Abstract
Purpose
Gestational diabetes mellitus (GDM) and thyroid dysfunction during gestation (GTD) are the two most prevalent endocrinopathies during pregnancy. The aim of the present review is to provide an overview of the peculiar aspects of GDM and GTD, to highlight the potential interactions and clinical consequences of these two frequent clinical conditions.
Methods
A literature review regarding GDM and GTD was carried out with particular interest on meta-analyses and human studies dealing with the (i) shared risk factors between GDM and GTD, (ii) the epidemiological link between GTD and GDM, (iii) physiopathologic link between GTD and GDM, (iv) clinical consequences of GDM and GTD, and (v) post-partum implications of GDM and GTD.
Results
The association between GDM and GTD is common and may be explained by the insulin-resistance state due to maternal GTD, to alterations in the placentation process or to the many shared risk factors. Discrepant results of epidemiologic studies can be explained, at least in part, by the changes in diagnostic criteria and screening strategies throughout the years for both conditions. GDM and GTD impact pregnancy outcome and have post-partum long-term consequences, but more studies are needed to prove an additional adverse effect.
Conclusions
Based on the epidemiological and physio-pathological link between GDM and GTD, it could be suggested that a diagnosis of GTD could lead to screen GDM and the other way round.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During pregnancy, profound hormonal modifications occur, involving both glucose homeostasis and thyroid function adaptation. Several compensatory mechanisms are required to meet the gestation-induced neo-equilibrium [1, 2]. Based on the above, pregnancy may be viewed as a challenging event, which implies that even slight pre-conception reduction in functional reserve (i.e., insulin-resistance or reduced thyroidal reserve) may lead to impaired adaptive responses and subsequent pathological states. This is the case of gestational diabetes mellitus (GDM) and of a wide spectrum of thyroid conditions, including subclinical hypothyroidism (SCH), presence of thyroid autoantibodies in euthyroid women and low maternal FT4 levels, which, for the aim of this review, will be referred to as thyroid dysfunctions during gestation (GTD).
GDM and GTD represent the most prevalent endocrinopathies during pregnancy [3, 4]. Taking into account the diversified screening approach and given the different risk according to ethnicity and body mass index (BMI), the prevalence of GDM ranges between 1 and 28% [5,6,7]. Reported prevalence for thyroid dysfunction also varies widely across studies according to different diagnostic criteria and included population, going from 0 to 13% for overt hypothyroidism and from 1.5 to 42.9% for SCH [8, 9]. A recent meta-analysis based on the latest American Thyroid Association (ATA) recommendations estimated a prevalence of 0.50% for overt hypothyroidism, 3.47% for SCH, and 2.05% for isolated low maternal fT4 level among pregnant women [10]. The prevalence of thyroid autoantibody positivity also varies widely among studies, with an observed rate among unselected pregnant women ranging from 2 to 17% [11], with a higher rate observed in areas characterized by iodine deficiency [12].
The aim of the present review will be to provide an overview of the relationship between GDM and GTD. The two conditions may indeed share some risk factors for their development, maternal and fetal repercussions as well as long-term consequences. Particular emphasis will be put on the screening strategy of GDM or GTD when the other condition is present.
Materials and methods
A comprehensive narrative review was performed. We searched for relevant literature using Medline, Embase and Cochrane search and including the following terms: (“gestational diabetes” [MeSH Terms] OR (“gestational diabetes” [All Fields]) AND (“thyroid” [MeSH Terms] OR “thyroid” [All fields]). Publications from 1974 up to 2022 were included.
Physiopathology of GTD and GDM
During pregnancy, physiologic hormonal changings strongly affect both glucose metabolism and thyroid function. In the first part of gestation an anabolic state establishes in preparation for the energy demands of later pregnancy; this state is characterized by normal or slightly higher insulin sensitivity to promote the glucose uptake by liver and muscle and subsequent reduced fasting glycemia [13, 14]. After this period, human placental lactogen (hPL), human placental growth hormone (hPGH), prolactin and cortisol promote the utilization of glucose by the feto-placental unit, establishing a catabolic status where hepatic gluconeogenesis and insulin resistance progressively increase (≈30% and 50% by late gestation, respectively) [1, 15]. These metabolic adaptations stress β-cells, causing augmented insulin secretion to maintain euglycemia and subsequent β-cell hypertrophy. Pre-pregnancy insulin resistance and/or β-cell dysfunction can trigger a GDM [16].
Pregnancy has also a profound impact on both thyroid gland morphology and function. First, the pre-pregnancy thyroid hormones steady-state equilibrium is markedly modified because of the high circulating levels of human chorionic gonadotropin (hCG) with its thyrotropic action. Moreover, an increase in iodothyronine deiodination activity of the placenta and in the serum concentration of T4-binding globulin occurs [2, 17]. An increase in thyroid volume, due to the stimulation by hCG, is observed throughout gestation. The degree of thyroid volume increase is directly proportional to the degree of iodine deficiency (≈10% in iodine replete countries and ≈20–40% in areas of iodine deficiency) [18]. This increase in thyroid volume is usually reversible at the end of pregnancy [19, 20], but can lead to permanent goiter in areas characterized by iodine deficiency. In the meantime, the production of thyroid hormones increases by nearly 50%, which is paralleled by a ≈50% increase in the daily iodine requirement. These physiological changes are not challenging in thyroid-disease-free women, but different degrees of thyroid dysfunction may occur even in euthyroid women with pre-pregnancy subtle thyroid abnormalities.
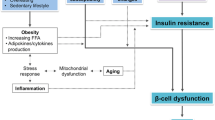
Studies performed in non-pregnant women suggest a close link between thyroid function and glucose homeostasis. First, several epidemiologic studies showed that non-pregnant patients with type 2 diabetes (T2D are characterized by a higher prevalence of thyroid dysfunction as compared to the general population [21]. On the other hand, several studies report that, in adult subjects, subtle changes in thyroid hormones levels, even within the normal range, are not free of metabolic repercussions [22,23,24,25,26,27].
In particular, in the presence of subclinical or overt hypothyroidism, muscle and adipose tissue become resistant to insulin, with a reduced insulin-stimulated glucose uptake in these tissues due to impaired translocation of GLUT4 glucose transporters on the plasma membrane [28, 29].
Given the above, it could be hypothesized that thyroid dysfunction, even if subclinical, could promote a state of insulin resistance also during gestation, and that this phenomenon could contribute to the pathogenesis of GDM during the second part of pregnancy [30].
Another interesting hypothesis regarding the possible physio-pathological link between GTD and GDM stems from the pivotal role of the placenta as a fetal endocrine organ. The placenta has a central role in determining insulin resistance during pregnancy via its secretion of cytokines and hormones, including hCG, hPL, and hPGH, into the maternal circulation [13, 31]. Moreover, the placenta is the main barrier between maternal and fetal environments and regulates the amount of nutrients reaching the fetus.
Indeed, thyroid dysfunction and thyroid autoimmunity in early pregnancy seem to cause an impairment in the development of the feto-placental unit [32]. Recent studies showed that pregnant women with SCH, even in the absence of thyroid autoimmunity, show important abnormalities in surrogate markers of defective placentation, including increased values of the uterine artery pulsatility index and an increased rate uterine artery Doppler velocimetry index abnormalities [33]. Women with thyroid dysfunction also display typical alterations in placenta histological features, including decidual vasculopathy, maternal vascular malperfusion, retroplacental hematoma and subchorionic thrombi [32, 34].
Data coming from histologic analysis of the placenta of women with GDM show a wide array of abnormalities, including placental overgrowth, villous immaturity and edema, vascular abnormalities and altered contractile and vasodilatory responses [35]. Moreover, recent studies suggest that alterations in gene expression and DNA methylation occur in the placental tissue of GDM women [36]. Interestingly, several studies have demonstrated that the placenta is able to secrete circulating microRNAs in maternal blood flow that can influence gene expression in other maternal organs [37, 38].
hCG is a placental hormone that plays a central role in prolonging the progesterone-secreting activity of the corpus luteum and in stimulating trophoblast differentiation, uterine angiogenesis and immunotolerance [39]. Low β-hCG levels are considered a marker of impaired development of the feto-placental unit [40, 41]. Furthermore, a higher hCG in early pregnancy is negatively associated with GDM risk [42,43,44]. Although no study directly investigated this issue, it could be hypothesized that a defective placentation process in the early phases of gestation due to the presence of thyroid dysfunction could impair placental hormone secretion (including the secretion of beta-hCG) and favor insulin resistance and eventually GDM in the second part of pregnancy.
Moreover, it was recently demonstrated that women with positive anti-TPO antibodies (TPO Ab) display an impaired thyroidal response to hCG in terms of reduced increase in circulating thyroid hormones [45]. The resulting lower FT4 levels could induce a state of insulin resistance, favoring the development of GDM. On the other hand, also pre-existing and/or early pregnancy insulin resistance may affect the placentation process, resulting in lower hCG and subsequent reduced thyroid stimulation with reduced thyroid hormones secretion.
GDM and GTD: common risk factors?
A possible association between GDM and GTD could be sustained by shared risks factors, as illustrated in the following paragraphs.
Non-modifiable risk factors
Genetic background, ethnicity and environmental exposure
A family history of T2D or hypothyroidism is a known risk factor of GDM [46, 47] or GTD [48], respectively. This phenomenon could reflect the combination of genetic susceptibility and shared environmental exposure and/or lifestyle among households.
For GDM, candidate gene studies identified the association of risk variants and polymorphisms for T2D with GDM, thereby confirming the genetic similarity between GDM and T2D [49, 50]. A shared genetic background for thyroid autoimmunity is suggested by studies on monozygotic twins, with an observed 55% concordance rate [51]. Nevertheless, the role of shared incorrect dietary habits among families [52,53,54], including a high in fat and sugar diet and a reduced iodine intake, could contribute to the observed association.
There is a general consensus about the increased prevalence of GDM in non-Caucasian populations [55] and in specific ethnic groups (i.e., South Asian, Caribbean, and Middle East [46, 56, 57]). The impact of ethnicity on thyroid dysfunction during pregnancy is less univocal. While some studies suggest a different prevalence of GTD among different ethnic groups [58, 59], other studies do not show any difference [60]. Recent evidence suggests that using ethnic-specific reference ranges for thyroid function parameters during pregnancy would allow to correctly identify pregnant patients with a SCH [61, 62]. A further confounding effect could be due to differences in iodine intake among different ethnic groups due to varying dietary habits and access to multivitamin supplementation [63, 64].
Lastly, the role of the exposure to environmental pollutants during pregnancy in increasing the risk of both GDM [65, 66] and GTD [67, 68] among subjects living in the same area cannot be excluded.
Age
The prevalence of GDM increases with age [69, 70], and an age above 35 is often considered as a criterion for GDM screening [70,71,72].
The most recent ATA guidelines regarding GTD suggest that women older than 30 years should be specifically targeted for thyroid dysfunction screening during pregnancy [48]. Indeed, several studies showed an increase in the prevalence of hypothyroidism with age, reaching a prevalence of ≈10% among women older than 55 years [73, 74]. Although data in the general population suggest that hypothyroidism should be more frequent in older pregnant women, a cohort study specifically designed to evaluate this hypothesis failed to show any increase in the prevalence of hypothyroidism in women older than 30 years when compared with younger women [75].
Modifiable risk factors
Body mass index
It is widely recognized that obesity is a risk factor for both T2D and GDM [70, 76]. On the other hand, to our knowledge, no specific study evaluated if obesity is a risk factor for hypothyroidism during pregnancy. Although this relationship could be hypothesized, literature data coming from the non-pregnant general population suggest that this topic is more complex than what is commonly observed in GDM [77]. Indeed, some population studies seem to show a relationship between BMI and thyroid function [22, 78]. Many authors instead suggest that subjects living with obesity, especially morbid, experience an increase in TSH levels that probably does not reflect a hypothyroid state, but rather a compensatory “hypometabolic” mechanism due to the high fat mass, that quickly recedes after weight loss [77]. A possible role of leptin signaling was suggested to explain this phenomenon [79]. Similarly, while some authors suggest that obesity could be a risk factor for thyroid autoimmunity [80], other showed that the rate of thyroid antibodies positivity was comparable between hypothyroid subjects living with obesity and normo-weight euthyroid controls. Moreover, the male/female ratio commonly found in patients with hypothyroidism was not observed among subjects living with obesity and with higher levels of TSH but no thyroid antibodies [77].
Specific studies in the pregnant population are needed to assess whether obesity has any impact on the risk of hypothyroidism or thyroid autoimmunity during pregnancy, although the case finding ATA screening strategy recommends to test for serum TSH if the pregnant woman has a BMI ≥ 40 kg/m2.
Vitamin D deficiency
The association between GDM and vitamin D deficiency is still controversial. A recent meta-analysis on pregnant women suggests that vitamin D deficiency could increase the risk of GDM, but the included studies were highly heterogeneous [81]. Some studies suggest that vitamin D supplementation could help reducing fasting plasma glucose and improve insulin resistance in pregnant women [82, 83], although a recent meta-analysis highlighted how the available randomized controlled trials (RCTs) are all of insufficient quality [84]. In conclusion, available evidence is not sufficient to recommend a vitamin D supplementation in GDM-complicated pregnancy to ameliorate the metabolic state and more high-quality RCTs are needed.
Similarly, the association between vitamin D deficiency and hypothyroidism or thyroid autoimmunity during pregnancy is not completely elucidated. Although several studies suggest that non-pregnant subjects with autoimmune thyroid disease have lower serum vitamin D levels as compared to healthy controls [85], only few retrospective studies assessed the correlation between thyroid function and vitamin D deficiency during pregnancy. A recent cohort study, involving 277 pregnant women at 13–28 gestational week (GW), showed that vitamin D levels were positively correlated with TSH, but negatively correlated with free thyroid hormones [86]. On the contrary, other retrospective studies found no association between vitamin D levels and thyroid function parameters throughout gestation [87, 88]. Another study showed a direct linear association between vitamin D serum levels and circulating thyroid hormones, although the association was not seen in the second and third trimester [89]. The postulated mechanism underlying the possible association between thyroid function and Vitamin D levels could be due to the presence of 1,25(OH)D receptors on anterior pituitary [90] and thyroid cells [91], but also to its well-known immune-modulating effect [92, 93]. Nevertheless, the lack of data coming from RCTs does not allow to draw firm conclusions regarding the effects of vitamin D supplementation during pregnancy on thyroid function.
Selenium
Selenium has antioxidant properties and is involved in immune function [94]. During pregnancy, there is an increased requirement for selenium due to fetal growth which induces a decrease in maternal blood levels.
Some evidence exists suggesting that selenium levels may be significantly lower in women with GDM than in those without, but the negative correlation has been found only with glycemia and not with insulin levels [95, 96].
Interventional studies in women with GDM gave controversial results: in one RCT the supplementation of 200 μg/d selenium for 6 weeks from 24 to 28 GW was able to reduce fasting plasma glucose, insulin levels and Homeostasis model assessment—insulin resistance (HOMA-IR) [97]. Another RCT showed that a supplementation of 50 µg/d of selenium for 12 weeks starting from the second trimester of pregnancy could reduce fasting plasma glucose [98]. On the contrary, another trial in which pregnant women received 100 µg/d of selenium no effect on fasting plasma glucose, 2-h post-prandial blood glucose, HbA1C, insulin level, and HOMA-IR could be observed [99]. In conclusion, available evidence is not sufficient to recommend a selenium supplementation in GDM-complicated pregnancies to ameliorate the metabolic state and more RCTs are needed.
The thyroid is one of the organs with the highest selenium content because it expresses several specific selenoproteins, including glutathione peroxidases, thioredoxin reductases, iodothyronine deiodinases (responsible for the activation and degradation of T3), and selenoprotein P. The synthesis of thyroid hormones needs adequate levels of selenium [100,101,102]. Although the role of selenium supplementation as a preventive strategy for post-partum thyroid dysfunction was suggested by a clinical trial [103], no data support the role of selenium deficiency in the pathogenesis of GTD during pregnancy.
Myo-inositol
Inositols have insulin-mimetic properties and are able to lower post-prandial blood glucose, delaying carbohydrate digestion and absorption. Furthermore, they can modulate insulin sensitivity [104].
Myo-inositol is one of the predominant forms under which inositols can be found in nature and that have shown to have therapeutic effects in human health; the dysregulation of myo-inositol metabolism is associated with insulin resistance and long-term microvascular complication of diabetes [105]. Because of reduced intake, increased catabolism and excretion, decreased biosynthesis and inhibition of intestinal absorption of myo-inositol, the need of this nutrient is increased during pregnancy [106, 107]. A RCT conducted in women with GDM, comparing the effects of 8-week treatment of different dosage and combinations of inositol stereoisomers on insulin resistance, showed a significant amelioration in HOMA-IR and a lower variation in average weight gain in the treated group [108]. Although some RCTs suggested that myo-inositol supplementation could reduce the incidence of GDM in high-risk women [109,110,111,112,113], a Cochrane review in 2016 concluded that the evidence sustaining the role of myo-inositol supplementation in reducing insulin requirements, reducing plasma glucose and preventing adverse neonatal and pregnancy outcome is still insufficient [114]. A possible explanation for these inconclusive results could be the lack of a universally accepted definition of myo-inositol deficiency, making difficult for the clinicians to specifically target myo-inositol deficient women. Based on the hypothesis that myo-inositol supplementation could limit the need for insulin therapy in women with GDM [115], a double-blind randomized study is ongoing (ClinicalTrials.gov Identifier: NCT03875755).
Myo-inositol is a precursor of inositol triphosphate which plays an important role in TSH in thyroid cell [116]. The effects of myo-inositol supplementation on thyroid function are also not univocal and there is no study evaluating a possible role of myo-inositol supplementation on the incidence of hypothyroidism in pregnancy. Some intervention studies performed in adult non-pregnant subjects showed that a myo-inositol (in association with selenium) supplementation was able to decrease TSH and anti-thyroid antibodies titers in patients with chronic autoimmune thyroiditis [117]. Nevertheless, these results should be confirmed by larger RCTs [116, 118].
Cigarette smoking
Available evidence does not suggest that the cigarette smoking during pregnancy is a risk factor for GDM [119, 120].
Some studies performed on non-pregnant subjects suggest that cigarette smoking could reduce TSH, increase thyroid hormones and reduce the probability of developing positivity for anti-thyroid antibodies [121, 122]. Nevertheless, data on pregnant women are few and controversial. Some studies suggest that women smoking during pregnancy display higher levels of FT3, while data on thyroid autoantibody positivity and TSH levels are discrepant among studies [123,124,125].
Is GTD a risk factor of GDM?
The problem of the diagnosis
Answering to the question if GTD is a risk factor of GDM presents several critical issues. Specifically, diagnostic criteria and screening strategies for both conditions may vary.
Diagnostic criteria
First, the diagnostic criteria for both GDM and GTD have changed during the last decades.
Diagnostic cutoff of GDM changed over time [126]. The commonly accepted diagnostic criteria for GDM have been defined only in 2010 by the International Association of Diabetes in Pregnancy Study Groups (IADPSG) [127], and then endorsed by the WHO in 2013 [128].
According to IADSPG recommendation, GDM was defined as any between fasting glycemia ≥ 5.2 mmol/l, 1 h OGTT glycemia ≥ 10 mmol/l or 2 h OGTT glycemia ≥ 8.5 mmol/l [127]. This definition encompassed the following sub-types: (i) early-diagnosed GDM, when screening is performed before 24 GW and fasting glycemia is 5.2–6.9 mmol/l, (ii) early diabetes in pregnancy, when before 24 GW fasting glycemia is ≥ 7 mmol/l, (iii) classical GDM, when OGTT is performed after 24 GW and fasting glycemia is 5.2–6.9 mmol/l, 1 h OGTT glycemia ≥ 10 mmol/l or 2 h OGTT glycemia is 8.5–11 mmol/l, and (iv) diabetes in pregnancy, when OGTT is performed after 24 GW and fasting glycemia is ≥ 7 mmol/l or 2 h OGTT glycemia is ≥ 11.1 mmol/l.
The most recent ADA guidelines recommend to define the so-called “early abnormal glucose metabolism” as a fasting glucose above 6.1 mmol/l or an HbA1c above 39 mmol/mol before 24 GW [129]. This change in the diagnostic criteria stems from the fact that the IADPSG diagnostic thresholds for GDM for the 75-g OGTT were not derived from data in the first half of pregnancy. These new cut offs may identify women at higher risk of adverse pregnancy and neonatal outcomes, need for insulin treatment, and post-24 GW GDM diagnosis.
It is evident how the definition of GDM includes a wide spectrum of clinical conditions, including several dysglycemic states during pregnancy according to gestational age and glucose values.
Similarly to what happened for GDM, also the definition of the most frequently observed form of GTD, i.e., SCH, has changed several times in the last decade. According to the most recent ATA Guidelines on Thyroid disease in pregnancy, in the absence of in-house established reference ranges for TSH, a reduction in the upper reference range by ≈ 0.5 mIU/l (corresponding in most cases to a value ≈ 4.0 mIU/l for most TSH assays) should be used to diagnose SCH in a pregnant patient during the first trimester, with a gradual return to pre-pregnancy reference ranges during the second and third trimesters [48]. This novel TSH cutoff actually raised the previous ones, i.e., 2.5 mIU/l during the first and 3.0 during the second and third trimesters. As a result of the increased TSH threshold for diagnosing SCH in pregnancy, a significant decrease in the prevalence of SCH was observed.
Screening strategies
Even after reaching an international agreement on the diagnostic criteria for diagnosis of GDM, the recommended screening strategy (universal vs selective, one vs two-step) still differs among different national guidelines [7, 70, 130]. Even when a selective screening is recommended, there is no agreement about which criteria should be followed to select high-risk patients. Some factors (BMI ≥ 25 kg/m2, advanced age, non-white ancestry, family history of T2D, previous history of GDM or macrosomia) are widely known to predict the development of GDM [69]. Others, such as parity, male fetus, multiple pregnancy, gestational weight gain, genetic factors, polycystic ovary syndrome, psychosocial factors (for example, depression in pregnancy), unhealthy dietary factors before pregnancy, physically inactive lifestyle before and during pregnancy, steroid or antipsychotic treatment and pregnancy following assisted reproductive technology are not routinely considered when a selective screening is chosen [131].
Also in the case of GTD, there is still no consensus regarding the screening strategy to be employed. Most guidelines recommend testing thyroid function only in women at increased risk, known as case finding, rather than universal screening [48]. According to the most recent ATA guidelines [48], TSH should be tested in case of: a history of hypothyroidism/hyperthyroidism or current symptoms/signs of thyroid dysfunction; known thyroid autoantibody positivity or presence of a goiter; history of head or neck radiation or prior thyroid surgery; age > 30 years, concomitant autoimmune disease; history of pregnancy loss, preterm delivery, or infertility, multiple prior pregnancies (≥ 2); family history of autoimmune thyroid disease or thyroid dysfunction; morbid obesity (BMI ≥ 40 kg/m2); use of amiodarone or lithium, or recent administration of iodinated radiologic contrast; residing in an area of known moderate to severe iodine insufficiency.
The case-finding approach overlooks a large number of women with abnormal thyroid function tests [132,133,134,135,136]. However, in a randomized trial, universal thyroid function screening and treatment did not improve overall pregnancy outcomes as compared with testing only high-risk women [137]. Similar findings were observed by a recent case control study, showing that although a case-finding approach may overlook some women with GTD, it is not associated with a higher risk of adverse pregnancy outcomes [138].
Is SCH associated to an increased risk of GDM?
Several studies evaluated whether the presence of SCH during pregnancy was predictive of development of GDM, as summarized in Table 1. Some studies showed a higher prevalence of SCH among women with GDM when compared with controls [139,140,141,142,143], while other studies [144,145,146,147,148] did not find this association. The varying results of these studies should be interpreted in light of differences in study design, different criteria for diagnosing SCH and GDM during pregnancy and timing of evaluation.
The majority of data come from retrospective cohort studies [139, 142,143,144,145, 149], while other studies [140, 147, 150, 151] were designed as case controls (comparing a group of women with GDM and a control of healthy pregnant women). Some authors used a TSH cutoff lower than non-pregnant normal value [143, 147, 150], while other used trimester-specific ranges [139, 142, 144, 149, 151]. In addition, timing of evaluation was not uniform among studies: some studies evaluated thyroid function parameters during the first trimester [139, 142], others during second trimester (usually at the same time of OGTT) [140, 150], or during the third trimester [144, 151].
Keeping these important discrepancies in mind, the main finding that emerges from available studies is that SCH seems to have a predictive role for GDM especially when a higher threshold for defining SCH is considered (TSH > 4.0 mIU/l), particularly in patients with positive thyroid autoantibodies. This finding was confirmed by two recent meta-analyses [152, 153]. The meta-analysis by Kent et al. in particular highlighted how when considering studies using a TSH level < 4.0 mIU/l for SCH diagnosis, no association between SCH and GDM was observed, unless patients were thyroid autoantibody positive. At difference, when a TSH cutoff > 4.0 mIU/l was used, a significant increase in the odds of GDM, regardless of thyroid autoantibody status [153] could be observed. Interestingly, another recent meta-analysis by Maraka et al. failed to show an association between SCH and GDM, but in this case, a TSH value above 2.5 mIU/l was considered, and no correction for the presence of thyroid autoantibodies was performed [154].
A review [155] encompassing several published meta-analyses evaluating risk factors for GDM showed that hypothyroidism (either overt or subclinical) was, surprisingly, the only strongly predictive risk factor for GDM besides obesity, even after correcting for possible biases, although this finding was based on a single meta-analysis including only seven studies [156]. Moreover, the authors were not able to differentiate subclinical from overt hypothyroidism.
The evaluation of the predictive role of TSH as a continuous variable, irrespective from a pre-fixed normality threshold, led to contrasting results. While some studies showed a direct, linear correlation between higher TSH values and GDM risk, even after correction for possible confounders [142, 143, 146], the majority of authors failed to observe a direct correlation between TSH values and GDM risk [42, 144, 148, 151, 157, 158]. Moreover, no direct correlation between TSH levels and metabolic parameters, including, BMI, HbA1c, triglycerides, or HOMA-IR, could be found [144].
In conclusion, the relationship between TSH values and risk of GDM seems to be not linear, with a sharp increase in risk with TSH levels above 4 mU/l and with the additional risk given by the presence of thyroid autoantibodies.
Can subtle alterations in circulating FT4 increase the risk of GDM, even when TSH is normal?
Several recent studies have highlighted how subtle changes in circulating FT3 and FT4, even with normal TSH, could be linked to an increased incidence of GDM. The most consistently observed finding is a correlation between a combination of low FT4 levels, high FT3 and, consequently, a high FT3/F4 ratio, and a higher risk of developing GDM in late gestation [148, 157,158,159]. The inverse relationship between FT4 levels and risk of GDM was also confirmed by a recent meta-analysis [160]. Some studies show that FT3 and FT3/FT4 ratio are positively associated with higher fasting glucose levels among women with GDM [159, 161]. Even among healthy pregnant women without GDM a lower FT4 and a higher FT3/FT4 ratio could be related to a worse metabolic profile (i.e., higher BMI, post-OGTT glucose, HbA1c, fasting insulin, HOMA-IR, triglycerides and placental weight) [144, 162].
The physiopathologic reasons underlying of this relationship are not fully understood. One of the possible explanations comes from the role of deiodinases in determining active thyroid hormone availability. T4 is the prohormone of T3 and exerts its effect mainly by converting to T3 through the action of several deiodinase enzymes [163]. For this reason, low levels of FT4 (the metabolically inactive prohormone) and a high FT3/FT4 ratio can be considered as markers for increased deiodinase activity. Obesity has been associated with low FT4 levels [161, 164,165,166], although through mechanisms that are not completely understood. An increased body weight can increase deiodinase activity, possibly through the action of leptin, resulting in lower FT4 and higher FT3/FT4 ratio [161, 167]. This effect would be even more marked in areas characterized by iodine deficiency [168]. This hypothesis is supported by a recent study demonstrating that a higher expression and activity of Type 3 Deiodinase can be observed in the placental tissue of mothers with GDM [141]. According to this hypothesis, alterations in circulating thyroid hormones would be a consequence, rather than a cause, of the higher BMI typical of women with GDM.
Another interesting hypothesis was suggested by a recent study including more than 18,000 women in China aimed at identifying if there was a relationship between hCG levels in early pregnancy and the risk of GDM, and if this was mediated by FT4 levels. The results showed that higher hCG in early pregnancy were associated with a lower GDM risk in TPO Ab negative women, but not in TPO Ab positive ones. The authors hypothesized that lower hCG in early pregnancy and/or the presence of TPO Ab could lead to alterations in the placentation process and reduced hCG-mediated thyroid stimulation with a subsequent lowering in FT4 levels, both leading to an increased risk of insulin resistance and GDM [42].
Is thyroid autoantibody positivity a risk factor for GDM?
Few and weak evidences supported an independent association of thyroid autoantibodies positivity and GDM [169]. An early report by Olivieri et al. suggested that women with multiple risk factors of GDM were more frequently positive for thyroid autoantibodies (16% vs 11.7%), independently from thyroid function [170]. No difference in thyroid autoantibodies prevalence was instead observed in GDM vs healthy pregnant women in several more recent studies [145, 157, 171, 172]. Moreover, Karakosta et al. [149] showed that the sole presence of thyroid autoantibodies in euthyroid women did not confer an increased risk of GDM.
A meta-analysis published in 2015 specifically aimed at assessing the link between GDM and the presence of thyroid autoantibodies, showed only a weak association between thyroid autoantibodies and GDM. The sub-group meta-analysis highlighted that a significant positive association could be found in women with concurrent thyroid dysfunction, but not in euthyroid women [173].
In conclusion, available literature suggests that an association between thyroid autoantibodies positivity and GDM can be observed only when a thyroid dysfunction is present.
Do GDM and GTD impact on the same pregnancy-related outcomes?
The pregnancy complications of the two conditions are summarized in Table 2.
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study [174] demonstrated that maternal hyperglycemia increases the risk of adverse pregnancy outcomes for both the newborn (including macrosomia, shoulder dystocia, neonatal hypoglycemia, intensive neonatal care and hyperbilirubinemia) and the mother (including preeclampsia, cesarean section and premature delivery). Indeed, different GDM sub-types and/or increases in different parameters of OGTT (fasting, 1 h or 2 h post-load) may have different prognostic relevance in terms of adverse pregnancy outcomes [127, 175]. Treating GDM can prevent, at least in part, the occurrence of these complications [127, 176, 177].
Evidence about the efficacy of screening and treating early-pregnancy abnormal glucose metabolism is conflicting and this depends on small sample size, different screening strategies with different diagnostic thresholds, heterogeneity in age, BMI and ethnicity of studied populations [178,179,180]. A recent meta-analysis [181] did not find any difference in several pregnancy outcomes among all the trials comparing early screening and treatment of dysglycemia to routine care, even if a sub-group analysis of trials that performed a universal screening, as opposed to only including GDM high-risk women, demonstrated a lower rate of large-for-gestational-age with early screening and treatment for GDM. Two large RCTs, (TESGO study/Taiwan, NCT 03523143 and LEMA_GDM, NCT04451915) are ongoing to better clarify the matter [182].
Overt maternal hypothyroidism was long known to be associated with an increased risk of adverse pregnancy complications [183] and subsequent impairment of fetal neurocognitive development [184]. More specifically, overtly hypothyroid pregnant women display increased risks of gestational hypertension, premature birth, low birth weight, pregnancy loss, and lower offspring intelligence quotient (IQ) [185, 186]. Based on the above findings, it is clear that overt hypothyroidism negatively affects the maternal–fetal unit.
On the other hand, GTD has been less consistently associated to adverse pregnancy outcomes which, at least in part may depend upon different TSH cutoffs used for defining SCH and by the non-systematic evaluation of thyroid autoantibodies status. In particular, the notion that thyroid autoantibodies positivity per se may affect pregnancy outcome independently from thyroid function has further complicated the issue.
The main end-points for which the role of GTD was evaluated include: effects on pregnancy outcome (i.e., pregnancy loss), adverse perinatal outcomes (i.e., premature delivery and hypertensive disorders), and neurocognitive outcomes in offspring. Although the results of the published studies remain contrasting, according to a meta-analysis evaluating the risk of pregnancy complications (pregnancy loss, preterm delivery, and placental abruption) in relation to maternal thyroid status, a significant association with SCH during early pregnancy would actually be present. It should be highlighted that SCH was variably defined across the included studies [154]. A recent cohort study including more than 8000 pregnant women showed that a maternal TSH above 4 mIU/l was associated with an approximately twofold increased risks of prematurity and respiratory distress syndrome in the offspring [187].
Post-partum implications
GDM
Mother
After delivery, in most cases, euglycemia is restored, but GDM will recur in 30–84% of subsequent pregnancies, according to ethnicity [188]. Weight gain between pregnancies and post-partum weight are also important risk factors for GDM recurrence [188].
Moreover, a history of GDM confers a sevenfold increased risk of T2D in the mother [188,189,190], and a post-partum OGTT and life-long metabolic follow-up are recommended to screen a persistent dysglycemia or a new onset of prediabetes or diabetes, respectively [191]. Furthermore, GDM diagnosed by different criteria (IADSPG vs Carpenter&Coustan) was found differently associated to persistent prediabetes or overt diabetes, being IADSPG criteria less predictive [192].
An interesting meta-analysis found that the risk of T2D is increased mostly in case of raised fasting glucose, need of insulin during pregnancy, and mostly in case of early-diagnosed GDM [193]. Moreover, women with a previous GDM display an elevated cardiovascular risk, even in the absence of development of T2D and women with a history of GDM who become diabetic after pregnancy have an increased risk of microvascular complications as compared to T2D women without a previous GDM [194, 195].
Offspring
Maternal GDM could affect the metabolic status of offspring all life-long. In fact, a higher risk of obesity and dysglycemia during infancy and adulthood has been shown in the sons and daughters of women with GDM [196,197,198,199,200]. Several studies agree about the role of fetal GDM exposure on the development of neuro-psychiatric disorders (substance use disorders, schizophrenia, mood and anxiety disorders, eating disorders, intellectual and developmental disorders) in childhood and adulthood [201, 202]. It should be emphasized that a part of this effect could be due to preeclampsia, a condition that is known to be associated to increased risk of psychiatric disorders [203].
GTD
Mother
According to a recent study, almost 40% of patients with SCH during pregnancy showed a persistent hypothyroidism after delivery during a median follow-up of 11 months, with anti-thyroid antibody positivity during pregnancy and persistency at 6 weeks after delivery as the significant risk factors for long-term hypothyroidism. Moreover, almost one-third of women with normal thyroid function 6-week post-partum were found to have hypothyroidism during the follow-up [204]. In another study, where women were followed for 20 years, overt hypothyroidism and TPOAb increased the risk for subsequent thyroid disease 17- and 4.2-fold, respectively, showing a synergic effect [30].
It is established that family history of thyroid dysfunction is a risk factor for personal thyroid dysfunction. Genetic background is important, but fetal programming may have a role [205]. Experimental data from animal suggest that deficiencies of thyroid hormones during gestation and lactation can alter the thyroid function and the metabolic status of the fetus [206].
Offspring
The effects of maternal SCH on fetal neurocognitive development are by far less clear. Some previous studies indicated a slight but significant reduction in IQ among children as well as a delay in motor skill development, language development, and attention at 7–9 years of age in children born to untreated women with gestational SCH compared to euthyroid controls [184]. Some studies suggest that even the positivity for thyroid autoantibodies, in the absence of SCH, could cause detrimental effects on children neurological development, even if there is great discrepancies among studies [207]. However, intervention studies in pregnant women with SCH receiving placebo or LT4 did not differ for maternal and fetal outcomes. Potential limitations of these negative results were suggested to be related to the delayed initiation of treatment with LT4 (approximately at 13 and 16 weeks of pregnancy, respectively) [208, 209].
Association of GTD and GDM
Data regarding the long-term consequences of GDM and GTD when the two conditions are associated are still scanty. The presence of overt hypothyroidism during pregnancy seems to confer a sixfold higher risk for developing T2D throughout a 20-year follow-up, even after adjustment for age, BMI, parity and a history of GDM [30]. Moreover, some evidence suggests that post-partum thyroiditis is more frequent in women with a history of GDM than in healthy pregnant women [150].
Conclusions
GDM and GTD are the most common endocrinopathies found in pregnant women. Several studies suggest that these two conditions often co-occur. This association may be explained by the induced insulin resistance state due to the action of thyroid hormones on the mother. Another possible explanation resides on the alterations in the placentation process which are typical of both GDM and GTD. Furthermore, the association between GDM and GTD could be sustained by several shared risk factors. Lastly, the possible role of GDM as a cause of thyroid dysfunction has not been investigated up to now, but could be the topic of future studies.
Based on the evidence of a possible epidemiological link between GDM and GTD, it could be suggested that a diagnosis of GTD could lead to screen GDM and the other way round.
Furthermore, both conditions are associated with an increased risk of pregnancy complications, most importantly preeclampsia and preterm delivery, so it could be hypothesized that the early management of the two could further improve pregnancy outcomes. Although in the past years, many studies have evaluated the epidemiological correlation between GDM and GTD, data regarding some specific clinical issues are lacking. These include the potential: (i) impact on the therapeutic management of GDM and GTD when the two conditions coexist; (ii) benefit of early treatment of GDM and GTD on pregnancy outcome; (iii) differences in terms of post-partum outcomes (both early and long-term) of GDM and GTD on the mother and the offspring when the two conditions are associated. Further prospective studies on these topics are needed to provide this essential information to the clinical community and to ameliorate the management of these two very common pregnancy-associated conditions. In the meanwhile, since it is highly probable that the coexistence of these two common disorders may result in a worse pregnancy outcome, these pregnant patients should be followed up with particular attention and care.
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Catalano PM, Tyzbir ED, Roman NM, Amini SB, Sims EA (1991) Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am J Obstet Gynecol 165(6 Pt 1):1667–1672
Burrow GH (1990) Thyroid status in normal pregnancy. J Clin Endocrinol Metab 71(2):274–275
Amin A, Robinson S, Teoh TG (2011) Endocrine problems in pregnancy. Postgrad Med J 87(1024):116–124
Modzelewski R, Stefanowicz-Rutkowska MM, Matuszewski W, Bandurska-Stankiewicz EM (2022) Gestational diabetes mellitus—recent literature review. J Clin Med 11(19):5736
Hillier TA, Pedula KL, Ogasawara KK, Vesco KK, Oshiro CES, Lubarsky SL et al (2021) A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med 384(10):895–904
Zhu Y, Zhang C (2016) Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep 16(1):7
Hod M, Kapur A, Sacks DA, Hadar E, Agarwal M, Di Renzo GC et al (2015) The international federation of gynecology and obstetrics (FIGO) initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet 131(Suppl 3):S173-211
Ahmed IZ, Eid YM, El Orabi H, Ibrahim HR (2014) Comparison of universal and targeted screening for thyroid dysfunction in pregnant Egyptian women. Eur J Endocrinol 171(2):285–291
Vaidya B, Anthony S, Bilous M, Shields B, Drury J, Hutchison S et al (2007) Detection of thyroid dysfunction in early pregnancy: universal screening or targeted high-risk case finding? J Clin Endocrinol Metab 92(1):203–207
Dong AC, Stagnaro-Green A (2019) Differences in diagnostic criteria mask the true prevalence of thyroid disease in pregnancy: a systematic review and meta-analysis. Thyroid 29(2):278–289
Moreno-Reyes R, Glinoer D, Van Oyen H, Vandevijvere S (2013) High prevalence of thyroid disorders in pregnant women in a mildly iodine-deficient country: a population-based study. J Clin Endocrinol Metab 98(9):3694–3701
Delshad H, Raeisi A, Abdollahi Z, Tohidi M, Hedayati M, Mirmiran P et al (2021) Iodine supplementation for pregnant women: a cross-sectional national interventional study. J Endocrinol Invest 44(10):2307–2314
Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH (2018) The pathophysiology of gestational diabetes mellitus. Int J Mol Sci 19(11):3342
Di Cianni G, Miccoli R, Volpe L, Lencioni C, Del Prato S (2003) Intermediate metabolism in normal pregnancy and in gestational diabetes. Diabetes Metab Res Rev 19(4):259–270
Catalano PM, Tyzbir ED, Wolfe RR, Roman NM, Amini SB, Sims EA (1992) Longitudinal changes in basal hepatic glucose production and suppression during insulin infusion in normal pregnant women. Am J Obstet Gynecol 167(4 Pt 1):913–919
Catalano PM, Huston L, Amini SB, Kalhan SC (1999) Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol 180(4):903–916
Glinoer D, de Nayer P, Bourdoux P, Lemone M, Robyn C, van Steirteghem A et al (1990) Regulation of maternal thyroid during pregnancy. J Clin Endocrinol Metab 71(2):276–287
Rotondi M, Amato G, Biondi B, Mazziotti G, Del Buono A, Rotonda Nicchio M et al (2000) Parity as a thyroid size-determining factor in areas with moderate iodine deficiency. J Clin Endocrinol Metab 85(12):4534–4537
Rasmussen NG, Hornnes PJ, Hegedüs L (1989) Ultrasonographically determined thyroid size in pregnancy and post partum: the goitrogenic effect of pregnancy. Am J Obstet Gynecol 160(5 Pt 1):1216–1220
Pedersen KM, Laurberg P, Iversen E, Knudsen PR, Gregersen HE, Rasmussen OS et al (1993) Amelioration of some pregnancy-associated variations in thyroid function by iodine supplementation. J Clin Endocrinol Metab 77(4):1078–1083
Biondi B, Kahaly GJ, Robertson RP (2019) Thyroid dysfunction and diabetes mellitus: two closely associated disorders. Endocr Rev 40(3):789–824
Knudsen N, Laurberg P, Rasmussen LB, Bülow I, Perrild H, Ovesen L et al (2005) Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab 90(7):4019–4024
Fernández-Real JM, López-Bermejo A, Castro A, Casamitjana R, Ricart W (2006) Thyroid function is intrinsically linked to insulin sensitivity and endothelium-dependent vasodilation in healthy euthyroid subjects. J Clin Endocrinol Metab 91(9):3337–3343
Roos A, Bakker SJ, Links TP, Gans RO, Wolffenbuttel BH (2007) Thyroid function is associated with components of the metabolic syndrome in euthyroid subjects. J Clin Endocrinol Metab 92(2):491–496
Kim BJ, Kim TY, Koh JM, Kim HK, Park JY, Lee KU et al (2009) Relationship between serum free T4 (FT4) levels and metabolic syndrome (MS) and its components in healthy euthyroid subjects. Clin Endocrinol (Oxf) 70(1):152–160
de Jesus Garduño-Garcia J, Alvirde-Garcia U, López-Carrasco G, Padilla Mendoza ME, Mehta R, Arellano-Campos O et al (2010) TSH and free thyroxine concentrations are associated with differing metabolic markers in euthyroid subjects. Eur J Endocrinol 163(2):273–278
Croce L, Pallavicini C, Crotti S, Coperchini F, Minnelli L, Magri F et al (2021) Basal and longitudinal changes in serum levels of TSH in morbid obese patients experiencing failure or success of dietary treatment. Eat Weight Disord 26(6):1949–1955
Dimitriadis G, Mitrou P, Lambadiari V, Boutati E, Maratou E, Panagiotakos DB et al (2006) Insulin action in adipose tissue and muscle in hypothyroidism. J Clin Endocrinol Metab 91(12):4930–4937
Maratou E, Hadjidakis DJ, Kollias A, Tsegka K, Peppa M, Alevizaki M et al (2009) Studies of insulin resistance in patients with clinical and subclinical hypothyroidism. Eur J Endocrinol 160(5):785–790
Männistö T, Vääräsmäki M, Pouta A, Hartikainen AL, Ruokonen A, Surcel HM et al (2010) Thyroid dysfunction and autoantibodies during pregnancy as predictive factors of pregnancy complications and maternal morbidity in later life. J Clin Endocrinol Metab 95(3):1084–1094
Barbour LA, McCurdy CE, Hernandez TL, Kirwan JP, Catalano PM, Friedman JE (2007) Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care 30(Suppl 2):S112–S119
Spinillo A, De Maggio I, Ruspini B, Bellingeri C, Cavagnoli C, Giannico S et al (2021) Placental pathologic features in thyroid autoimmunity. Placenta 112:66–72
Magri F, Bellingeri C, De Maggio I, Croce L, Coperchini F, Rotondi M et al (2022) A first-trimester serum TSH in the 4–10 mIU/L range is associated with obstetric complications in thyroid peroxidase antibody-negative women. J Endocrinol Invest. https://doi.org/10.1007/s40618-022-01996-z
Lavie A, Dahan M, Ton Nu TN, Balayla J, Gil Y, Machado-Gedeon A et al (2021) Maternal hypothyroidism and its effect on placental histopathology in singleton live births resulting from in vitro fertilization treatment. Hum Fertil (Camb). https://doi.org/10.1080/14647273.2021.1964102
Ehlers E, Talton OO, Schust DJ, Schulz LC (2021) Placental structural abnormalities in gestational diabetes and when they develop: a scoping review. Placenta 116:58–66
Reichetzeder C, Dwi Putra SE, Pfab T, Slowinski T, Neuber C, Kleuser B et al (2016) Increased global placental DNA methylation levels are associated with gestational diabetes. Clin Epigenetics 8:82
Zhao C, Dong J, Jiang T, Shi Z, Yu B, Zhu Y et al (2011) Early second-trimester serum miRNA profiling predicts gestational diabetes mellitus. PLoS ONE 6(8):e23925
Li J, Song L, Zhou L, Wu J, Sheng C, Chen H et al (2015) A MicroRNA signature in gestational diabetes mellitus associated with risk of macrosomia. Cell Physiol Biochem 37(1):243–252
Stern C, Schwarz S, Moser G, Cvitic S, Jantscher-Krenn E, Gauster M et al (2021) Placental endocrine activity: adaptation and disruption of maternal glucose metabolism in pregnancy and the influence of fetal sex. Int J Mol Sci 22(23):12722
Asvold BO, Vatten LJ, Tanbo TG, Eskild A (2014) Concentrations of human chorionic gonadotrophin in very early pregnancy and subsequent pre-eclampsia: a cohort study. Hum Reprod 29(6):1153–1160
Gungor K, Dokuzeylul GN (2021) Antithyroid antibodies may predict serum beta HCG levels and biochemical pregnancy losses in euthyroid women with IVF single embryo transfer. Gynecol Endocrinol 37(8):702–705
Liu Y, Guo F, Maraka S, Zhang Y, Zhang C, Korevaar TIM et al (2021) Associations between human chorionic gonadotropin, maternal free thyroxine, and gestational diabetes mellitus. Thyroid 31(8):1282–1288
Visconti F, Quaresima P, Chiefari E, Caroleo P, Arcidiacono B, Puccio L et al (2019) First trimester combined test (FTCT) as a predictor of gestational diabetes mellitus. Int J Environ Res Public Health 16(19):3654
Spencer K, Cowans NJ (2013) The association between gestational diabetes mellitus and first trimester aneuploidy screening markers. Ann Clin Biochem 50(Pt 6):603–610
Korevaar TI, Steegers EA, Pop VJ, Broeren MA, Chaker L, de Rijke YB et al (2017) Thyroid autoimmunity impairs the thyroidal response to human chorionic gonadotropin: two population-based prospective cohort studies. J Clin Endocrinol Metab 102(1):69–77
Cosson E, Cussac-Pillegand C, Benbara A, Pharisien I, Jaber Y, Banu I et al (2014) The diagnostic and prognostic performance of a selective screening strategy for gestational diabetes mellitus according to ethnicity in Europe. J Clin Endocrinol Metab 99(3):996–1005
Monod C, Kotzaeridi G, Linder T, Eppel D, Rosicky I, Filippi V et al (2023) Prevalence of gestational diabetes mellitus in women with a family history of type 2 diabetes in first- and second-degree relatives. Acta Diabetol. https://doi.org/10.1007/s00592-022-02011-w
Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C et al (2017) Guidelines of the American thyroid association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 27(3):315–389
Cao M, Zhang L, Chen T, Shi A, Xie K, Li Z et al (2020) Genetic susceptibility to gestational diabetes mellitus in a Chinese population. Front Endocrinol (Lausanne) 11:247
Jääskeläinen T, Klemetti MM (2022) Genetic risk factors and gene-lifestyle interactions in gestational diabetes. Nutrients 14(22):4799
Brix TH, Kyvik KO, Hegedüs L (2000) A population-based study of chronic autoimmune hypothyroidism in Danish twins. J Clin Endocrinol Metab 85(2):536–539
Mahmood L, Flores-Barrantes P, Moreno LA, Manios Y, Gonzalez-Gil EM (2021) The influence of parental dietary behaviors and practices on children’s eating habits. Nutrients 13(4):1138
Selzam S, McAdams TA, Coleman JRI, Carnell S, O’Reilly PF, Plomin R et al (2018) Evidence for gene-environment correlation in child feeding: links between common genetic variation for BMI in children and parental feeding practices. PLoS Genet 14(11):e1007757
van den Berg L, Henneman P, Willems van Dijk K, Delemarre-van de Waal HA, Oostra BA, van Duijn CM, et al (2013) Heritability of dietary food intake patterns. Acta Diabetol 50(5):721–726
Getahun D, Nath C, Ananth CV, Chavez MR, Smulian JC (2008) Gestational diabetes in the United States: temporal trends 1989 through 2004. Am J Obstet Gynecol 198(5):525.e1–5
Urquia M, Glazier RH, Berger H, Ying I, De Souza L, Ray JG (2011) Gestational diabetes among immigrant women. Epidemiology 22(6):879–880
Cripe SM, O’Brien W, Gelaye B, Williams MA (2011) Maternal morbidity and perinatal outcomes among foreign-born Cambodian, Laotian, and Vietnamese Americans in Washington State, 1993–2006. J Immigr Minor Health 13(3):417–425
Walker JA, Illions EH, Huddleston JF, Smallridge RC (2005) Racial comparisons of thyroid function and autoimmunity during pregnancy and the postpartum period. Obstet Gynecol 106(6):1365–1371
Benhadi N, Wiersinga WM, Reitsma JB, Vrijkotte TG, van der Wal MF, Bonsel GJ (2007) Ethnic differences in TSH but not in free T4 concentrations or TPO antibodies during pregnancy. Clin Endocrinol (Oxf) 66(6):765–770
Pearce EN, Oken E, Gillman MW, Lee SL, Magnani B, Platek D et al (2008) Association of first-trimester thyroid function test values with thyroperoxidase antibody status, smoking, and multivitamin use. Endocr Pract 14(1):33–39
Korevaar TI, Medici M, de Rijke YB, Visser W, de Muinck Keizer-Schrama SM, Jaddoe VW et al (2013) Ethnic differences in maternal thyroid parameters during pregnancy: the generation R study. J Clin Endocrinol Metab 98(9):3678–3686
Johnson N, Chatrani V, Taylor-Christmas AK, Choo-Kang E, Smikle M, Wright-Pascoe R et al (2014) Population reference values and prevalence rates following universal screening for subclinical hypothyroidism during pregnancy of an Afro-Caribbean cohort. Eur Thyroid J 3(4):234–239
Herrick KA, Perrine CG, Aoki Y, Caldwell KL (2018) Iodine status and consumption of key iodine sources in the U.S. population with special attention to reproductive age women. Nutrients 10(7):874
Magri F, Zerbini F, Gaiti M, Capelli V, Croce L, Bini S et al (2019) Poverty and immigration as a barrier to iodine intake and maternal adherence to iodine supplementation. J Endocrinol Invest 42(4):435–442
Tang X, Zhou JB, Luo F, Han Y, Heianza Y, Cardoso MA et al (2020) Air pollution and gestational diabetes mellitus: evidence from cohort studies. BMJ Open Diabetes Res Care 8(1):e000937
Eberle C, Stichling S (2022) Environmental health influences in pregnancy and risk of gestational diabetes mellitus: a systematic review. BMC Public Health 22(1):1572
Ghassabian A, Pierotti L, Basterrechea M, Chatzi L, Estarlich M, Fernández-Somoano A et al (2019) Association of exposure to ambient air pollution with thyroid function during pregnancy. JAMA Netw Open 2(10):e1912902
Coperchini F, Croce L, Ricci G, Magri F, Rotondi M, Imbriani M et al (2020) Thyroid disrupting effects of old and new generation PFAS. Front Endocrinol (Lausanne) 11:612320
Solomon CG, Willett WC, Carey VJ, Rich-Edwards J, Hunter DJ, Colditz GA et al (1997) A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA 278(13):1078–1083
Cosson E, Vicaut E, Sandre-Banon D, Gary F, Pharisien I, Portal JJ et al (2020) Performance of a selective screening strategy for diagnosis of hyperglycaemia in pregnancy as defined by IADPSG/WHO criteria. Diabetes Metab 46(4):311–318
Vambergue A (2010) Expert consensus on gestational diabetes mellitus. Diabetes Metab 36(6 Pt 2):511
Linea Guida della Società Italiana di Diabetologia (SID) e dell’Associazione dei Medici Diabetologi (AMD) La terapia del diabete mellito di tipo 2. SISTEMA NAZIONALE LINEE GUIDA DELL’ISTITUTO SUPERIORE DI SANITÀ. 2021. https://snlg.iss.it/?cat=6
Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA et al (2002) Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): national health and nutrition examination survey (NHANES III). J Clin Endocrinol Metab 87(2):489–499
Surks MI, Hollowell JG (2007) Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab 92(12):4575–4582
Potlukova E, Potluka O, Jiskra J, Limanova Z, Telicka Z, Bartakova J et al (2012) Is age a risk factor for hypothyroidism in pregnancy? An analysis of 5223 pregnant women. J Clin Endocrinol Metab 97(6):1945–1952
Zhang C, Ning Y (2011) Effect of dietary and lifestyle factors on the risk of gestational diabetes: review of epidemiologic evidence. Am J Clin Nutr 94(6 Suppl):1975S-S1979
Rotondi M, Leporati P, La Manna A, Pirali B, Mondello T, Fonte R et al (2009) Raised serum TSH levels in patients with morbid obesity: is it enough to diagnose subclinical hypothyroidism? Eur J Endocrinol 160(3):403–408
Asvold BO, Bjøro T, Vatten LJ (2009) Association of serum TSH with high body mass differs between smokers and never-smokers. J Clin Endocrinol Metab 94(12):5023–5027
Santini F, Marzullo P, Rotondi M, Ceccarini G, Pagano L, Ippolito S et al (2014) Mechanisms in endocrinology: the crosstalk between thyroid gland and adipose tissue: signal integration in health and disease. Eur J Endocrinol 171(4):R137–R152
Marzullo P, Minocci A, Tagliaferri MA, Guzzaloni G, Di Blasio A, De Medici C et al (2010) Investigations of thyroid hormones and antibodies in obesity: leptin levels are associated with thyroid autoimmunity independent of bioanthropometric, hormonal, and weight-related determinants. J Clin Endocrinol Metab 95(8):3965–3972
Tripathi P, Rao YK, Pandey K, Gautam KA (2019) Significance of vitamin D on the susceptibility of gestational diabetes mellitus—a meta-analysis. Indian J Endocrinol Metab 23(5):514–524
Jin S, Sha L, Dong J, Yi J, Liu Y, Guo Z et al (2020) Effects of nutritional strategies on glucose homeostasis in gestational diabetes mellitus: a systematic review and network meta-analysis. J Diabetes Res 2020:6062478
Akbari M, Moosazaheh M, Lankarani KB, Tabrizi R, Samimi M, Karamali M et al (2017) The effects of vitamin D supplementation on glucose metabolism and lipid profiles in patients with gestational diabetes: a systematic review and meta-analysis of randomized controlled trials. Horm Metab Res 49(9):647–653
Rodrigues MRK, Lima SAM, Mazeto GMFD, Calderon IMP, Magalhães CG, Ferraz GAR et al (2019) Efficacy of vitamin D supplementation in gestational diabetes mellitus: systematic review and meta-analysis of randomized trials. PLoS ONE 14(3):e0213006
Wang J, Lv S, Chen G, Gao C, He J, Zhong H et al (2015) Meta-analysis of the association between vitamin D and autoimmune thyroid disease. Nutrients 7(4):2485–2498
Pan Y, Zhong S, Liu Q, Wang CB, Zhu WH, Shen XA et al (2018) Investigating the relationship between 25-hydroxyvitamin D and thyroid function in second-trimester pregnant women. Gynecol Endocrinol 34(4):345–348
Ahi S, Adelpour M, Fereydooni I, Hatami N (2022) Correlation between maternal vitamin D and thyroid function in pregnancy with maternal and neonatal outcomes: a cross-sectional study. Int J Endocrinol 2022:6295775
Zhao Y, Miao W, Li C, Yu X, Shan Z, Guan H et al (2014) Dynamic changes in serum 25-hydroxyvitamin D during pregnancy and lack of effect on thyroid parameters. PLoS ONE 9(3):e90161
Wang H, Wang HJ, Jiao M, Han N, Xu J, Bao H et al (2022) Associations between dynamic vitamin D level and thyroid function during pregnancy. Nutrients 14(18):3780
Pérez-Fernandez R, Alonso M, Segura C, Muñoz I, García-Caballero T, Diguez C (1997) Vitamin D receptor gene expression in human pituitary gland. Life Sci 60(1):35–42
Berg JP, Liane KM, Bjørhovde SB, Bjøro T, Torjesen PA, Haug E (1994) Vitamin D receptor binding and biological effects of cholecalciferol analogues in rat thyroid cells. J Steroid Biochem Mol Biol 50(3–4):145–150
Mele C, Caputo M, Bisceglia A, Samà MT, Zavattaro M, Aimaretti G et al (2020) Immunomodulatory effects of vitamin D in thyroid diseases. Nutrients 12(5):1444
Rotondi M, Chiovato L (2013) Vitamin D deficiency in patients with Graves’ disease: probably something more than a casual association. Endocrine 43(1):3–5
Rayman MP (2000) The importance of selenium to human health. Lancet 356(9225):233–241
Tan M, Sheng L, Qian Y, Ge Y, Wang Y, Zhang H et al (2001) Changes of serum selenium in pregnant women with gestational diabetes mellitus. Biol Trace Elem Res 83(3):231–237
Hawkes WC, Alkan Z, Lang K, King JC (2004) Plasma selenium decrease during pregnancy is associated with glucose intolerance. Biol Trace Elem Res 100(1):19–29
Asemi Z, Jamilian M, Mesdaghinia E, Esmaillzadeh A (2015) Effects of selenium supplementation on glucose homeostasis, inflammation, and oxidative stress in gestational diabetes: Randomized, double-blind, placebo-controlled trial. Nutrition 31(10):1235–1242
Saifi H, Mabrouk Y, Saifi R, Benabdelkader M, Saidi M (2020) Influence of selenium supplementation on carbohydrate metabolism and oxidative stress in pregnant women with gestational diabetes mellitus. J Med Biochem 39(2):191–198
Sadat Najib F, Poordast T, Rezvan Nia M, Hossein DM (2019) Effects of selenium supplementation on glucose homeostasis in women with gestational diabetes mellitus: a randomized, controlled trial. Int J Reprod Biomed 18(1):57–64
Drutel A, Archambeaud F, Caron P (2013) Selenium and the thyroid gland: more good news for clinicians. Clin Endocrinol (Oxf) 78(2):155–164
Pirola I, Rotondi M, Cristiano A, Maffezzoni F, Pasquali D, Marini F et al (2020) Selenium supplementation in patients with subclinical hypothyroidism affected by autoimmune thyroiditis: results of the SETI study. Endocrinol Diabetes Nutr (Engl Ed) 67(1):28–35
Esposito D, Rotondi M, Accardo G, Vallone G, Conzo G, Docimo G et al (2017) Influence of short-term selenium supplementation on the natural course of Hashimoto’s thyroiditis: clinical results of a blinded placebo-controlled randomized prospective trial. J Endocrinol Invest 40(1):83–89
Negro R, Greco G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H (2007) The influence of selenium supplementation on postpartum thyroid status in pregnant women with thyroid peroxidase autoantibodies. J Clin Endocrinol Metab 92(4):1263–1268
Chatree S, Thongmaen N, Tantivejkul K, Sitticharoon C, Vucenik I (2020) Role of inositols and inositol phosphates in energy metabolism. Molecules 25(21):5079
Vitacolonna E, Masulli M, Palmisano L, Stuppia L, Franzago M (2022) Inositols, Probiotics, and gestational diabetes: clinical and epigenetic aspects. Nutrients 14(8):1543
Formoso G, Baldassarre MPA, Ginestra F, Carlucci MA, Bucci I, Consoli A (2019) Inositol and antioxidant supplementation: safety and efficacy in pregnancy. Diabetes Metab Res Rev 35(5):e3154
Scioscia M, Kunjara S, Gumaa K, McLean P, Rodeck CH, Rademacher TW (2007) Urinary excretion of inositol phosphoglycan P-type in gestational diabetes mellitus. Diabet Med 24(11):1300–1304
Fraticelli F, Celentano C, Zecca IA, Di Vieste G, Pintaudi B, Liberati M et al (2018) Effect of inositol stereoisomers at different dosages in gestational diabetes: an open-label, parallel, randomized controlled trial. Acta Diabetol 55(8):805–812
D’Anna R, Scilipoti A, Giordano D, Caruso C, Cannata ML, Interdonato ML et al (2013) myo-Inositol supplementation and onset of gestational diabetes mellitus in pregnant women with a family history of type 2 diabetes: a prospective, randomized, placebo-controlled study. Diabetes Care 36(4):854–857
D’Anna R, Di Benedetto A, Scilipoti A, Santamaria A, Interdonato ML, Petrella E et al (2015) Myo-inositol supplementation for prevention of gestational diabetes in obese pregnant women: a randomized controlled trial. Obstet Gynecol 126(2):310–315
Santamaria A, Di Benedetto A, Petrella E, Pintaudi B, Corrado F, D’Anna R et al (2016) Myo-inositol may prevent gestational diabetes onset in overweight women: a randomized, controlled trial. J Matern Fetal Neonatal Med 29(19):3234–3237
Matarrelli B, Vitacolonna E, D’Angelo M, Pavone G, Mattei PA, Liberati M et al (2013) Effect of dietary myo-inositol supplementation in pregnancy on the incidence of maternal gestational diabetes mellitus and fetal outcomes: a randomized controlled trial. J Matern Fetal Neonatal Med 26(10):967–972
Celentano C, Matarrelli B, Pavone G, Vitacolonna E, Mattei PA, Berghella V et al (2020) The influence of different inositol stereoisomers supplementation in pregnancy on maternal gestational diabetes mellitus and fetal outcomes in high-risk patients: a randomized controlled trial. J Matern Fetal Neonatal Med 33(5):743–751
Brown J, Crawford TJ, Alsweiler J, Crowther CA (2016) Dietary supplementation with myo-inositol in women during pregnancy for treating gestational diabetes. Cochrane Database Syst Rev 9:CD012048
Lubin V, Shojai R, Darmon P, Cosson E (2016) A pilot study of gestational diabetes mellitus not controlled by diet alone: first-line medical treatment with myoinositol may limit the need for insulin. Diabetes Metab 42(3):192–195
Fallahi P, Ferrari SM, Elia G, Ragusa F, Paparo SR, Caruso C et al (2018) Myo-inositol in autoimmune thyroiditis, and hypothyroidism. Rev Endocr Metab Disord 19(4):349–354
Pace C, Tumino D, Russo M, Le Moli R, Naselli A, Borzì G et al (2020) Role of selenium and myo-inositol supplementation on autoimmune thyroiditis progression. Endocr J 67(11):1093–1098
Paparo SR, Ferrari SM, Patrizio A, Elia G, Ragusa F, Botrini C et al (2022) Myoinositol in autoimmune thyroiditis. Front Endocrinol (Lausanne) 13:930756
Wang JW, Cao SS, Hu RY, Wang M (2020) Association between cigarette smoking during pregnancy and gestational diabetes mellitus: a meta-analysis. J Matern Fetal Neonatal Med 33(5):758–767
Wendland EM, Pinto ME, Duncan BB, Belizán JM, Schmidt MI (2008) Cigarette smoking and risk of gestational diabetes: a systematic review of observational studies. BMC Pregnancy Childbirth 8:53
Zhang Y, Shi L, Zhang Q, Peng N, Chen L, Lian X et al (2019) The association between cigarette smoking and serum thyroid stimulating hormone, thyroid peroxidase antibodies and thyroglobulin antibodies levels in Chinese residents: a cross-sectional study in 10 cities. PLoS ONE 14(11):e0225435
Krassas GE, Wiersinga W (2006) Smoking and autoimmune thyroid disease: the plot thickens. Eur J Endocrinol 154(6):777–780
Männistö T, Hartikainen AL, Vääräsmäki M, Bloigu A, Surcel HM, Pouta A et al (2012) Smoking and early pregnancy thyroid hormone and anti-thyroid antibody levels in euthyroid mothers of the Northern Finland Birth Cohort 1986. Thyroid 22(9):944–950
Shields B, Hill A, Bilous M, Knight B, Hattersley AT, Bilous RW et al (2009) Cigarette smoking during pregnancy is associated with alterations in maternal and fetal thyroid function. J Clin Endocrinol Metab 94(2):570–574
Andersen SL, Knøsgaard L, Handberg A, Vestergaard P, Andersen S (2021) Maternal adiposity, smoking, and thyroid function in early pregnancy. Endocr Connect 10(9):1125–1133
Houshmand A, Jensen DM, Mathiesen ER, Damm P (2013) Evolution of diagnostic criteria for gestational diabetes mellitus. Acta Obstet Gynecol Scand 92(7):739–745
Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P et al (2010) International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 33(3):676–682
Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a world health organization guideline (2014). Diabetes Res Clin Pract 103(3), 341–363.
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D et al (2023) 2. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care 46(Suppl 1):S19–S40
Benhalima K, Mathieu C, Van Assche A, Damm P, Devlieger R, Mahmood T et al (2016) Survey by the European board and college of obstetrics and gynaecology on screening for gestational diabetes in Europe. Eur J Obstet Gynecol Reprod Biol 201:197–202
McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P (2019) Gestational diabetes mellitus. Nat Rev Dis Primers 5(1):47
Taylor PN, Zouras S, Min T, Nagarahaj K, Lazarus JH, Okosieme O (2018) Thyroid screening in early pregnancy: pros and cons. Front Endocrinol (Lausanne) 9:626
Stagnaro-Green A, Dong A, Stephenson MD (2020) Universal screening for thyroid disease during pregnancy should be performed. Best Pract Res Clin Endocrinol Metab 34(4):101320
Horacek J, Spitalnikova S, Dlabalova B, Malirova E, Vizda J, Svilias I et al (2010) Universal screening detects two-times more thyroid disorders in early pregnancy than targeted high-risk case finding. Eur J Endocrinol 163(4):645–650
Nazarpour S, Tehrani FR, Simbar M, Tohidi M, AlaviMajd H, Azizi F (2016) Comparison of universal screening with targeted high-risk case finding for diagnosis of thyroid disorders. Eur J Endocrinol 174(1):77–83
Yang H, Shao M, Chen L, Chen Q, Yu L, Cai L et al (2014) Screening strategies for thyroid disorders in the first and second trimester of pregnancy in China. PLoS ONE 9(6):e99611
Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A (2010) Universal screening versus case finding for detection and treatment of thyroid hormonal dysfunction during pregnancy. J Clin Endocrinol Metab 95(4):1699–1707
Amiri M, Nazarpour S, Ramezani Tehrani F, Sheidaei A, Azizi F (2022) The targeted high-risk case-finding approach versus universal screening for thyroid dysfunction during pregnancy: thyroid-stimulating hormone (TSH) and/or thyroid peroxidase antibody (TPOAb) test? J Endocrinol Invest 45(9):1641–1651
Ying H, Tang YP, Bao YR, Su XJ, Cai X, Li YH et al (2016) Maternal TSH level and TPOAb status in early pregnancy and their relationship to the risk of gestational diabetes mellitus. Endocrine 54(3):742–750
Safian S, Esna-Ashari F, Borzouei S (2020) Thyroid dysfunction in pregnant women with gestational diabetes mellitus. Curr Diabetes Rev 16(8):895–899
Gutiérrez-Vega S, Armella A, Mennickent D, Loyola M, Covarrubias A, Ortega-Contreras B et al (2020) High levels of maternal total tri-iodothyronine, and low levels of fetal free L-thyroxine and total tri-iodothyronine, are associated with altered deiodinase expression and activity in placenta with gestational diabetes mellitus. PLoS ONE 15(11):e0242743
Fernández Alba JJ, Castillo Lara M, Jiménez Heras JM, Moreno Cortés R, González Macías C, Vilar Sánchez Á et al (2022) High first trimester levels of TSH as an independent risk factor for gestational diabetes mellitus: a retrospective cohort study. J Clin Med 11(13):3776
Tudela CM, Casey BM, McIntire DD, Cunningham FG (2012) Relationship of subclinical thyroid disease to the incidence of gestational diabetes. Obstet Gynecol 119(5):983–988
Knight BA, Shields BM, Hattersley AT, Vaidya B (2016) Maternal hypothyroxinaemia in pregnancy is associated with obesity and adverse maternal metabolic parameters. Eur J Endocrinol 174(1):51–57
Montaner P, Juan L, Campos R, Gil L, Corcoy R (2008) Is thyroid autoimmunity associated with gestational diabetes mellitus? Metabolism 57(4):522–525
Agarwal MM, Dhatt GS, Punnose J, Bishawi B, Zayed R (2006) Thyroid function abnormalities and antithyroid antibody prevalence in pregnant women at high risk for gestational diabetes mellitus. Gynecol Endocrinol 22(5):261–266
Vitacolonna E, Lapolla A, Di Nenno B, Passante A, Bucci I, Giuliani C et al (2012) Gestational diabetes and thyroid autoimmunity. Int J Endocrinol 2012:867415
Haddow JE, Craig WY, Neveux LM, Palomaki GE, Lambert-Messerlian G, Malone FD et al (2016) Free thyroxine during early pregnancy and risk for gestational diabetes. PLoS ONE 11(2):e0149065
Karakosta P, Alegakis D, Georgiou V, Roumeliotaki T, Fthenou E, Vassilaki M et al (2012) Thyroid dysfunction and autoantibodies in early pregnancy are associated with increased risk of gestational diabetes and adverse birth outcomes. J Clin Endocrinol Metab 97(12):4464–4472
Maleki N, Tavosi Z (2015) Evaluation of thyroid dysfunction and autoimmunity in gestational diabetes mellitus and its relationship with postpartum thyroiditis. Diabet Med 32(2):206–212
Chen GD, Gou XY, Pang TT, Li PS, Zhou ZX, Lin DX et al (2022) Associations between thyroid function and gestational diabetes mellitus in Chinese pregnant women: a retrospective cohort study. BMC Endocr Disord 22(1):44
Jia M, Wu Y, Lin B, Shi Y, Zhang Q, Lin Y et al (2019) Meta-analysis of the association between maternal subclinical hypothyroidism and gestational diabetes mellitus. Int J Gynaecol Obstet 144(3):239–247
Kent NL, Young SL, Akison LK, Cuffe JSM (2021) Is the link between elevated TSH and gestational diabetes mellitus dependant on diagnostic criteria and thyroid antibody status: a systematic review and meta-analysis. Endocrine 74(1):38–49
Maraka S, Ospina NM, O'Keeffe DT, Espinosa De Ycaza AE, Gionfriddo MR, Erwin PJ, et al (2016) Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid 26(4):580–590
Giannakou K, Evangelou E, Yiallouros P, Christophi CA, Middleton N, Papatheodorou E et al (2019) Risk factors for gestational diabetes: an umbrella review of meta-analyses of observational studies. PLoS ONE 14(4):e0215372
Gong LL, Liu H, Liu LH (2016) Relationship between hypothyroidism and the incidence of gestational diabetes: a meta-analysis. Taiwan J Obstet Gynecol 55(2):171–175
Yang S, Shi FT, Leung PC, Huang HF, Fan J (2016) Low thyroid hormone in early pregnancy is associated with an increased risk of gestational diabetes mellitus. J Clin Endocrinol Metab 101(11):4237–4243
Wang Y, Sun F, Wu P, Huang Y, Ye Y, Yang X et al (2022) A prospective study of early-pregnancy thyroid markers, lipid species, and risk of gestational diabetes mellitus. J Clin Endocrinol Metab 107(2):e804–e814
Rawal S, Tsai MY, Hinkle SN, Zhu Y, Bao W, Lin Y et al (2018) A longitudinal study of thyroid markers across pregnancy and the risk of gestational diabetes. J Clin Endocrinol Metab 103(7):2447–2456
Zhuo L, Wang Z, Yang Y, Liu Z, Wang S, Song Y (2022) Obstetric and offspring outcomes in isolated maternal hypothyroxinaemia: a systematic review and meta-analysis. J Endocrinol Invest. https://doi.org/10.1007/s40618-022-01967-4
Haddow JE, Metzger BE, Lambert-Messerlian G, Eklund E, Coustan D, Catalano P et al (2019) Maternal BMI, Peripheral deiodinase activity, and plasma glucose: relationships between white women in the HAPO study. J Clin Endocrinol Metab 104(7):2593–2600
Bassols J, Prats-Puig A, Soriano-Rodríguez P, García-González MM, Reid J, Martínez-Pascual M et al (2011) Lower free thyroxin associates with a less favorable metabolic phenotype in healthy pregnant women. J Clin Endocrinol Metab 96(12):3717–3723
Luongo C, Dentice M, Salvatore D (2019) Deiodinases and their intricate role in thyroid hormone homeostasis. Nat Rev Endocrinol 15(8):479–488
Han C, Li C, Mao J, Wang W, Xie X, Zhou W et al (2015) High body mass index is an indicator of maternal hypothyroidism, hypothyroxinemia, and thyroid-peroxidase antibody positivity during early pregnancy. Biomed Res Int 2015:351831
Männistö T, Surcel HM, Ruokonen A, Vääräsmäki M, Pouta A, Bloigu A et al (2011) Early pregnancy reference intervals of thyroid hormone concentrations in a thyroid antibody-negative pregnant population. Thyroid 21(3):291–298
Pop VJ, Biondi B, Wijnen HA, Kuppens SM, Lvader H (2013) Maternal thyroid parameters, body mass index and subsequent weight gain during pregnancy in healthy euthyroid women. Clin Endocrinol (Oxf) 79(4):577–583
Zimmermann-Belsing T, Brabant G, Holst JJ, Feldt-Rasmussen U (2003) Circulating leptin and thyroid dysfunction. Eur J Endocrinol 149(4):257–271
Gowachirapant S, Melse-Boonstra A, Winichagoon P, Zimmermann MB (2014) Overweight increases risk of first trimester hypothyroxinaemia in iodine-deficient pregnant women. Matern Child Nutr 10(1):61–71
Bitterman O, Bongiovanni M, Giuliani C, Roma G, Toscano V, Napoli A (2014) Anti thyroperoxidase and anti thyroglobulin antibodies in diabetic pregnancies. J Endocrinol Invest 37(10):911–915
Olivieri A, Magnani F, Valensise H, D’Archivio M, Medda E, Baccarini S et al (1997) Thyroid autoimmunity in pregnant women at risk for GDM. Ann Ist Super Sanita 33(3):447–450
Lapolla A, Betterle C, Sanzari M, Zanchetta R, Pfeifer E, Businaro A et al (1996) An immunological and genetic study of patients with gestational diabetes mellitus. Acta Diabetol 33(2):139–144
Ortega-González C, Liao-Lo A, Ramírez-Peredo J, Cariño N, Lira J, Parra A (2000) Thyroid peroxidase antibodies in Mexican-born healthy pregnant women, in women with type 2 or gestational diabetes mellitus, and in their offspring. Endocr Pract 6(3):244–248
Yang Y, Li Q, Wang Q, Ma X (2015) Thyroid antibodies and gestational diabetes mellitus: a meta-analysis. Fertil Steril 104(3):665–71.e3
Group HSCR (2002) The hyperglycemia and adverse pregnancy outcome (HAPO) study. Int J Gynaecol Obstet 78(1):69–77
Cosson E, Bentounes SA, Nachtergaele C, Berkane N, Pinto S, Sal M et al (2021) Prognosis associated with sub-types of hyperglycaemia in pregnancy. J Clin Med 10(17):3904
Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B et al (2009) A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 361(14):1339–1348
Crowther CA, Samuel D, McCowan LME, Edlin R, Tran T, McKinlay CJ et al (2022) Lower versus higher glycemic criteria for diagnosis of gestational diabetes. N Engl J Med 387(7):587–598
Harper LM, Jauk V, Longo S, Biggio JR, Szychowski JM, Tita AT (2020) Early gestational diabetes screening in obese women: a randomized controlled trial. Am J Obstet Gynecol 222(5):495.e1-495.e8
Cosson E, Vicaut E, Sandre-Banon D, Gary F, Pharisien I, Portal JJ et al (2019) Early screening for gestational diabetes mellitus is not associated with improved pregnancy outcomes: an observational study including 9795 women. Diabetes Metab 45(5):465–472
Raets L, Beunen K, Benhalima K (2021) Screening for gestational diabetes mellitus in early pregnancy: what is the evidence? J Clin Med 10(6):1257
McLaren RA, Ruymann KR, Ramos GA, Osmundson SS, Jauk V, Berghella V (2022) Early screening for gestational diabetes mellitus: a meta-analysis of randomized controlled trials. Am J Obstet Gynecol MFM 4(6):100737
Simmons D, Hague WM, Teede HJ, Cheung NW, Hibbert EJ, Nolan CJ et al (2018) Hyperglycaemia in early pregnancy: the treatment of booking gestational diabetes mellitus (TOBOGM) study. A randomised controlled trial. Med J Aust 209(9):405–406
van den Boogaard E, Vissenberg R, Land JA, van Wely M, Ven der Post JA, Goddijn M, et al (2016) Significance of (sub)clinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Hum Reprod Update 22(4):532–533
Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J et al (1999) Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 341(8):549–555
Abalovich M, Gutierrez S, Alcaraz G, Maccallini G, Garcia A, Levalle O (2002) Overt and subclinical hypothyroidism complicating pregnancy. Thyroid 12(1):63–68
Leung AS, Millar LK, Koonings PP, Montoro M, Mestman JH (1993) Perinatal outcome in hypothyroid pregnancies. Obstet Gynecol 81(3):349–353
Lee SY, Cabral HJ, Aschengrau A, Pearce EN (2020) Associations between maternal thyroid function in pregnancy and obstetric and perinatal outcomes. J Clin Endocrinol Metab 105(5):e2015–e2023
Kim C, Berger DK, Chamany S (2007) Recurrence of gestational diabetes mellitus: a systematic review. Diabetes Care 30(5):1314–1319
Kim C, Newton KM, Knopp RH (2002) Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 25(10):1862–1868
Bellamy L, Casas JP, Hingorani AD, Williams D (2009) Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 373(9677):1773–1779
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D et al (2023) 15. Management of diabetes in pregnancy: standards of care in diabetes-2023. Diabetes Care 46(Supplement_1):S254–S266
Lowe WL, Scholtens DM, Lowe LP, Kuang A, Nodzenski M, Talbot O et al (2018) Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA 320(10):1005–1016
Rayanagoudar G, Hashi AA, Zamora J, Khan KS, Hitman GA, Thangaratinam S (2016) Quantification of the type 2 diabetes risk in women with gestational diabetes: a systematic review and meta-analysis of 95,750 women. Diabetologia 59(7):1403–1411
Retnakaran R, Shah BR (2017) Role of type 2 diabetes in determining retinal, renal, and cardiovascular outcomes in women with previous gestational diabetes mellitus. Diabetes Care 40(1):101–108
Tranidou A, Dagklis T, Tsakiridis I, Siargkas A, Apostolopoulou A, Mamopoulos A et al (2021) Risk of developing metabolic syndrome after gestational diabetes mellitus - a systematic review and meta-analysis. J Endocrinol Invest 44(6):1139–1149
Meigs JB, Cupples LA, Wilson PW (2000) Parental transmission of type 2 diabetes: the Framingham offspring study. Diabetes 49(12):2201–2207
Lawlor DA, Fraser A, Lindsay RS, Ness A, Dabelea D, Catalano P et al (2010) Association of existing diabetes, gestational diabetes and glycosuria in pregnancy with macrosomia and offspring body mass index, waist and fat mass in later childhood: findings from a prospective pregnancy cohort. Diabetologia 53(1):89–97
Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM et al (2000) Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 49(12):2208–2211
Lowe WL, Scholtens DM, Kuang A, Linder B, Lawrence JM, Lebenthal Y et al (2019) Hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care 42(3):372–380
Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J et al (2008) High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care 31(2):340–346
Nogueira Avelar e Silva R, Yu Y, Liew Z, Vested A, Sørensen HT, Li J (2021) Associations of maternal diabetes during pregnancy with psychiatric disorders in offspring during the first 4 decades of life in a population-based Danish birth cohort. JAMA Netw Open 4(10):e2128005
Li M, Fallin MD, Riley A, Landa R, Walker SO, Silverstein M et al (2016) The association of maternal obesity and diabetes with autism and other developmental disabilities. Pediatrics 137(2):e20152206
Kong L, Chen X, Liang Y, Forsell Y, Gissler M, Lavebratt C (2022) Association of preeclampsia and perinatal complications with offspring neurodevelopmental and psychiatric disorders. JAMA Netw Open 5(1):e2145719
Li N, Yang J, Chen X, Huang J, Lai M, Fang F et al (2020) Postpartum follow-up of patients with subclinical hypothyroidism during pregnancy. Thyroid 30(11):1566–1573
Andersen SL, Olsen J, Laurberg P (2015) Foetal programming by maternal thyroid disease. Clin Endocrinol (Oxf) 83(6):751–758
Tapia-Martínez J, Torres-Manzo AP, Franco-Colín M, Pineda-Reynoso M, Cano-Europa E (2019) Maternal thyroid hormone deficiency during gestation and lactation alters metabolic and thyroid programming of the offspring in the adult stage. Horm Metab Res 51(6):381–388
Amouzegar A, Pearce EN, Mehran L, Lazarus J, Takyar M, Azizi F (2022) TPO antibody in euthyroid pregnant women and cognitive ability in the offspring: a focused review. J Endocrinol Invest 45(2):425–431
Cooper DS, Pearce EN (2017) Subclinical hypothyroidism and hypothyroxinemia in pregnancy—still no answers. N Engl J Med 376(9):876–877
Brent GA (2012) The debate over thyroid-function screening in pregnancy. N Engl J Med 366(6):562–563
Funding
Open access funding provided by Università degli Studi di Pavia within the CRUI-CARE Agreement. This paper was not supported by any grant or funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Laura Croce is Member of the Editorial Board of the Journal of Endocrinological Investigation.
Research involving human participants and/or animals
The present Review study did not involve reasearch on human partecipants and/or animals.
Informed consent
For this type of study, consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pinto, S., Croce, L., Carlier, L. et al. Thyroid dysfunction during gestation and gestational diabetes mellitus: a complex relationship. J Endocrinol Invest 46, 1737–1759 (2023). https://doi.org/10.1007/s40618-023-02079-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-023-02079-3