Abstract
Background
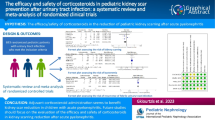
Acute pyelonephritis is a common infection in children that may cause renal scarring. The aim of this systematic review and meta-analysis was to analyse the use of corticosteroid treatment to prevent renal scarring.
Methods
We searched the PubMED, SCOPUS, Cochrane CENTRAL and Web of Science databases in June 2022 for (corticosteroid* or dexamethasone or prednisolone* or prednisone* or hydrocortisone*) AND pyelonephritis. Randomised controlled trials focusing on children were included. The intervention was corticosteroid treatment with antibiotics compared to antibiotics with or without a placebo. The main outcome was the presence of renal scars on dimercaptosuccinic acid scanning at follow-up. The evidence quality was assessed using the GRADE methodology and risk of bias 2.0 tool. We calculated the risk ratio (RR), absolute risk difference (RD) with 95% confidence intervals (CI) and the number needed to treat (NNT). We applied a fixed effects model due to low heterogeneity.
Results
We screened 872 abstracts and included five full texts. Renal scarring at follow-up was found in 31/220 (14.1%) patients in the corticosteroid groups and 76/278 (27.3%) in the control groups (RR 0.65, CI 0.44–0.96, RD − 13.2%, NNT 8). The evidence quality was moderate. Two studies reported adverse events with no differences between the groups. The risk of bias analysis showed some concerns in four studies.
Conclusion
We found moderate quality evidence that adjuvant corticosteroid treatment could prevent renal scarring. Adverse events were insufficiently reported, and more research on their effectiveness and harm is therefore needed before using corticosteroids in clinical settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary tract infections (UTIs) are one of the most common bacterial infections in childhood as approximately 4% of 1-year-olds and 10% of 6-year-olds have an episode of UTI [1]. Acute pyelonephritis may cause renal scarring, and renal scarring has been detected by dimercaptosuccinic acid (DMSA) scanning in approximately 15–57% of patients after the acute phase [2, 3]. Renal scarring may cause later renal sequalae, such as hypertension and kidney insufficiency; however, the relationship is not completely straightforward [4, 5]. Some countries routinely use DMSA scanning, and some countries do not use it at all.
Researchers have sought potential treatments to prevent scarring, and strategies such as corticosteroid therapy, vitamin supplementation and antibiotic treatment have been assessed. Antibiotic treatment alone does not offer sufficient protection from scarring as renal scars seem to develop regardless of the appropriately started antibiotic treatment [3, 6]. The formation of scars seems to originate from the inflammatory process in the kidneys rather than the bacterial infection, thus corticosteroids could potentially offer protection from renal scarring. In recent randomised studies, corticosteroid treatment has produced controversial results with respect to renal scarring. A meta-analysis published in 2021 showed the effects against scarring when corticosteroid therapy was combined with routine antibiotic treatment [7]. Since then, novel studies have been published, and we therefore decided to update the evidence.
The aim of this systematic review and meta-analysis was to analyse whether adjuvant corticosteroid treatment can prevent renal scarring in acute pyelonephritis in children.
Methods
Search strategy
We searched the PubMed (MEDLINE), Cochrane CENTRAL, Scopus and Web of Science databases in June 2022. The full search strategy is described in Supplement 1. We included only studies published in English. We did not use any time or other filters. The search results were then uploaded for screening in Covidence software (Covidence, Melbourne, Australia) (Table 1).
Inclusion and exclusion criteria
We included randomised controlled trials (RCTs) that compared adjuvant corticosteroid treatment to standard antibiotic treatment with or without a placebo, as well as studies conducted on children aged older than 28 days but younger than 18 years. Animal studies were excluded as were observational studies and all other studies that did not present original data (reviews, editorials, letters, etc.).
Review process
Two authors (JJ and IK) individually screened the abstracts and conflicts were resolved by the third author (MR). Full texts were assessed independently by two authors (IK and JJ). The outcomes data was then extracted into an Excel spreadsheet. The Cochrane risk of bias 2.0 tool was used to assess risk of bias in the included studies. The risk of bias figures were created by using the robvis package in R version 4.0.3. The evidence quality was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology [8] (Fig. 1).
Outcomes measures
Our primary outcome was the presence of renal scarring on DMSA scans after the acute phase. Our secondary outcomes were the severity of the scarring on DMSA scans and adverse events related to the initial corticosteroid treatment (Table 2).
Statistics
We used Review Manager version 5.4 for the meta-analysis. The data analyses were performed according to the Cochrane Handbook of Systematic Review Guidelines [9]. We calculated the risk ratios (RR) with 95% confidence intervals (CI) for the dichotomous outcomes and the absolute risk difference (RD) and number needed to treat (NNT) for the primary outcome. A forest plot was presented for the primary outcome and a funnel plot was used to analyse possible publication bias. As the secondary outcomes were measured differently, we decided not to pool them to a single estimate. Instead, they are presented in accordance with the Synthesis Without Meta-analysis (SWiM) guidelines [10]. We analysed the inconsistency index (I2) statistics for heterogeneity, and as heterogeneity was low (less than 40%), we used a fixed effects model.
We reported our systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Supplement 2) [11].
Protocol registration
We registered our protocol in Prospero (CRD42022339721).
Results
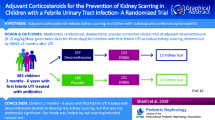
We initially screened 872 abstracts and assessed 16 full texts, with 5 studies selected for the final analysis. These 5 studies included a total of 498 children, with a mean age ranging from 7.4 to 34.19 months [12,13,14,15,16]. The majority of patients were girls, and the most common causative bacteria was Escherichia coli (Table 3). A total of 227 children underwent baseline DMSA scan. Da Dalt et al. performed a follow-up DMSA at 6 months after diagnosis, Ghaffari et al. between 4 and 6 months, Huang et al. between 6 and 38 months, Rius-Gordillo et al. at 6 months and Shaikh et al. between 5 and 24 months after diagnosis. Altogether, 182 children were lost to follow up; 130 children in Shaik et al., 21 children in Rius-Gordillo et al., 22 children in DaDalt et al. and 1 child in Huang et al.. Graffari et al. did not specify why 8 children dropped out of the study. Da Dalt, Ghaffari, Rius-Gordillo and Shaikh et al. used dexamethasone as intervention in their RCTs, and doses were 0.15 mg/kg every 12 h for 4 days, 0.15 mg/kg every 6 h for 4 days, 0.30 mg/kg every 12 h for 3 days and 0.15 mg/kg every 12 h for 3 days, respectively. Huang et al. used methylprednisolone 1.6 mg/kg/day with maximum dose of 48 mg/day in divided doses every 6 h for 3 days.
Risk of bias
The overall risk of bias was considered low in one study although there were some concerns regarding bias in four studies (Fig. 2A). Most of the bias was due to missing outcomes data and the selection of the reported results (Fig. 2B).
Renal scarring
Renal scarring was found in 31 (14.1%) of the 220 children in the corticosteroid groups and 76 (27.3%) of the 278 children in the control groups (RR 0.65 [CI 0.44–0.96], absolute RD − 13.2%, NNT 8; Fig. 3, Table 4). We ranked the evidence quality as moderate due to the risk of bias (Table 4). The funnel plot was symmetrical, and publication bias was not observed (Fig. 4).
Renal scarring severity
Renal scarring severity was reported in three studies with 428 children [12, 14, 16]. Analysis of renal scarring volume in the study by Huang et al. found that the median volume was 0.0 ml (range 0.0–4.5 ml) in the corticosteroid group and 1.5 ml (range 0–14.8 ml) in the control group (p < 0.01) [12]. In the Shaikh et al. study, kidney scarring was present in 1.9 segments in the corticosteroid group and in 2.4 segments in the control group (p = 0.22). Rius-Gordillo et al. analysed renal damage severity scores (range 0–8) in their study. In the follow-up DMSA scans, the score was 0.41 in the corticosteroid group and 0.32 in the control group (p = 0.848) [14, 16].
Adverse events
Two studies comprising a total of 272 children reported adverse events [14, 15]. In the Shaikh et al. study, the non-severe and severe adverse outcome rates were similar between the corticosteroid and control groups, and the serious adverse events (i.e., hospitalisations and bacteraemia findings) were similar in both groups (2.6% vs. 2.9%, respectively) [14]. Da Dalt et al. reported that there was only one case of transient behavioural change in the corticosteroid group in their study, and no other adverse outcomes were observed [15].
Discussion
This meta-analysis consisted of five studies that included 498 children, 220 of whom received adjuvant corticosteroid treatment. Renal scarring was found in 14.1 and 27.3% of the patients in the corticosteroid and control groups, respectively. Dexamethasone was the most used steroid in the RCTs included and it seemed to reduce the risk of renal scarring. Methylprednisolone was used in Huang et al. and they found that it reduced renal scaring in the study group [12]. The route of administration of steroids does not seem to affect the risk reduction as both oral and intravenous corticosteroids reduced renal scar formation. When possible, oral administration of corticosteroids should be primary as it is non-invasive. Meena et al. [7] found a reduction in kidney scarring in their fixed-effects meta-analysis (RR 0.57, CI 0.36–0.91), which was similar to the findings of our meta-analysis (RR 0.65, CI 0.44–0.96) [7]. The Meena et al. meta-analysis included three RCTs with 389 children, and those three RCTs were also included in our meta-analysis [12,13,14]. Although it seems that corticosteroids reduced kidney scarring, the reporting of adverse events was poor. Only two studies [14, 15] reported adverse events: fussiness was more common in the corticosteroid group, and one case of transient behavioural change was reported in the corticosteroid group in one study. Although we found no significant differences in the serious adverse events between the corticosteroid and control groups (2.6% vs 2.9%, respectively) in our meta-analysis, these were only reported in one study [14]. Follow-up DMSA scans were done approximately 6 months after recruitment. Long follow-up periods may lead to increased number of dropouts and reinfections. Shaikh et al. had the highest number of patients lost to follow up, and outcome assessment was done 5–24 months after diagnosis [14]. At the 6-month follow up, the rate of dropouts and reinfections remained moderate.
The follow-up studies suggest that renal scarring may lead to the development of hypertension, renal insufficiency and an increased risk of preeclampsia during pregnancy [4, 5, 17]. Jacobson et al. found a significant reduction in the glomerular filtration rate (GFR) and higher diastolic blood pressure in the patients with kidney scars than in the control patients [4]. However, in more recent studies such findings have not been as clear [18]. Wennerström et al. did not find any significant difference in ambulatory blood pressure between the children with non-obstructive renal scarring after the first UTI and the reference group without renal scarring [5]. Notwithstanding, there was an increase in atrial natriuretic peptin in the study group, which suggests that patients with renal scars could have an ongoing counter-regulatory mechanism that prevents blood pressure from increasing. After two decades, the GFR was well preserved, but when looking at the function of a single kidney, the GFR was significantly lower in the scarred kidney than in the healthy one [17]. Evidence suggests that children with kidney scars are at a higher risk of developing hypertension and kidney insufficiency later in life.
We were able to conduct our review in line with our protocol, which is a strength of our study. The limitations of this meta-analysis arose mostly from the original publications. The main limitation of the included studies was the limited number of participants and high dropout rates. First, one of the studies changed the prespecified analysis plan to the Bayesian approach during the study process [15]. Second, most of the studies did not discuss potential adverse events or harm stemming from corticosteroid treatment. Only one of the studies included confirmation of pyelonephritis via DMSA scans, and in one study, pyelonephritis was confirmed via either DMSA scans or renal sonography [12, 16]. Although the incidence of pyelonephritis from febrile UTIs has been shown to be approximately 70%, in the three studies where pyelonephritis was defined as febrile UTIs, one third of the patients included may not have had true acute pyelonephritis [6, 13,14,15]. This could have affected the final results in those three studies. The lack of baseline DMSA scans makes it impossible to discover if the scars were due to acute pyelonephritis or if the children were born with them.
Based on the findings of this meta-analysis, adjuvant corticosteroid therapy could prevent renal scarring in acute pyelonephritis. However, many issues have to be clarified before recommending the use of corticosteroids in clinical settings. First, the diagnosis of pyelonephritis should be sound before considering steroids. Second, we need more information on the side effects of the steroid treatment with this indication. Furthermore, clinical studies are needed to determine the optimal dose of corticosteroids with ideal risk/benefit ratio. Third, the real clinical impact of the scars should be elucidated with effective and long monitoring. Baseline DMSA scans before the infection episode would probably not be a realistic aim, but an appropriate randomization process is needed to undertake this and other baseline biases. These issues should be taken into consideration in future trials.
Conclusions
We found moderate quality evidence supporting that adjuvant corticosteroid treatment could prevent renal scarring in acute pyelonephritis in children. The NNT to avoid single renal scarring was eight in our analysis. However, the reporting of adverse events was considered to be insufficient, and more research on the effectiveness and potential harm of corticosteroid treatment is therefore needed before considering routinely using corticosteroids in all clinical settings.
Data availability
All the applied materials and analyses are provided in the Supplements or can be requested directly from the corresponding author.
References
Ladomenou F, Bitsori M, Galanakis E (2015) Incidence and morbidity of urinary tract infection in a prospective cohort of children. Acta Paediatr 104(7):324. https://doi.org/10.1111/apa.12992
Leung AKC, Wong AHC, Leung AAM, Hon KL (2019) Urinary tract infection in children. Recent Pat Inflamm Allergy Drug Disc 13(1):2–18. https://doi.org/10.2174/1872213X13666181228154940
Shaikh N, Ewing AL, Bhatnagar S, Hoberman A (2010) Risk of renal scarring in children with a first urinary tract infection: a systematic review. Pediatrics 126(6):1084. https://doi.org/10.1542/peds.2010-0685
Jacobson SH, Eklöf O, Eriksson CG, Lins LE, Tidgren B, Winberg J (1989) Development of hypertension and uraemia after pyelonephritis in childhood: 27 year follow up. BMJ 299(6701):703–706. https://doi.org/10.1136/bmj.299.6701.703
Wennerstro M, Hansson S, Hedner T, Himmelmann A, Jodal U (2000) Ambulatory blood pressure 16 ± 26 years after the ®rst urinary tract infection in childhood. J Hypertens 18:485
Lin K, Chiu N, Chen M, et al (2003) Acute pyelonephritis and sequelae of renal scar in pediatric first febrile urinary tract infection. Pediatr Nephrol (Berlin, West). 18(4):362–365. https://www.ncbi.nlm.nih.gov/pubmed/12700963. https://doi.org/10.1007/s00467-003-1109-1
Meena J, Kumar J (2021) Adjuvant corticosteroids for prevention of kidney scarring in children with acute pyelonephritis: a systematic review and meta-analysis. Arch Dis Child 106(11):1081–1086. https://doi.org/10.1136/archdischild-2020-320591
Guyatt GH, Oxman AD, Vist GE et al. (2008) GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336(7650):924–926. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2335261/. Accessed Jul 17, 2022. https://doi.org/10.1136/bmj.39489.470347.AD
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (2022) Cochrane handbook for systematic reviews of interventions version 6.3. . www.training.cochrane.org/handbook. https://training.cochrane.org/handbook/current. Updated 2022
Campbell M, Mckenzie JE, Sowden A et al (2020) Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. https://doi.org/10.1136/bmj.l6890
Page MJ, Mckenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. https://doi.org/10.1136/bmj.n71
Huang YY, Chen MJ, Chiu NT, Chou HH, Lin KY, Chiou YY (2011) Adjunctive oral methylprednisolone in pediatric acute pyelonephritis alleviates renal scarring. Pediatrics 128(3):496. https://doi.org/10.1542/peds.2010-0297
Ghaffari J, Mohammadjafari H, Mohammadi GH, Mahdavi MR (2019) Assessment the effect of dexamethasone on urinary cytokines and renal scar in children with acute pyelonephritis. Iran J Kidney Dis 13(4):244–250 (4318/1078[pii])
Shaikh N, Shope TR, Hoberman A et al (2020) Corticosteroids to prevent kidney scarring in children with a febrile urinary tract infection: a randomized trial. Pediatr Nephrol 35(11):2113–2120. https://doi.org/10.1007/s00467-020-04622-3
Da Dalt L, Bressan S, Scozzola F et al (2021) Oral steroids for reducing kidney scarring in young children with febrile urinary tract infections: the contribution of Bayesian analysis to a randomized trial not reaching its intended sample size. Pediatr Nephrol 36(11):3681–3692. https://doi.org/10.1007/s00467-021-05117-5
Rius-Gordillo N, Ferre N, Gonzalez JD et al (2022) Dexamethasone to prevent kidney scarring in acute pyelonephritis: a randomized clinical trial. Pediatr Nephrol. https://doi.org/10.1007/s00467-021-05398-w
Wennerström M, Hansson S, Jodal U, Sixt R, Stokland E (2000) Renal function 16–26 years after the first urinary tract infection in childhood. Arch Pediatr Adolesc Med 154(4):339–345. https://doi.org/10.1001/archpedi.154.4.339
Honkila M, Hannula A, Pokka T et al (2020) Childhood urinary tract infections and pregnancy-related complications in adult women. Pediatrics. https://doi.org/10.1542/peds.2020-0610
Funding
Open access funding provided by University of Eastern Finland (UEF) including Kuopio University Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No financial or non-financial benefits have been or will be received from any party related directly or indirectly to the subject of this article.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Systematic review registration number: Prospero CRD42022339721.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jääskeläinen, J., Renko, M. & Kuitunen, I. Corticosteroids to prevent renal scarring in children with pyelonephritis: a systematic review and meta-analysis. J Nephrol 36, 1509–1518 (2023). https://doi.org/10.1007/s40620-022-01552-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-022-01552-1