipients. J Am Soc Nephrol 32(9):2153–2158. https://doi.org/10.1681/ASN.2021040490
Teijaro RJ, Farber D (2021) COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol 21:195–197. https://doi.org/10.1038/s41577-021-00526-x
Israelow B, Mao T, Klein J, et al (2021) Adaptive immune determinants of viral clearance and protection in mouse models of SARS-CoV-2. Sci Immunol 6(64):eabl4509. https://doi.org/10.1126/sciimmunol.abl4509
Sibbel S, McKeon K, Luo J et al (2022) Real-World Effectiveness and Immunogenicity of BNT162b2 and mRNA-1273 SARS-CoV-2 Vaccines in Patients on Hemodialysis. J Am Soc Nephrol 33(1):49–57. https://doi.org/10.1681/ASN.2021060778
Oliver MJ, Thomas D, Balamchi S et al (2022) Vaccine effectiveness against SARS-CoV-2 infection and severe outcomes in the maintenance dialysis population in Ontario. Canada J Am Soc Nephrol 33(4):839–849. https://doi.org/10.1681/ASN.2021091262
El Karoui K, Hourmant M, Ayav C et al (2022) Vaccination and COVID-19 dynamics in dialysis patients. Clin J Am Soc Nephrol 17(3):395–402. https://doi.org/10.2215/CJN.10300721
Aslam S, Adler E, Mekeel K, Little SJ (2021) Clinical effectiveness of COVID-19 vaccination in solid organ transplant recipients. Transpl Infect Dis23(5):e13705. https://doi.org/10.1111/tid.13705
Lacson E Jr, Argyropoulos CP, Manley HJ et al (2021) Immunogenicity of SARS-CoV-2 vaccine in dialysis. J Am Soc Nephrol 32(11):2735–2742. https://doi.org/10.1681/ASN.2021040432
Van Praet J, Reynders M, De Bacquer D, et al (2021) Predictors and Dynamics of the Humoral and Cellular Immune Response to SARS-CoV-2 mRNA Vaccines in Hemodialysis Patients: A Multicenter Observational Study [published online ahead of print, 2021 Sep 29]. J Am Soc Nephrol 32(12):3208–3220. https://doi.org/10.1681/ASN.2021070908
Quiroga B, Soler MJ, Ortiz A et al (2022) Loss of humoral response 3 months after SARS-CoV-2 vaccination in the CKD spectrum: the multicentric SENCOVAC study. Nephrol Dial Transplant 37(5):994–999. https://doi.org/10.1093/ndt/gfac007
Quiroga B, Soler MJ, Ortiz A et al (2022) Safety and immediate humoral response of COVID-19 vaccines in chronic kidney disease patients: the SENCOVAC study. Nephrol Dial Transplant 37(10):1868–1878. https://doi.org/10.1093/ndt/gfab313
Quiroga B, Soler MJ, Ortiz A et al (2022) Humoral response to third dose of SARS-CoV-2 vaccines in the CKD spectrum. Clin J Am Soc Nephrol 17(6):872–876. https://doi.org/10.2215/CJN.01770222
Hsu CM, Weiner DE, Manley HJ et al (2022) Seroresponse to SARS-CoV-2 vaccines among maintenance dialysis patients over 6 Months. Clin J Am Soc Nephrol 17(3):403–413. https://doi.org/10.2215/CJN.12250921
Karakizlis H, Agarwal V, Aly M, et al (2022) Humoral and cellular immune responses to the mRNA-1273 SARS-CoV-2 vaccine booster in patients on maintenance dialysis [published online ahead of print, 2022 Jun 22]. J Nephrol. 1–4. https://doi.org/10.1007/s40620-022-01371-4
Boyarsky BJ, Werbel WA, Avery RK et al (2021) Antibody response to 2-Dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 325(21):2204–2206. https://doi.org/10.1001/jama.2021.7489
Yau K, Chan CT, Abe KT et al (2022) Differences in mRNA-1273 (Moderna) and BNT162b2 (Pfizer-BioNTech) SARS-CoV-2 vaccine immunogenicity among patients undergoing dialysis. CMAJ 194(8):E297–E305. https://doi.org/10.1503/cmaj.211881
Verleye A, Wijtvliet V, Abrams S et al (2022) Seroconversion rate after primary vaccination with two doses of BNT162b2 versus mRNA-1273 in solid organ transplant recipients: a systematic review and meta-analysis. Nephrol Dial Transplant 37(8):1566–1575. https://doi.org/10.1093/ndt/gfac174
Sanders JF, Bemelman FJ, Messchendorp AL et al (2022) The RECOVAC immune-response study: the immunogenicity, tolerability, and safety of COVID-19 vaccination in patients with chronic kidney disease, on dialysis, or living with a kidney transplant. Transplantation 106(4):821–834. https://doi.org/10.1097/TP.0000000000003983
Grupper A, Sharon N, Finn T et al (2021) Humoral response to the Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol 16(7):1037–1042. https://doi.org/10.2215/CJN.03500321
Chavarot N, Ouedrani A, Marion O et al (2021) Poor Anti-SARS-CoV-2 humoral and T-cell responses after 2 injections of mRNA vaccine in kidney transplant recipients treated with belatacept. Transplantation 105(9):e94–e95. https://doi.org/10.1097/TP.0000000000003784
Korth J, Jahn M, Dorsch O, et al (2021) Impaired Humoral Response in Renal Transplant Recipients to SARS-CoV-2 Vaccination with BNT162b2 (Pfizer-BioNTech). Viruses 13(5):756. Published 2021 Apr 25. https://doi.org/10.3390/v13050756
Rozen-Zvi B, Yahav D, Agur T et al (2021) Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: a prospective cohort study. Clin Microbiol Infect 27(8):1173.e1-1173.e4. https://doi.org/10.1016/j.cmi.2021.04.028
Benotmane I, Gautier-Vargas G, Cognard N et al (2021) Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int 99:1498–1500. https://doi.org/10.1016/j.kint.2021.04.005
Manothummetha K, Chuleerarux N, Sanguankeo A, et al (2022) Immunogenicity and Risk Factors Associated With Poor Humoral Immune Response of SARS-CoV-2 Vaccines in Recipients of Solid Organ Transplant: A Systematic Review and Meta-Analysis. JAMA Netw Open 5(4):e226822. Published 2022 Apr 1. https://doi.org/10.1001/jamanetworkopen.2022.6822
Espi M, Charmetant X, Barba T et al (2022) A prospective observational study for justification, safety, and efficacy of a third dose of mRNA vaccine in patients receiving maintenance hemodialysis. Kidney Int 101(2):390–402. https://doi.org/10.1016/j.kint.2021.10.040
Lesny P, Anderson M, Cloherty G et al (2021) Immunogenicity of a first dose of mRNA- or vector-based SARS-CoV-2 vaccination in dialysis patients: a multicenter prospective observational pilot study. J Nephrol 34(4):975–983. https://doi.org/10.1007/s40620-021-01076-0
Espi M, Koppe L, Fouque D, Thaunat O (2020) Chronic Kidney Disease-Associated Immune Dysfunctions: Impact of Protein-Bound Uremic Retention Solutes on Immune Cells. Toxins (Basel) 12(5):300. Published 2020 May 6. https://doi.org/10.3390/toxins12050300
Rincon-Arevalo H, Choi M, Stefanski AL, et al (2021) Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci Immunol 6(60):eabj1031. https://doi.org/10.1126/sciimmunol.abj1031
Kovacic V, Sain M, Vukman V (2002) Efficient haemodialysis improves the response to hepatitis B virus vaccination. Intervirology 45(3):172–176. https://doi.org/10.1159/000065873
Sattler A, Schrezenmeier E, Weber UA, et al (2021)) Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J Clin Invest 131(14):e150175. https://doi.org/10.1172/JCI150175
Cucchiari D, Egri N, Rodriguez-Espinosa D, et al (2022) Humoral and cellular immune responses after a 3-dose course of mRNA-1273 COVID-19 vaccine in kidney transplant recipients: a prospective cohort study. Transplant Direct 8(11):e1389. Published 2022 Oct 7. https://doi.org/10.1097/TXD.0000000000001389
Sahin U, Muik A, Vogler I et al (2021) BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 595(7868):572–577. https://doi.org/10.1038/s41586-021-03653-6
Schrezenmeier E, Rincon-Arevalo H, Stefanski AL et al (2021) B and T cell responses after a third dose of SARS-CoV-2 vaccine in kidney transplant recipients. J Am Soc Nephrol 32(12):3027–3033. https://doi.org/10.1681/ASN.2021070966
Bertoletti A, Le Bert N, Tan AT (2022) SARS-CoV-2-specific T cells in the changing landscape of the COVID-19 pandemic. Immunity 55(10):1764–1778. https://doi.org/10.1016/j.immuni.2022.08.008
Skelly DT, Harding AC, Gilbert-Jaramillo J et al (2021) Two doses of SARS-CoV-2 vaccination induce robust immune responses to emerging SARS-CoV-2 variants of concern. Nat Commun 12(1):5061. https://doi.org/10.1038/s41467-021-25167-5
McMahan K, Yu J, Mercado NB et al (2021) Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 590(7847):630–634. https://doi.org/10.1038/s41586-020-03041-6
Tangye SG; COVID Human Genetic Effort consortium. Impact of SARS-CoV-2 infection and COVID-19 on patients with inborn errors of immunity. J Allergy Clin Immunol 2022 Dec 13:S0091–6749(22)01568–8. https://doi.org/10.1016/j.jaci.2022.11.010.
Le Bert N, Tan AT, Kunasegaran K et al (2020) SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 584(7821):457–462. https://doi.org/10.1038/s41586-020-2550-z
Anft M, Blazquez-Navarro A, Stervbo U et al (2021) Detection of pre-existing SARS-CoV-2-reactive T cells in unexposed renal transplant patients. J Nephrol 34(4):1025–1037. https://doi.org/10.1007/s40620-021-01092-0
Thieme CJ, Blazquez-Navarro A, Safi L, et al (2021) Impaired Humoral but Substantial Cellular Immune Response to Variants of Concern B1.1.7 and B.1.351 in Hemodialysis Patients after Vaccination with BNT162b2. J Am Soc Nephrol 32(11):2725–2727. https://doi.org/10.1681/ASN.2021050672
Imhof C, Messchendorp AL, van der Heiden M, et al (2022) SARS-CoV-2 Spike-specific IFN-γ T-cell Response After COVID-19 Vaccination in Patients With Chronic Kidney Disease, on Dialysis, or Living With a Kidney Transplant. Transplant Direct 8(11):e1387. Published 2022 Oct 18. https://doi.org/10.1097/TXD.0000000000001387
Brunelli SM, Sibbel S, Karpinski S, et al (2022) Comparative Effectiveness of mRNA-based BNT162b2 Vaccine versus Adenovirus Vector-Based Ad26.COV2.S Vaccine for the Prevention of COVID-19 among Dialysis Patients. J Am Soc Nephrol 33(4):688–697. https://doi.org/10.1681/ASN.2021101395
Bell S, Campbell J, Lambourg E et al (2022) The impact of vaccination on incidence and outcomes of SARS-CoV-2 infection in patients with kidney failure in Scotland. J Am Soc Nephrol 33:677–686. https://doi.org/10.1681/ASN.2022010046
Hall VG, Ferreira VH, Ku T et al (2021) Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med 385(13):1244–1246. https://doi.org/10.1056/NEJMc2111462
Quiroga B, Soler MJ, Ortiz A, et al (2022) Humoral response after the fourth dose of the SARS-CoV-2 vaccine in the CKD spectrum: a prespecified analysis of the SENCOVAC study [published online ahead of print, 2022 Nov 24]. Nephrol Dial Transplant ;gfac307. https://doi.org/10.1093/ndt/gfac307
Osmanodja B, Ronicke S, Budde K et al (2022) Serological response to three, four and five doses of SARS-CoV-2 vaccine in kidney transplant recipients. J Clin Med 11(9):2565. https://doi.org/10.3390/jcm11092565
Munro APS, Janani L, Cornelius V, et al (2021) Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial [published correction appears in Lancet. 2021 Dec 18;398(10318):2246]. Lancet. 398(10318):2258-2276. https://doi.org/10.1016/S0140-6736(21)02717-3
Meijers B, Goedgezelschap A, Peeters D et al (2022) Heterologous versus homologous triple anti-COVID-19 vaccine regimens in patients on maintenance haemodialysis. Nephrol Dial Transplant 37(7):1384–1386. https://doi.org/10.1093/ndt/gfac033
Kho MML, Messchendorp AL, Frölke SC, et al (2022) Alternative strategies to increase the immunogenicity of COVID-19 vaccines in kidney transplant recipients not responding to two or three doses of an mRNA vaccine (RECOVAC): a randomised clinical trial [published online ahead of print, 2022 Oct 27]. Lancet Infect Dis. 2022;S1473–3099(22)00650–8. https://doi.org/10.1016/S1473-3099(22)00650-8
Reindl-Schwaighofer R, Heinzel A, Mayrdorfer M et al (2022) Comparison of SARS-CoV-2 antibody response 4 weeks after homologous vs heterologous third vaccine dose in kidney transplant recipients: a randomized clinical trial. JAMA Intern Med 182(2):165–171. https://doi.org/10.1001/jamainternmed.2021.7372
Irrgang P, Gerling J, Kocher K, et al (2023) Class switch toward noninflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination. Sci Immunol 2023 Jan 27;8(79):eade2798. https://doi.org/10.1126/sciimmunol.ade2798.
Buhre JS, Pongracz T, Künsting I et al (2023) mRNA vaccines against SARS-CoV-2 induce comparably low long-term IgG Fc galactosylation and sialylation levels but increasing long-term IgG4 responses compared to an adenovirus-based vaccine. Front Immunol 13:1020844. https://doi.org/10.3389/fimmu.2022.1020844
Allison AC (2005) Mechanisms of action of mycophenolate mofetil. Lupus 14(Suppl 1):s2–s8. https://doi.org/10.1191/0961203305lu2109oa
Furer V, Eviatar T, Zisman D, et al (2021) Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study [published correction appears in Ann Rheum Dis. 2022 Jul;81(7):e133]. Ann Rheum Dis 80(10):1330–1338. https://doi.org/10.1136/annrheumdis-2021-220647
Grupper A, Rabinowich L, Schwartz D et al (2021) Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transpl 21(8):2719–2726. https://doi.org/10.1111/ajt.16615
Al Fatly Z, Betjes MGH, Messchendorp AL et al (2022) COVID-19 vaccination response in kidney transplant recipients with and without mycophenolate mofetil: follow-up of a randomized controlled trial. Kidney Int Rep 7(6):1433–1434. https://doi.org/10.1016/j.ekir.2022.04.002
Mulley WR, Visvanathan K, Hurt AC et al (2012) Mycophenolate and lower graft function reduce the seroresponse of kidney transplant recipients to pandemic H1N1 vaccination. Kidney Int 82:212–219. https://doi.org/10.1038/ki.2012.106
Schrezenmeier E, Rincon-Arevalo H, Jens A, et al (2022) Temporary antimetabolite treatment hold boosts SARS-CoV-2 vaccination-specific humoral and cellular immunity in kidney transplant recipients. JCI Insight 7(9):e157836. Published 2022 May 9. https://doi.org/10.1172/jci.insight.157836
Connolly CM, Chiang TP, Boyarsky BJ et al (2022) Temporary hold of mycophenolate augments humoral response to SARS-CoV-2 vaccination in patients with rheumatic and musculoskeletal diseases: a case series. Ann Rheum Dis 81(2):293–295. https://doi.org/10.1136/annrheumdis-2021-221252
Kantauskaite M, Müller L, Hillebrandt J, et al (2022) Immune response to third SARS-CoV-2 vaccination in seronegative kidney transplant recipients: Possible improvement by mycophenolate mofetil reduction. Clin Transplant. 36(11):e14790. https://doi.org/10.1111/ctr.14790
Chavarot N, Morel A, Leruez-Ville M et al (2021) Weak antibody response to three doses of mRNA vaccine in kidney transplant recipients treated with belatacept. Am J Transpl 21(12):4043–4051. https://doi.org/10.1111/ajt.16814
Netti GS, Infante B, Troise D et al (2022) mTOR inhibitors improve both humoral and cellular response to SARS-CoV-2 messenger RNA BNT16b2 vaccine in kidney transplant recipients. Am J Transpl 22(5):1475–1482. https://doi.org/10.1111/ajt.16958
Lammert A, Schnuelle P, Rabenau HF, et al (2022) SARS-CoV-2 Vaccination in Kidney Transplant Recipients-Stratified Analysis of the Humoral Immune Response. Transplant Direct 8(11):e1384. Published 2022 Oct 14. https://doi.org/10.1097/TXD.0000000000001384
Khoury DS, Cromer D, Reynaldi A et al (2021) Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 27(7):1205–1211. https://doi.org/10.1038/s41591-021-01377-8
Jahrsdörfer B, Kroschel J, Ludwig C et al (2021) Independent side-by-side validation and comparison of 4 serological platforms for SARS-CoV-2 antibody testing. J Infect Dis 223(5):796–801. https://doi.org/10.1093/infdis/jiaa656
Wand O, Nacasch N, Fadeela A, et al (2022)Humoral response and breakthrough infections with SARS-CoV-2 B.1.617.2 variant in vaccinated maintenance hemodialysis patients. J Nephrol 35(5):1479–1487. https://doi.org/10.1007/s40620-022-01245-9
Anand S, Montez-Rath ME, Han J et al (2022) SARS-CoV-2 vaccine antibody response and breakthrough infection in patients receiving dialysis. Ann Intern Med 175(3):371–378. https://doi.org/10.7326/M21-4176
Caillard S, Chavarot N, Bertrand D et al (2021) Occurrence of severe COVID-19 in vaccinated transplant patients. Kidney Int 100(2):477–479. https://doi.org/10.1016/j.kint.2021.05.011
Boudhabhay I, Serris A, Servais A et al (2022) COVID-19 outbreak in vaccinated patients from a haemodialysis unit: antibody titres as a marker of protection from infection. Nephrol Dial Transplant 37(7):1357–1365. https://doi.org/10.1093/ndt/gfac016
Pillai S. (2023) Is it bad, is it good, or is IgG4 just misunderstood? Sci Immunol, Ahead of Print https://doi.org/10.1126/sciimmunol.adg7327
Andrews N, Tessier E, Stowe J et al (2022) Duration of protection against mild and severe disease by Covid-19 vaccines. N Engl J Med 386:340–350
Perkins GB, Tunbridge M, Salehi T et al (2022) Concurrent vaccination of kidney transplant recipients and close household cohabitants against COVID-19. Kidney Int 101(5):1077–1080. https://doi.org/10.1016/j.kint.2022.02.015
Medigeshi GR, Batra G, Murugesan DR, et al (2022) Sub-optimal neutralisation of omicron (B.1.1.529) variant by antibodies induced by vaccine alone or SARS-CoV-2 Infection plus vaccine (hybrid immunity) post 6-months. EBioMedicine 78:103938. https://doi.org/10.1016/j.ebiom.2022.103938
Benotmane I, Velay A, Gautier-Vargas G et al (2022) Pre-exposure prophylaxis with 300 mg Evusheld elicits limited neutralizing activity against the Omicron variant. Kidney Int 102(2):442–444. https://doi.org/10.1016/j.kint.2022.05.008
Yetmar ZA, Bhaimia E, Razonable RR (2022) Antispike monoclonal antibodies for prevention and treatment of coronavirus disease-2019 in solid organ transplant recipients. Yetmar ZA et al. Curr Opin Organ Transplant 27:269–276. https://doi.org/10.1097/MOT.0000000000000981
Seija M, Rammauro F, Santiago J et al (2021) Comparison of antibody response to SARS-CoV-2 after two doses of inactivated virus and BNT162b2 mRNA vaccines in kidney transplant. Clin Kidney J 15(3):527–533. https://doi.org/10.1093/ckj/sfab291
Zimmermann P, Curtis N (2019) Factors that influence the immune response to vaccination. Clin Microbiol Rev 32:e00084-e118. https://doi.org/10.1128/CMR.00084-18
Palucka K, Banchereau J, Mellman I (2010) Designing vaccines based on biology of human dendritic cell subsets. Immunity 33:464–478. https://doi.org/10.1016/j.immuni.2010.10.007
Roozen GVT, Prins MLM, van Binnendijk R et al (2022) Safety and Immunogenicity of intradermal fractional dose administration of the mRNA-1273 vaccine: a proof-of-concept study. Ann Intern Med 175(12):1771–1774. https://doi.org/10.7326/M22-2089
Willicombe M, Scanlon M, Loud F, Lightstone L (2022) Should we be clinically assessing antibody responses to covid vaccines in immunocompromised people? BMJ 377:o966. https://doi.org/10.1136/bmj.o966
Acknowledgements
This article is based on a lecture given at the 59th ERA Congress, Paris, May 2022.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical statement
Ethical statement is not applicable for this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
All Authors Participated in the Research and Preparation of the Manuscript. The Manuscript Has Not Been Published and Is Not Being Considered for Publication Elsewhere, in Whole Or in Part, in Any Language.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rossi, M., Pessolano, G. & Gambaro, G. What has vaccination against COVID-19 in CKD patients taught us?. J Nephrol 36, 1257–1266 (2023). https://doi.org/10.1007/s40620-023-01640-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-023-01640-w