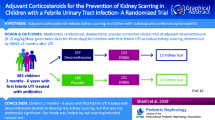
Abstract
Background
Acute pyelonephritis (APN) in pediatric patients may lead to kidney scarring and is one of the main causes of permanent kidney damage. The incidence of kidney scarring after one febrile urinary tract infection (UTI) is reported to range from 2.8 to 15%, with the percentage rising to 28.6% after ≥ 3 febrile UTIs. Corticosteroids may have a role in the reduction of kidney scar formation and urine cytokine levels. The possible benefit of adjuvant corticosteroid administration in the reduction of kidney scar formation in children with APN has been recently examined in randomized controlled trials (RCTs).
Objectives
The aim of this meta-analysis was to provide a summary of the current literature about the efficacy and safety of adjuvant corticosteroid administration in the reduction of kidney scar formation in children with APN.
Data sources
An extensive literature search through major databases (PubMed/MEDLINE and Scopus) was carried out for RCTs from inception until October 12, 2022, investigating the efficacy and safety of adjuvant corticosteroids in preventing kidney scarring in children with APN. A risk ratio with 95% CI was used for dichotomous outcomes.
Results
In total, 5 RCTs with 918 pediatric patients with APN were included in the study. Adjuvant corticosteroid treatment revealed a statistically significant reduction in kidney scarring (95% CI 0.42–0.95, p = 0.03), without increasing the risk of adverse events like bacteremia, prolonged hospitalization, or recurrence of UTI.
Limitations
There were limitations regarding sample size (n = 498 children), different classes of corticosteroids (methylprednisolone or dexamethasone), different routes of corticosteroid administration (intravenous or oral), and different day courses (3-day or 4-day course).
Conclusions
Adjuvant corticosteroid administration seems to have a beneficial effect on kidney scar reduction in children with APN. Future studies should focus on the evaluation of the efficacy and safety of corticosteroids in kidney scarring reduction after APN to strengthen the results of our study.
Graphical Abstract

A higher resolution version of the Graphical abstract is available as Supplementary information
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Acute pyelonephritis (APN) in pediatric patients may lead to kidney scarring and is listed as one of the important causes of permanent kidney damage [1, 2]. Acquired scarring because of APN seems to be more common in girls and usually is associated with lower-grade vesicoureteral reflux (VUR) and better outcomes [3]. Kidney scarring may lead to hypertension, proteinuria, and the risk of chronic kidney disease increases when high-grade VUR background is present [1, 2, 4, 5]. The incidence of kidney scarring after one febrile urinary tract infection (UTI) is reported to range from 2.8% to 15%, with the percentage rising to 28.6% after three or more febrile UTIs [5, 6]. Risk factors for kidney scarring are multiple APN episodes, high-grade VUR, bacterial virulence, and delay of treatment with antibiotics, especially in infants with non-specific UTI signs [7, 8]. Adequate antibiotic treatment is the most efficient treatment option for UTI, but it may not be sufficient to prevent kidney scarring [9].
Corticosteroids may have a role in reducing kidney scar formation and urine cytokine levels [10]. Cytokines may predict the severity of kidney damage, playing a key role in kidney scarring after APN as they represent the mediators of an inflammatory process in response to an infection [11,12,13,14]. A few studies have attempted to examine the hypothesis that corticosteroids may affect cytokine response and decrease kidney damage after APN, with promising results [12, 15]. Recent randomized controlled studies (RCTs) and a meta-analysis demonstrated that a short period of adjuvant corticosteroids may decrease the risk of kidney scar formation after APN [10, 13, 14]. These results and minimal adverse events make adjuvant corticosteroid administration to antibiotics a promising future treatment option for children with pyelonephritis.
We conducted a systematic review and meta-analysis to clarify the role of adjuvant administration of corticosteroids to antibiotic treatment for kidney scar prevention after APN in pediatric patients.
Methods
Study registration
We conducted this meta-analysis according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) and the Cochrane Handbook for Systematic Reviews of Interventions [16, 17]. On October 12, 2022, a prespecified review protocol was registered in OSF (https://osf.io/gw8b3/).
Search strategy
An extensive literature search through major databases was carried out for RCTs from inception until October 12, 2022, investigating the efficacy and safety of adjuvant corticosteroids in preventing kidney scarring in children with APN. Our search strategy was based on the electronic search by three reviewers (NG, AG, MM) of the available literature in the main medical e-databases (PubMed/MEDLINE and Scopus) (Supplementary Table 1), including relevant terms for kidney scars, pyelonephritis, corticosteroids, and children. Clinicaltrials.com and OSF were screened for additional data. There were no limitations regarding publication year and language. Finally, we screened all the references from the included studies for additional studies.
Eligibility criteria
The research question was defined using the following criteria [18]: articles were RCTs published in the English language with no limitation on the publication year; pediatric patients with UTI were over two months of age; adjuvant corticosteroid administration to antibiotics in the prevention of kidney scarring and placebo plus antibiotics were administered to the subjects of the intervention and control groups accordingly; the primary outcomes were the incidence of kidney scarring on dimercaptosuccinic acid scan (DMSA scan) after the intervention with corticosteroids in comparison to placebo administration; the secondary outcomes were mean change in clinical, serological, and imaging parameters; non-RCT studies, studies that included bagged urine collection, and studies that involved patients with a previous history of UTI, urinary tract anomalies, kidney failure, kidney scarring, and taking antibiotics before admission were excluded.
Data collection and extraction
Two authors (AG and MM) independently performed the search of the literature. The records were extracted and imported into a reference management tool (rayan.qcri.org) and duplicates were removed [19]. Then, they independently screened the retrieved studies (title and abstract) according to the inclusion criteria. The eligibility of the remaining studies was assessed independently by full-text screening and in case of disagreements, a third reviewer (NG) made the final decision. Finally, three reviewers (TV, TD, and PM) independently extracted the data of the eligible studies (publication year, study location, identification number, “NCT” number, number of patients in each study, intervention, and patients’ characteristics) into a pre-specified data extraction form. If any study missed data, corresponding authors were contacted to obtain sufficient data.
Quality assessment
The risk of bias was assessed by two independent-working examiners (NG and PM) using the revised Cochrane risk-of-bias tool (RoB 2.0 version 5.4.1) for randomized trials for each outcome [17, 20]. The RoB tool consists of five domains: randomization process; deviations from intended interventions; missing outcome data; measurement of the outcome; selection of the reported results. Studies were graded as low risk when all domains were classified as “low risk,” “some concerns,” or “high risk” in studies which had one domain classified as “high risk,” or three domains were classified as “some concerns.” In case of any disagreement, a third senior reviewer (DT) made the final decision.
Outcome measurements
The primary outcome was kidney scarring incidence after the administration of corticosteroids in pediatric patients with APN. Kidney scarring was defined as a photopenic cortical defect with or without loss of volume or contour. Secondary outcomes were mean change in the following parameters: procalcitonin (PCT), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), creatinine levels, urinary interleukin-6 (UIL-6) and UIL-8. Incidence of VUR, fever duration, kidney damage severity score at the early DMSA (early RDSS), hospitalization duration, risk of bacteremia, ultrasonographic pathologic features in the acute phase, and incidence of kidney scarring on the DMSA scan were also examined. Finally, we evaluated the distribution of a variety of adverse events.
Statistical analysis
Review manager software 5.4 (RevMan 5.4) was used for statistical analyses [17]. Data from intention-to-treat analyses (ITT) were used when available. Mean values and standard deviations (SD) were used for quantitative data analysis. Qualitative data were analyzed using a 95% confidence interval (95% CI) and risk ratio (RR) or risk difference (RD) when trials with no outcome events in both treatment and control arms were included [21].
Heterogeneity between the studies was assessed using the I2 test as < 40% may be low, 30–60% as moderate, 50–90% as substantial, and 75–100% as considerable [17]. When I2 was > 50%, the random effect model was applied. For the analyses, a p-value < 0.05 was considered statistically significant.
Finally, subgroup analyses were conducted based on the corticosteroid of use (dexamethasone).
Results
Search results
In total, we identified 6592 records from our initial search. After duplicate removal and title and abstract screening, 8 studies remained for full-text assessment for eligibility, with 5 studies included in the meta-analysis. In total, 693 randomized patients who met the inclusion criteria of the meta-analysis and 498 patients that completed the study (intervention and control groups) were included in the meta-analysis (Fig. 1) [13,14,15, 22, 23].
Baseline characteristics
Participants’ mean age ranged from 8.3 (7.9) to 50.55 (44.41) months (Table 1). In four studies, intervention with dexamethasone was made [13, 15, 22, 23] and in only one study [14] methylprednisolone was used as an adjuvant corticosteroid to antibiotic treatment for UTI/APN in pediatric patients. In three studies, the duration of intervention with corticosteroids was for 3 days [14, 22, 23], and in two studies [13, 15], corticosteroids were administered for 4 days. The diagnosis of UTI/APN was made with positive urine culture in three studies [13, 15, 23], and in two studies [14, 22], the diagnosis was made after positive urine culture and DMSA scan evaluation. Finally, in only three studies pediatric patients exclusively with APN were evaluated [14, 15, 22].
Risk of bias
Four of the five studies included in our meta-analysis were evaluated to be at “low risk of bias” [13, 14, 22, 23]. Only one study was evaluated to be at “some concerns” regarding the lack of well-described blinding processes [15]. A summary of the risk of bias assessment is described in Fig. 2.
Primary outcome
Co-intervention of corticosteroids with antibiotics showed a significant effect on the incidence of kidney scarring after UTI/APN (RR 0.64, 95% CI 0.42–0.98, I2 = 7%, p = 0.04) (Fig. 3).
Secondary outcomes
The risk of bacteremia remained the same between the two study groups (RD 0.00, 95% CI –0.01 to 0.01, I2 = 0%, p = 0.99) (Fig. 4). Regarding the length of hospitalization, corticosteroid administration did not lead to any significant change between the two study groups (RR 0.82, 95% CI 0.58–1.14, I2 = 0%, p = 0.24) (Fig. 5). Finally, corticosteroids did not lead to a recurrence of febrile UTI (RD − 0.01, 95% CI − 0.04 to 0.02, I2 = 0%, p = 0.60) (Fig. 6).
As no sufficient data were found for PCT, ESR, CRP, creatine levels, UIL-6/UIL-8, incidence of VUR, fever duration, and early RDSS, we could not come up with a meta-analysis of these endpoints and draw any conclusion.
Subgroup analysis
Dexamethasone administration
Evaluation of the subset of studies that used adjuvant dexamethasone to antibiotics in pediatric patients with APN did not show a significant effect on kidney scarring incidence after UTI/APN (Supplementary Fig. 2).
Discussion
Our meta-analysis evaluated the effectiveness of adjuvant corticosteroids to adequate antibiotic treatment in the reduction of kidney scar formation after APN/UTI in the pediatric population. The results of our meta-analysis showed that adjuvant corticosteroids to antibiotics led to a statistically significant reduction in kidney scarring incidence after APN/UTI in pediatric patients, without raising the risk of prolonged hospitalization, bacteremia, or recurrence of UTI.
UTI pathogens play a key role in inflammation, with the activation of local and systematic routes after the bacterial invasion [12]. Animal studies have shown that the activation of cytokines during APN can cause damage to the kidney tissue leading to kidney dysfunction [12, 24]. Kidney scarring is the result of the acute inflammation process and although APN is treated with adequate and aggressive antibiotic treatment, there is a high risk of kidney scar formation [12, 25, 26]. Anti-inflammatory agent administration in animal studies has shown statistical significance in the reduction of kidney scarring after APN [25, 27, 28]. Corticosteroids are one of the most used anti-inflammatory agents and the most studied option for kidney scar prevention after APN [29].
A few studies have investigated the effects of corticosteroids for the prevention of kidney scarring after pediatric APN or UTI [10, 12,13,14,15, 22, 23]. Meena et al. conducted the first meta-analysis to assess the efficacy and safety of adjuvant corticosteroids for preventing kidney scar formation in children with APN [10]. They included 529 randomized subjects from three RCTs drawing the conclusion that corticosteroids are effective in kidney scarring reduction compared with placebo.
We conducted an extended literature search based on the available literature in the main medical electronic databases (PubMed/MEDLINE and Scopus) with no limitations regarding publication year and language. Our meta-analysis included only well-designed, placebo-controlled RCTs that focused on the pediatric population. Additionally, the analysis was performed with the help of the most recent RoB 2.0 tool and the review procedure was done in accordance with the Cochrane Handbook for Systematic Reviews of Interventions [17, 20]. Moreover, our meta-analysis was characterized by low heterogeneity for all outcomes assessed. Finally, only one study was evaluated to be at “some concerns” with all remaining studies evaluated to be at “low risk of bias” in the quality assessment.
The main advantages of the present systematic review and meta-analysis include the larger population number of included pediatric patients (5 RCTs with 693 randomized patients who met the inclusion criteria of the meta-analysis and 498 patients who completed the study follow-up). We also investigated the effectiveness of corticosteroids based on the corticosteroid of use (dexamethasone).
Our meta-analysis had also some limitations that have to be acknowledged. Corticosteroids used in the RCTs of our analysis do not belong to the same classes, with one study [14] including methylprednisolone and the other four [13, 15, 22, 23] dexamethasone as the corticosteroid of choice. According to the “Coopman classification,” methylprednisolone belongs to class A and dexamethasone to class B corticosteroids [30]. They were also administered via different routes (intravenous or oral) and for different day courses (3-day or 4-day courses). Other limitations are that the total number of subjects who completed the study is relatively small (n = 498 children) and that the diagnosis of APN was not confirmed with DMSA in all RCTs, and therefore in three of them [13, 15, 23], the UTI episodes cannot be recorded with certainty as APN. Ghaffari et al. was the only study that evaluated the modification of interleukin levels in the urine, which can be helpful in the estimation of treatment response [15]. Finally, the outcomes of our meta-analysis are limited due to the incompatibility of the possible comparisons between the study outcomes of different RCTs; thus, adverse events and inflammation marker trends before and after co-intervention with corticosteroids could not be thoroughly evaluated.
The subgroup that received dexamethasone did not reach any significant result in kidney scarring reduction. It is believed that this result was influenced by the dynamics of the studies, as that of Huang et al. was the only RCT that led to a significant reduction of kidney scarring after methylprednisolone administration [14]. This study was not included in the subgroup of dexamethasone administration. When all RCTs were included in the meta-analysis, Huang et al. received a large weighting (32.5%), influencing the result. In conclusion, differences in corticosteroid classes may have a key role in these results.
As reported by the results of our meta-analysis, corticosteroids—a well-known and routinely used, inexpensive, and relatively safe agent in moderate short-course dosages—could lead to the reduction of the risk of kidney scarring in children with APN, without causing any serious adverse effects. Although there are data that support corticosteroid administration in kidney scarring prevention, current evidence is still insufficient. Further RCTs should evaluate the benefit of corticosteroids in fever duration after their initiation, urinary interleukins, and other serum/urine biomarker levels before and after the intervention and a variety of adverse events.
Conclusion
In conclusion, adjuvant corticosteroid treatment seems to benefit kidney scar reduction in children with APN. Further well-designed clinical studies examining the efficacy and safety of corticosteroids on kidney scarring reduction after APN should be conducted in the future to strengthen the results of our meta-analysis.
References
Shaikh N, Ewing AL, Bhatnagar S, Hoberman A (2010) Risk of renal scarring in children with a first urinary tract infection: a systematic review. Pediatrics 126:1084–1091. https://doi.org/10.1542/peds.2010-0685
‘t Hoen LA, Bogaert G, Radmayr C, Dogan HS, Nijman RJM, Quaedackers J, Rawashdeh YF, Silay MS, Tekgul S, Bhatt NR, Stein R (2021) Update of the EAU/ESPU guidelines on urinary tract infections in children. J Pediatr Urol 17:200–207. https://doi.org/10.1016/j.jpurol.2021.01.037
Mattoo TK (2011) Vesicoureteral reflux and reflux nephropathy. Adv Chronic Kidney Dis 18:348. https://doi.org/10.1053/J.ACKD.2011.07.006
Tullus K (2015) Outcome of post-infectious renal scarring. Pediatr Nephrol 30:1375–1377
Shaikh N, Craig JC, Rovers MM, Da Dalt L, Gardikis S, Hoberman A, Montini G, Rodrigo C, Taskinen S, Tuerlinckx D, Shope T (2014) Identification of children and adolescents at risk for renal scarring after a first urinary tract infection: a meta-analysis with individual patient data. JAMA Pediatr 168:893–900. https://doi.org/10.1001/jamapediatrics.2014.637
Shaikh N, Haralam MA, Kurs-Lasky M, Hoberman A (2019) Association of renal scarring with number of febrile urinary tract infections in children. JAMA Pediatr 173:949–952. https://doi.org/10.1001/jamapediatrics.2019.2504
Karavanaki KA, Soldatou A, Koufadaki AM, Tsentidis C, Haliotis FA, Stefanidis CJ (2017) Delayed treatment of the first febrile urinary tract infection in early childhood increased the risk of renal scarring. Acta Paediatr 106:149–154. https://doi.org/10.1111/apa.13636
Leung AKC, Wong AHC, Leung AAM, Hon KL (2019) Urinary tract infection in children. Recent Pat Inflamm Allergy Drug Discov 13:2–18. https://doi.org/10.2174/1872213X13666181228154940
Hewitt IK, Zucchetta P, Rigon L, Maschio F, Molinari PP, Tomasi L, Toffolo A, Pavanello L, Crivellaro C, Bellato S, Montini G (2008) Early treatment of acute pyelonephritis in children fails to reduce renal scarring: data from the Italian Renal Infection Study Trials. Pediatrics 122:486–490. https://doi.org/10.1542/peds.2007-2894
Meena J, Kumar J (2021) Adjuvant corticosteroids for prevention of kidney scarring in children with acute pyelonephritis: a systematic review and meta-analysis. Arch Dis Child 106:1081–1086. https://doi.org/10.1136/archdischild-2020-320591
Tramma D, Hatzistylianou M, Gerasimou G, Lafazanis V (2012) Interleukin-6 and interleukin-8 levels in the urine of children with renal scarring. Pediatr Nephrol 27:1525–1530. https://doi.org/10.1007/S00467-012-2156-2
Sharifian M, Anvaripour N, Karimi A, Fahimzad A, Mohkam M, Dalirani R, Gholikhani F, Rafiee MA (2008) The role of dexamethasone on decreasing urinary cytokines in children with acute pyelonephritis. Pediatr Nephrol 23:1511–1516. https://doi.org/10.1007/s00467-008-0864-4
Da Dalt L, Bressan S, Scozzola F, Vidal E, Gennari M, La Scola C, Anselmi M, Miorin E, Zucchetta P, Azzolina D, Gregori D, Montini G (2021) Oral steroids for reducing kidney scarring in young children with febrile urinary tract infections: the contribution of Bayesian analysis to a randomized trial not reaching its intended sample size. Pediatr Nephrol 36:3681–3692. https://doi.org/10.1007/s00467-021-05117-5
Huang Y-Y, Chen M-J, Chiu N-T, Chou H-H, Lin K-Y, Chiou Y-Y (2011) Adjunctive oral methylprednisolone in pediatric acute pyelonephritis alleviates renal scarring. Pediatrics 128:e496–e504. https://doi.org/10.1542/peds.2010-0297
Ghaffari J, Mohammadjafari H, Mohammadi GH, Mahdavi MR (2019) Assessment the effect of dexamethasone on urinary cytokines and renal scar in children with acute pyelonephritis. Iran J Kidney Dis 13:244–250
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(264–269):W64. https://doi.org/10.7326/0003-4819-151-4-200908180-00135
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J (2019) Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 10:ED000142
Schardt C, Adams MB, Owens T, Keitz S, Fontelo P (2007) Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak 7:16. https://doi.org/10.1186/1472-6947-7-16
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A (2016) Rayyan-a web and mobile app for systematic reviews. Syst Rev 5:210. https://doi.org/10.1186/s13643-016-0384-4
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898. https://doi.org/10.1136/bmj.l4898
Friedrich JO, Adhikari NKJ, Beyene J (2007) Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med Res Methodol 7:5. https://doi.org/10.1186/1471-2288-7-5
Rius-Gordillo N, Ferré N, González JD, Ibars Z, Parada-Ricart E, Fraga MG, Chocron S, Samper M, Vicente C, Fuertes J, Escribano J, DEXCAR Study Group (2022) Dexamethasone to prevent kidney scarring in acute pyelonephritis: a randomized clinical trial. Pediatr Nephrol 37:2109–2118. https://doi.org/10.1007/s00467-021-05398-w
Shaikh N, Shope TR, Hoberman A, Muniz GB, Bhatnagar S, Nowalk A, Hickey RW, Michaels MG, Kearney D, Rockette HE, Charron M, Lim R, Majd M, Shalaby-Rana E, Kurs-Lasky M, Cohen DM, Wald ER, Lockhart G, Pohl HG, Martin JM (2020) Corticosteroids to prevent kidney scarring in children with a febrile urinary tract infection: a randomized trial. Pediatr Nephrol 35:2113–2120. https://doi.org/10.1007/s00467-020-04622-3
Glauser MP, Meylan P, Bille J (1987) The inflammatory response and tissue damage. The example of renal scars following acute renal infection. Pediatr Nephrol 1:615–622. https://doi.org/10.1007/BF00853599
Pohl HG, Rushton HG, Park JS, Chandra R, Majd M (1999) Adjunctive oral corticosteroids reduce renal scarring: the piglet model of reflux and acute experimental pyelonephritis. J Urol 162:815–820. https://doi.org/10.1097/00005392-199909010-00067
Huang A, Palmer LS, Hom D, Anderson AE, Kushner L, Franco I (1999) Ibuprofen combined with antibiotics suppresses renal scarring due to ascending pyelonephritis in rats. J Urol 162:1396–1398
BahatÖzdoǧan E, Özdemir T, ArslansoyuÇamlar S, Imamoğlu M, Cobanoğlu Ü, Sönmez B, Tosun İ, Doğan I (2014) Could pyelonephritic scarring be prevented by anti-inflammatory treatment? An experimental model of acute pyelonephritis. Biomed Res Int 2014:134940. https://doi.org/10.1155/2014/134940
Haraoka M, Matsumoto T, Takahashi K, Kubo S, Tanaka M, Kumazawa J (1994) Suppression of renal scarring by prednisolone combined with ciprofloxacin in ascending pyelonephritis in rats. J Urol 151:1078–1080. https://doi.org/10.1016/s0022-5347(17)35187-x
Murugapoopathy V, McCusker C, Gupta IR (2020) The pathogenesis and management of renal scarring in children with vesicoureteric reflux and pyelonephritis. Pediatr Nephrol 35:349–357. https://doi.org/10.1007/s00467-018-4187-9
Coopman S, Degreef H, Dooms-Goossens A (1989) Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol 121:27–34. https://doi.org/10.1111/J.1365-2133.1989.TB01396.X
Funding
Open access funding provided by HEAL-Link Greece.
Author information
Authors and Affiliations
Contributions
Nikolaos Gkiourtzis: conception and design of the work, analysis, and interpretation of data, drafted the work, approved the version to be published, agreed to be accountable for all aspects of the work; Agni Glava: analysis and interpretation of data, revised the work critically, approved the version to be published, agreed to be accountable for all aspects of the work; Maria Moutafi: analysis and interpretation of data, revised the work critically, approved the version to be published, agreed to be accountable for all aspects of the work; Theopisti Vasileiadou: analysis and interpretation of data, revised the work critically, approved the version to be published, agreed to be accountable for all aspects of the work; Theodora Delaporta: analysis and interpretation of data, revised the work critically, approved the version to be published, agreed to be accountable for all aspects of the work; Panagiota Michou: analysis and interpretation of data, revised the work critically, approved the version to be published, agreed to be accountable for all aspects of the work; Nikoleta Printza: analysis and interpretation of data, drafted and revised the work critically, approved the version to be published, agreed to be accountable for all aspects of the work; Kali Makedou: analysis and interpretation of data, drafted and revised the work critically, approved the version to be published, agreed to be accountable for all aspects of the work; Despoina Tramma: analysis and interpretation of data, drafted and revised the work critically, approved the version to be published, agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gkiourtzis, N., Glava, A., Moutafi, M. et al. The efficacy and safety of corticosteroids in pediatric kidney scar prevention after urinary tract infection: a systematic review and meta-analysis of randomized clinical trials. Pediatr Nephrol 38, 3937–3945 (2023). https://doi.org/10.1007/s00467-023-05922-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-023-05922-0