Abstract
Objective
To examine the association between isolated maternal hypothyroxinaemia (IMH) and adverse obstetric outcomes and offspring outcomes and also investigate the effects of levothyroxine therapy on IMH for the above outcomes.
Methods
We systematically searched PubMed, EMBASE, and Cochrane Library, and the reference lists of key reviews were hand searched on June 9, 2021. Two authors independently screened titles/abstracts. Full articles were further assessed if the information suggested that the study met the inclusion/exclusion criteria, and two researchers performed data extraction and risk-of-bias assessment using standardized tables. Summary relative risks or the mean difference between maternal effects and offspring outcomes were calculated by a random-effects model.
Results
We identified 38 eligible articles (35 cohort studies and two randomized controlled trials [RCT]). Meta-analysis showed that maternal IMH was associated with increased gestational diabetes mellitus, preterm premature rupture of membranes, preterm birth, fetal distress, and macrosomia outcomes in IMH compared to euthyroid women, and the relative risks were 1.42 (1.03–1.96), 1.50 (1.05–2.14), 1.33 (1.15–1.55), 1.75 (1.16–2.65) and 1.62 (1.35–1.94), respectively. IMH was not associated with placenta previa, gestational hypertension, pre-eclampsia, intrauterine growth restriction, and offspring outcomes like birth weight, low birth weight infants, fetal macrosomia, neonatal intensive care, neonatal death, or fetal head circumference. In addition, we did not find an association between IMH and adverse offspring cognitive defects. Due to insufficient data for meta-analysis, it failed to pool the evidence of levothyroxine’s therapeutic effect on IMH and their offspring.
Conclusions and relevance
IMH in pregnancy may relate to a few maternal and offspring outcomes. Moreover, there is currently no sufficient evidence that levothyroxine treatment during pregnancy reduces adverse maternal outcomes and disability in offspring. Further investigation to explore the beneficial effects of levothyroxine therapy is warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The thyroid hormone regulates metabolism, growth, and development in most human body tissues, and the hormone levels during pregnancy are crucial to fetal and neonatal neuropsychological development [1]. The fetus is entirely dependent on maternal thyroid hormones via the placenta in the first trimester since the fetal thyroid cannot secrete thyroid hormones before 12–14 weeks of gestation [2, 3]. Maternal thyroid dysfunction may lead to adverse fetal and neonatal outcomes, such as miscarriage, placenta abruption, pre-eclampsia, premature delivery, and even reduced offspring intelligence [4].
Defined by American and European Thyroid Associations’ Guidelines, isolated maternal hypothyroxinaemia (IMH) is with free thyroxine (FT4) concentration in the lower 2.5–5th percentile of the pregnancy-related reference range in conjunction with a normal maternal thyroid-stimulating hormone (TSH) concentration [5, 6]. Emerging evidence suggests that IMH during pregnancy has increased in recent years [7, 8]. The prevalence of IMH in the pregnant population ranges from 1.3 to 23.9%, depending on the study [9], with the most frequently reported percentage being 8–10% [10, 11]. The causes of as well as potential consequences of IMH have not yet been fully elucidated, and the current evidence does not adequately explain the reasons for IMH, which is speculated to be mainly caused by iodine deficiency [7, 12] and iron deficiency [13, 14].
Several studies have investigated the effects of IMH, and the results show conflicting evidence regarding adverse obstetric and neurodevelopmental outcomes. Recent studies have revealed that IMH in pregnancy is associated with unfavorable pregnancy outcomes, such as preterm delivery [8, 15], preterm premature rupture of the membranes [16], spontaneous abortion [17, 18], and gestational hypertension [19, 20], etc. Also, a couple of studies have suggested a similar point of view [19, 21, 22]. Beyond that, some other researchers hold different perspectives [23,24,25,26,27]. What is worth mentioning, according to a recent systematic review that included 19 cohorts, is suggested that IMH was not associated with gestational hypertension or pre-eclampsia [28]. Moreover, due to inconsistent research results and insufficient evidence for adverse pregnancy outcomes with increased IMH, levothyroxine (LT4) treatment is not recommended in China or America [5, 29]. However, LT4 replacement is only advocated for this condition in the first trimester of pregnancy in Europe [6]. So, the application of LT4 therapy during pregnancy remains controversial.
Our study aims to evaluate the association between IMH and pregnancy and offspring outcomes and further investigate the effects of LT4 supplementation on pregnant women with IMH and the offspring's outcomes through a systematic review and meta-analysis.
Materials and methods
Data sources and searches
To identify studies for inclusion, we conducted a systematic literature search for articles on the association of IMH with adverse maternal and offspring outcomes and the effect of LT4 treatment published from database inception to June 9, 2021, using PubMed, Embase, and Cochrane Library databases. Controlled vocabulary terms (e.g., MeSH term) were used for each concept and keyword synonyms for the search strategies (Supplementary Table S1). In addition, we also manually searched the references of included studies and previous systematic reviews to identify further relevant studies.
Study selection
Studies were included if they met all of the following criteria: (1) IMH pregnant women with offspring or IMH maternal accepted LT4 therapy during pregnancy; (2) referring estimation of the maternal and offspring outcomes, including but not limited to preterm birth of premature rupture of membranes (PROM), placenta previa, gestational hypertension, pre-eclampsia, intrauterine growth restriction, and gestational diabetes mellitus (GDM), as well as offspring birth weight, number of low birth weight infants, total malformations, fetal macrosomia, fetal distress, neonatal intensive care, neonatal death, and fetal head circumference and cognitive outcome [intelligence quotient (IQ)]. Studies were excluded if they: (1) were published as an abstract, letter to the editor, case report, or review, (2) failed to provide sufficient data or information for analysis, and (3) duplicated studies.
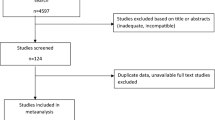
Potential studies were independently assessed via titles, keywords, and abstracts for suitability by ZL, LZX, and YY. Full texts were referred to when information in the records was inadequate for determination. Records not meeting the inclusion criteria were excluded, and the remaining were examined thoroughly. Any disagreement was resolved by discussion with a third author (WSF and SYF). The article and the flow chart were developed based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement.
Data extraction and quality assessment
Two investigators extracted data independently using a pre-designed extraction form modified following a pilot test and assessed each included study's risk of bias (ZL and LZX). The revised extraction form comprised four parts: general information, methodological quality, clinical characteristics, and outcomes. Precisely, the extraction form consists of the first author, year of publication, country, study design, patient characteristics, age, thyroid status reference values, thyroid status, thyroid hormone (TH) values (exposure for cohort analysis), and LT4 supplementation (intervention for RCTs), as well as maternal and offspring outcomes, which were listed in the ‘Study Selection’ part of this manuscript. The Newcastle–Ottawa Scale (NOS) for assessing the quality of observational studies and the Cochrane risk-of-bias tool was used to evaluate cohort studies’ quality and randomized trials, respectively. The studies from the same cohort will be assessed carefully, and then the suitable ones (published lately and with a larger sample size) will be selected for the quantitative combination of outcomes.
Data synthesis and analysis
All analyses were performed with Review Manager 5.3, and all statistical tests were two-sided. All outcomes analyses were carried out using a two-step approach with random-effect models to pool estimates of the studies and assess heterogeneity across studies using the I2 statistic and 95% CIs. The study weight was calculated using the inverse variance method. The risk ratio (RR) or mean difference was used to measure the relative risk/ risk difference between the two groups in selected studies. The forest plot was used to display the results visually. Results were considered statistically significant if the p value was < 0.05. The power of each original study result was calculated if the result was not proven to be statistically significant (i.e., p > 0.05). Heterogeneity between studies was tested with Cochran’s Q test (P < 0.10 was considered significant heterogeneity) and the I2 statistic (values of 25, 50, and 75% were considered low, moderate, and high degrees of heterogeneity, respectively).
Details of the protocol for this systematic review are registered on PROSPERO and can be accessed at https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021278471.
Role of the funding sources
This study was funded by the National Natural Science Foundation (81,922,016, 81,870,607), Peking University Medicine Fund of Fostering Young Scholars’ Scientific AND Technological Innovation (BMU2022PYB035), Fundamental Research Funds for the Central University, Key Clinical Projects of Peking University Third Hospital (BYSYZD2021030), and Shandong Provincial Natural Science Foundation (ZR2019JQ25, ZR2020ZD14) of China. The study’s funders played no role in the study design, data collection, data analysis, data interpretation, or report writing.
Results
Search results and the general characteristics of the included studies
From 6213 published reports, 1159 were excluded after the duplicate deletion, and 54 remained eligible for inclusion based on the title and abstract screening. After reading the full text, a total of 35 cohorts and 2 RCTs matched the eligibility criteria (Fig. 1). The general characteristics of the 38 included studies published between 2003 and 2021 are presented in Table 1. Overall, the relevant studies were conducted in Netherlands (n = 12) [30,31,32,33,34,35,36,37,38,39,40,41] and China (n = 8) [8, 17, 19, 20, 42,43,44,45]. The geographic distribution of included studies can be found in Figure S1.
Included cohorts
In the cohort study part, the study population comprised 179,980 participants, of whom 8111 (4.36%) had IMH. After reading the full article thoroughly, we found multiple studies from the same cohort. First published in 2010, seven studies reported the source cohort as the Generation R Study, a prospective population-based cohort study from fetal life until young adulthood in a multi-ethnic urban population in the Netherlands [32,33,34, 36, 38, 40, 46]. In addition, three studies sourced from the ABCD cohort analyzed the association between IMH in early pregnancy and offspring cognitive outcomes [35, 39, 41]. Explicitly speaking, Pop (2003) [30], Kooistra (2006) [31], and Oostenbroek (2017) [41]were from the cohort established between 1997 and 1998 in Veldhoven, Netherlands. Additionally, it was also reported the source from NFBC 1986, China-Anhui Birth Defects and Child Development cohort, Exeter Family Study, FASTER trial funded by NICHD, SHEP, The Avon Longitudinal Study of Parents and Children, The INMA, the MABC, TTPs and electronic medical records from local hospitals.
Of the 35 cohorts, all of the studies included in the cohort analysis scored more than six stars, suggesting a low risk of bias (Table S2). All of the studies were with low risk on the representativeness of the exposed cohort, selection of nonexposed cohort, ascertainment of exposure, and outcome assessment. Around 77.14% (27/35) of the studies were at high risk of cohorts’ comparability based on the design or analysis. Most of the studies were fully and adequately followed up.
Included RCTs
In the two RCT studies, 937 pregnant women with IMH received LT4 supplementation compared throughout the pregnancy. Initially from two randomized controlled trials: (1) Randomized Trial of Thyroxine Therapy for Subclinical Hypothyroidism and Randomized Trial of Thyroxine Therapy and Hypothyroxinemia Diagnosed During Pregnancy, in which LT4 was compared with placebo [47, 48], and (2) in Controlled Antenatal Thyroid Screening (CATS), compared to no-treatment control [49]. No significant selection, performance, detection, attrition, reporting, or other biases were detected among the included articles (Figure S2A, S2B).
Association between IMH and pregnancy /offspring outcomes
Pregnancy outcomes
The association between IMH and pregnancy outcomes had wide variations in the amplitude of findings between studies included in this review. (1) In the 98,190 pregnancies from 15 cohorts, the risk of preterm birth was 6.66% (182 out of 2,733 IMH) vs 4.95% (4725 of 95,457 euthyroid women) in euthyroid women (RR [95% CI]; 1.33 [1.15, 1.55], P < 0.001), with a low heterogeneity (I2 = 3%, p = 0.42) (Fig. 2A). (2) In the PROM analysis, there was a statistically significant increase in PROM in the IMH group (RR [95% CI]; 1.50 [1.05, 2.14], P < 0.05), with no heterogeneity (I2 = 0%, p = 0.67) (Figure S3 A). (3) In the eight cohorts, that observed gestational diabetes, the meta-synthesis result showed that the IMH group had a higher risk of developing gestational diabetes (RR [95% CI]; 1.42 [1.03, 1.96], P < 0.05) (Figure S3 B). (4) In addition, in the nine studies that reported gestational hypertension as a maternal outcome, we found no association with IMH (RR [95% CI]; 1.21 [0.99–1.48]). Meanwhile, compared to euthyroid pregnancies, IMH did not significantly grow in placenta previa, pre-eclampsia, abruptio placentae, and intrauterine growth restriction of babies (Table 2 and Figure S3 C-G).
Forest plots of studies on the effect of IMH on the premature rupture of gestational diabetes mellitus, preterm premature rupture of membranes, preterm birth, fetal distress and macrosomia. A Forest plots of studies on the effect of IMH for preterm birth. B Forest plots of studies on the effect of IMH for total malformations. C Forest plots of studies on the effect of IMH for fetal distress
Fetal outcomes
Among 11 studies on birth weight outcomes, the studies Pop (2003) [30] and Kooistra (2006) [31] were from the same cohort, so Kooistra (2006) was included in the meta-analysis considering the sample size and published year. There was no significant difference in birth weight between babies born to IMH and euthyroid mothers (Table 2 and Figure S3 H). Similarly, the proportion of small for gestational age and low birth weight infants was no different between IMH and euthyroid mothers (Table 2 and Figure S3 I, J).
Within 8101 pregnancies, the fetal head circumference was 31.9 and 33.5 cm in IMH and euthyroid groups, respectively (P = 0.92) (Table 2 and Figure S3 K). In the three studies identified with intelligence evaluation, the IQ score of IMH offsprings was lower than euthyroid offsprings (105.4 vs 110, in IMH and euthyroid offsprings respectively; MD [95% CI]; − 4.43 [− 11.35, 2.50], P = 0.21). However, the means were not statistically different, and the heterogeneity between studies was 85% (Table 2 and Figure S3 L).
Of the meta-analysis in 8 cohorts, there was a statistically significant increase in macrosomic infants in the IMH group (RR [95% CI]; 1.62 [1.35, 1.94], P < 0.001) compared with the euthyroid group, with a moderate heterogeneity (I2 = 38%, P = 0.13) (Fig. 2B). Women with IMH had a higher risk of fetal distress babies vs euthyroid women (6.23 vs 1.94%, respectively; RR [95% CI]; 1.75 [1.16, 2.65], P < 0.05) (Fig. 2C). No differences were found in the rest of the analyses in neonatal intensive care and neonatal/fetal death outcomes between IMH and euthyroid women. (Table 2 and Figure S3 M–N).
Power analysis
In the power analysis, only two out of 18 (11.1%) outcomes (i.e., birth weights of offspring and intelligence score) achieved power over 0.5. No difference was found between the IMH and euthyroid group on the intelligence score outcomes, and the power was 0.999. Most of the rest of the outcomes obtained low to moderate power. Even the outcomes referring to gestational hypertension and pre-eclampsia only achieved a power lower than 0.10.
The studies on the effect of LT4 supplementation on pregnancy outcomes/cognitive outcomes in individuals with isolated maternal hypothyroxinaemia
Based on the eligibility criteria, only two published RCTs were included for LT4 treatment in pregnant women with IMH. However, owing to the absence of further available randomized trials demonstrating the benefit of levothyroxine treatment for maternal hypothyroxinemia, screening for FT4 cannot be advocated.
Casey et al. [47] was secondary analyses of data from Randomized Trial of Thyroxine Therapy for Subclinical Hypothyroidism and Randomized Trial of Thyroxine Therapy for Hypothyroxinemia Diagnosed During Pregnancy at 15 centers within the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. In Casey et al. [47], it was reported that 526 hypothyroxinaemia cases at 17.8 weeks were included. They found no significant differences in neurodevelopmental outcomes in children whose mothers had received LT4 treatment for IMH during pregnancy and the control groups (265/261) through a comprehensive battery of tests through 5 years of age. Using the same population data, Varner et al. [48] concluded no difference in neonatal TSH values between 461 newborns from pregnant women with IMH and those born to euthyroid women who were administered 50 \(\mathrm{\mu g}\) of L-T4 supplementation during pregnancy from the same population, which was excluded as a duplicate study.
The result is consistent with the Controlled Antenatal Thyroid Screening (CATS) study conducted by Lazarus and his colleagues [49], which randomized 21 846 women recruited in early pregnancy (between 11 and 16 weeks gestation) to test TSH and FT4 levels during pregnancy versus serum sample storage and measurement after pregnancy. The subanalysis of the 411 mothers with IMH (defined as FT4 levels < P 2.5) found no significant improvement in cognitive function in children. Based on the current evidence, pregnancy outcomes and neonatal outcomes might not differ significantly between IMH with LT4 treatment and those without LT4 therapy during pregnancy. However, further studies are needed to validate these findings.
Discussion
The effects of IMH on pregnancy and offspring outcomes are still controversial. This study performed a systematic review and meta-analysis to examine the differences in IMH and euthyroidism in pregnancy. Outcomes related to maternal health and neonatals’/offsprings’ growth and development were also summarized. Furthermore, we intended to verify the effects of LT4 supplementation on pregnancy outcomes and cognitive outcomes in children born to mothers with IMH through this systematic review.
In this systematic review and meta-analysis, IMH was associated with a higher risk of preterm birth than euthyroidism (6.66 vs 4.95%; RR, 1.33 [1.15, 1.55], P < 0.001). This finding was similar to a previous meta-analysis that included 47,045 pregnant women (containing 904 isolated hypothyroxinemia) individual data [15], which reported that among IMH women, the risk of preterm birth was 7.1 vs 5.0% in euthyroid women (OR, 1.46 [95% CI, 1.12–1.90]). IMH during pregnancy was also associated with a higher risk of maternal PROM, gestational diabetes, fetal macrosomia, and fetal distress compared with euthyroidism. Furthermore, there was no association between IMH and any other studied outcomes. Similar to previous studies [28], IMH was not associated with gestational hypertension. The above effects convincingly replicate the results of previous observational studies and systematic reviews.
The precise etiology of IMH is not fully understood. The iodine deficiency was recognized as a major high-risk factor of IMH because pregnant women with iodine deficiency had a significantly higher prevalence of hypothyroxinemia than those without [7, 12], and iodine deficiency would probably lead to a prior production of T3 to T4. Furthermore, some studies [13, 50, 51], reported that iron deficiency was another potential risk factor for IMH because the insufficient supply of iron deficiency in pregnant women had a significantly higher prevalence of hypothyroxinemia than those without. Some previous studies [52,53,54] proved that iron deficiency has multiple affects on the thyroid axis and was of crucial importance for thyroid hormone synthesis by reducing the activity of thyroid peroxidase. What’s more, environmental pollutants were found to be a significant inverse association with FT4 levels and no significant association with FT4 levels [55]. Besides these, including overweight/obesity [13, 45], maternal age [13], angiogenic factors, and thyroid peroxidase antibodies (TPOAb) [11, 20], were also reported as independent risk factors for IMH.
However, the mechanisms that cause this change and how to eliminate these risk factors remain unclear. Thyroid hormones regulate metabolism procedures and energy homeostasis [56]. Maternal thyroid hormones are essential for average physical growth and neurocognitive development from conception to adulthood [57]. Pop et al. [58] and Haddow et al. [59] reported that FT4 negatively correlates with body weight during pregnancy. Moreover, it has been confirmed that thyroid hormone therapy can improve blood glucose and insulin sensitivity in animal models [60]. The current study provides evidence that there was no consensus on the adverse effects of IMH on maternal–fetal outcomes, except for preterm birth, PROM, fetal macrosomia, and fetal distress. Moreover, we did not find many positive results, possibly because the conversion of T4 to T3 plays a role in ensuring a sufficient amount of T3. It is believed that lower FT4 levels could be compensated by higher peripheral deiodinase activity, resulting in a higher conversion rate of FT4 into active thyroid hormone FT3 and a higher FT3/FT4 ratio [59]. Those might lead to increased placental nutrition transfer or metabolism of fatty acids to the fetus, contributing to fetal macrosomia in the latter part of gestation [61]. Besides, the thyroid hormone plays a vital role in fetal neurodevelopment, associated with maternal thyroid hormones in the late first or early second trimester [57, 62, 63]. The fetus’s thyroid gland develops at 12 weeks and functions around 18 to 20 weeks of gestation. Therefore, even a mild decrease in thyroid hormone during the critical period will adversely affect brain development.
However, we still observed some differences from the published results. In our study, IMH was not associated with a higher risk for offspring’s intelligence and achieved a power greater than 0.99. In a meta-analysis of individual participant data from 9036 mother–child pairs from three prospective population-based birth cohorts, [22] FT4 < 2.5th percentile was associated with a 3.9-point (95% CI, − 5.7 to − 2.2) lower nonverbal IQ and a 2.1-point (95% CI, − 4.0 to − 0.1) lower verbal IQ. A suggestive association of hypothyroxinemia with a greater risk of autistic traits was observed.
The conflicting results of the above studies on the effects of IMH can be partly explained by various sources of heterogeneity between the individual studies. First of all, there are substantial differences in the definition of IMH, including the FT4 lower-range cutoffs and the normal ranges for TSH and FT4 between studies, which may affect the interpretation of the study results. Furthermore, the timing of the diagnosis of IMH differed considerably, ranging from 10 to 13 weeks up until 20 weeks of pregnancy [20, 64]. As discussed above, this might be important, as the study by Cleary-Goldman et al. suggested the trimester-specific effects of IMH [64]. In addition, the differences in covariates and the degree of endpoint measurements between studies could not be ignored. For example, orientated from the Generation R Study, Mil [33] and his colleagues described the maternal education level and delivery times in detail; In the study of follow-up of more than 40,000 pregnant women [8], the authors categorized premature delivery in detail, such as preterm birth (birth before 37 weeks gestation), very early preterm birth (birth before 34 weeks gestation), preterm premature rupture of membranes (preterm birth with spontaneous rupture of the membranes at less than 37 weeks gestation and before the onset of contractions), spontaneous preterm birth with intact membranes (spontaneous preterm birth with intact membranes), and medically-induced preterm birth (preterm birth after labor induction or cesarean delivery for maternal or fetal indications). In comparison, most studies are not able to differentiate between subgroups. Lastly, as mentioned, there are substantial differences in sample sizes between studies, with many studies working with only a limited number of cases leading to underpowered analyses, while some studies were based on a large population [8, 30, 31, 45]. Besides, albeit small power for most negative outcomes is also noteworthy.
Still, there is no consensus that the correction of IMH in pregnant women with LT4 treatment is beneficial [5, 6]. It is reported that the efficacy of levothyroxine treatment in reducing obstetric risk may vary by medical history and prior risk factors as well as by the timing of commencement of therapy. Treatment for hypothyroxinaemia beginning between 8 and 20 weeks of gestation has no significantly better cognitive outcomes in children through 5 years of age than no treatment for those conditions [47]. Based on the previous evidence, the pregnancy and neonatal outcomes are not significantly different between IMH with LT4 therapy and those without LT4 treatment [20, 47]. In the current study, only two RCTs were included in LT4 supplementation in pregnant women with IMH, and they were not suitable for synthesizing the outcomes quantitatively. Furthermore, more RCTs and clinical studies, especially with larger sample sizes using population- and pregnancy-specific reference ranges of FT4 and TSH levels, are still required to provide evidence for the efficacy of LT4 therapy. What also deserves expecting is new related studies in preparation and progress [65].
To the best of our knowledge, well designed and conducted, the current study is a systematic review and meta-analysis that roundly evaluated the association between IMH and pregnancy and offspring outcomes, while previous studies focused on a limited number of outcomes. Most of the previously reviewed studies focused on the effects of IMH on mental development in early life. As some of these cohorts have continued follow-up of their participants over the years, they have also studied cognitive performance in later life. Our study found no significant differences in most pregnancy outcomes or cognitive function in offspring between pregnant women with IMH and individuals with euthyroid, except for gestational diabetes mellitus, PROM, preterm birth, fetal distress, and macrosomia. In addition, given the existing interventional data, IMH might not be routinely treated during pregnancy.
There are some limitations to our study. First, we failed to specify the pregnancy period for enrolled pregnant women strictly. The fetal thyroid does not secrete thyroid hormones before 12–14 weeks of gestation during the first trimester, which may risk pregnancy outcomes and child cognitive development and neurodevelopmental disorders [66, 67]. In euthyroid pregnant women, FT4 levels increase, and TSH levels decrease, regulated by the increased human chorionic gonadotropin (hCG) levels during early gestation. The pregnant women with IMH in this study were in different stages of pregnancy, which might have affected the results. Besides, few trials could be included to synthesize quantitatively for each parameter of fetal-maternal and neonatal outcomes, despite extensive database searches. Finally, there were some limitations in those RCT studies. In Casey et al. [68] study, pregnant women earlier than eight weeks of gestation were not enrolled since they might have an early miscarriage. In Varner et al. [48] study, the newborn TSH levels were detected soon after birth at only a single point in time, which may not reflect thyroid function either in utero or later in life. Furthermore, the newborn TSH levels cannot be compared with those whose parents had a biochemical abnormality with euthyroid controls.
Conclusions
Taken together, isolated maternal hypothyroxinaemia may be associated with gestational diabetes mellitus, preterm premature rupture of membranes and preterm birth in maternal, and fetal distress and macrosomia in offspring. The results of the current study provide further insights into the potential risks of isolated maternal hypothyroxinemia that may help optimize clinical decision-making strategies. Meanwhile, large detailed, and sufficiently powered studies using consistent definitions for IMH and adverse pregnancy outcomes still be required to clarify further the exact effects of IMH on pregnancy and offspring complications and elucidate the underlying pathophysiology of IMH. At the same time, the need for a sufficiently powered, placebo-controlled RCT to treat IMH pregnancies is emphasized.
Abbreviations
- IMH:
-
Isolated maternal hypothyroxinaemia
- IH:
-
Isolated hypothyroxinaemia
- RCT:
-
Randomized clinical trial
- TSH:
-
Thyroid-stimulating hormone
- L-T4:
-
Levothyroxine
- FT4:
-
Free thyroxine
- PROM:
-
Premature rupture of membranes
- IQ:
-
Intelligence quotient
- NOS:
-
The Newcastle–Ottawa Scale
- TH:
-
Thyroid hormone
- RR:
-
Risk ratio
- BMI:
-
Body mass index
- FPG:
-
Fasting blood glucose
- HOMA-IR:
-
Homeostasis model assessment-insulin resistance
- GDM:
-
Gestational diabetes mellitus
- LBW:
-
Low birth weight
- HCG:
-
Human chorionic gonadotropin
- PTB:
-
Preterm birth
- MeSH:
-
Medical subject headings
References
Ge GM, Leung MTY, Man KKC, Leung WC, Ip P, Li GHY et al (2020) Maternal thyroid dysfunction during pregnancy and the risk of adverse outcomes in the offspring: a systematic review and meta-analysis. J Clin Endocrinol Metab 105(12):dgaa555
de Escobar GM, Obregón MJ, del Rey FE (2004) Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab 18(2):225–248
de Escobar GM, Obregón MJ, del Rey FE (2007) Iodine deficiency and brain development in the first half of pregnancy. Public Health Nutr 10(12a):1554–1570
Fetene DM, Betts KS, Alati R (2017) MECHANISMS IN ENDOCRINOLOGY: Maternal thyroid dysfunction during pregnancy and behavioural and psychiatric disorders of children: a systematic review. Eur J Endocrinol 177(5):R261-r273
Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C et al (2017) 2017 guidelines of the American thyroid association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 27(3):315–389
Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B (2014) 2014 European thyroid association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J 3(2):76–94
López-Muñoz E, Mateos-Sánchez L, Mejía-Terrazas GE, Bedwell-Cordero SE (2019) Hypothyroidism and isolated hypothyroxinemia in pregnancy, from physiology to the clinic. Taiwan J Obstet Gynecol 58(6):757–763
Yang X, Yu Y, Zhang C, Zhang Y, Chen Z, Dubois L et al (2020) The Association between isolated maternal hypothyroxinemia in early pregnancy and preterm birth. Thyroid 30(12):1724–1731
Dosiou C, Medici M (2017) Management of endocrine disease: isolated maternal hypothyroxinemia during pregnancy: knowns and unknowns. Eur J Endocrinol 176(1):R21–R38
Knight BA, Shields BM, Hattersley AT, Vaidya B (2016) Maternal hypothyroxinaemia in pregnancy is associated with obesity and adverse maternal metabolic parameters. Eur J Endocrinol 174(1):51–57
Etemadi A, Amouzegar A, Mehran L, Tohidi M, Azizi F, Moradi K et al (2020) Isolated hypothyroxinemia in iranian pregnant women, the role of iodine deficiency: a population-based cross-sectional study. Thyroid: Off J Am Thyroid Assoc 30(2):262–269
Opazo MC, Coronado-Arrázola I, Vallejos OP, Moreno-Reyes R, Fardella C, Mosso L et al (2020) The impact of the micronutrient iodine in health and diseases. Crit Rev Food Sci Nutr 62(6):1–14
Liu Y, Li G, Guo N, Liu X, Huang S, Du Q (2022) Association between maternal characteristics and the risk of isolated maternal hypothyroxinemia. Front Endocrinol 13:843324
Yu X, Shan Z, Li C, Mao J, Wang W, Xie X et al (2015) Iron deficiency, an independent risk factor for isolated hypothyroxinemia in pregnant and nonpregnant women of childbearing age in China. J Clin Endocrinol Metab 100(4):1594–1601
Korevaar TIM, Derakhshan A, Taylor PN, Meima M, Chen L, Bliddal S et al (2019) Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA 322(7):632–641
Nazarpour S, Ramezani Tehrani F, Rahmati M, Amiri M, Azizi F (2022) Effects of isolated maternal hypothyroxinemia on adverse pregnancy outcomes. Arch Gynecol Obstet 305(4):903–911
Su PY, Huang K, Hao JH, Xu YQ, Yan SQ, Li T et al (2011) Maternal thyroid function in the first twenty weeks of pregnancy and subsequent fetal and infant development: a prospective population-based cohort study in China. J Clin Endocrinol Metab 96(10):3234–3241
Ramezani Tehrani F, Nazarpour S, Behboudi-Gandevani S (2021) Isolated maternal hypothyroxinemia and adverse pregnancy outcomes: a systematic review. J Gynecol Obstet Hum Reprod 50(7):102057
Su X, Zhao Y, Cao Z, Yang Y, Duan T, Hua J (2019) Association between isolated hypothyroxinaemia in early pregnancy and perinatal outcomes. Endocr Connect 8(4):435–441
Gong X, Liu A, Li Y, Sun H, Li Y, Li C et al (2019) The impact of isolated maternal hypothyroxinemia during the first and second trimester of gestation on pregnancy outcomes: an intervention and prospective cohort study in China. J Endocrinol Invest 42(5):599–607
Furnica RM, Gruson D, Lazarus JH, Maiter D, Bernard P, Daumerie C (2017) First trimester isolated maternal hypothyroxinaemia: adverse maternal metabolic profile and impact on the obstetrical outcome. Clin Endocrinol (Oxf) 86(4):576–583
Levie D, Korevaar TIM, Bath SC, Dalmau-Bueno A, Murcia M, Espada M et al (2018) Thyroid function in early pregnancy, child IQ, and autistic traits: a meta-analysis of individual participant data. J Clin Endocrinol Metab 103(8):2967–2979
Gu Y, Su X, Li Y, Tang Y, Bao Y, Ying H (2019) Do free thyroxine levels influence the relationship between maternal serum ferritin and gestational diabetes mellitus in early pregnancy? Diabetes Res Clin Pract 151:114–119
Huang K, Xu Y, Yan S, Li T, Xu Y, Zhu P et al (2019) Isolated effect of maternal thyroid-stimulating hormone, free thyroxine and antithyroid peroxidase antibodies in early pregnancy on gestational diabetes mellitus: a birth cohort study in China. Endocr J 66(3):223–231
Nelson SM, Haig C, McConnachie A, Sattar N, Ring SM, Smith GD et al (2018) Maternal thyroid function and child educational attainment: prospective cohort study. BMJ 360:k452
Päkkilä F, Männistö T, Hartikainen AL, Suvanto E (2018) Maternal thyroid function during pregnancy and the child’s linguistic and sensory development in the Northern Finland Birth Cohort 1986. Front Endocrinol (Lausanne) 9:127
Chan S, Boelaert K (2015) Optimal management of hypothyroidism, hypothyroxinaemia and euthyroid TPO antibody positivity preconception and in pregnancy. Clin Endocrinol 82(3):313–326
Toloza FJK, Derakhshan A, Männistö T, Bliddal S, Popova PV, Carty DM et al (2022) Association between maternal thyroid function and risk of gestational hypertension and pre-eclampsia: a systematic review and individual-participant data meta-analysis. Lancet Diabetes Endocrinol 10(4):243–252
Edition AHWC CSOEC (2019) Chinese society of perinatology CMA guideline on diagnosis and management of thyroid diseases during pregnancy and postpartum (2nd edition). Chin J Perinat Med 8:505–539
Pop VJ, Brouwers EP, Vader HL, Vulsma T, van Baar AL, de Vijlder JJ (2003) Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol (Oxf) 59(3):282–288
Kooistra L, Crawford S, van Baar AL, Brouwers EP, Pop VJ (2006) Neonatal effects of maternal hypothyroxinemia during early pregnancy. Pediatrics 117(1):161–167
Henrichs J, Bongers-Schokking JJ, Schenk JJ, Ghassabian A, Schmidt HG, Visser TJ et al (2010) Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the generation R study. J Clin Endocrinol Metab 95(9):4227–4234
van Mil NH, Steegers-Theunissen RP, Bongers-Schokking JJ, El Marroun H, Ghassabian A, Hofman A et al (2012) Maternal hypothyroxinemia during pregnancy and growth of the fetal and infant head. Reprod Sci 19(12):1315–1322
Korevaar TI, Schalekamp-Timmermans S, de Rijke YB, Visser WE, Visser W, de Muinck Keizer-Schrama SM et al (2013) Hypothyroxinemia and TPO-antibody positivity are risk factors for premature delivery: the generation R study. J Clin Endocrinol Metab 98(11):4382–4390
Finken MJ, van Eijsden M, Loomans EM, Vrijkotte TG, Rotteveel J (2013) Maternal hypothyroxinemia in early pregnancy predicts reduced performance in reaction time tests in 5- to 6-year-old offspring. J Clin Endocrinol Metab 98(4):1417–1426
Román GC, Ghassabian A, Bongers-Schokking JJ, Jaddoe VW, Hofman A, de Rijke YB et al (2013) Association of gestational maternal hypothyroxinemia and increased autism risk. Ann Neurol 74(5):733–742
Medici M, Ghassabian A, Visser W, de Muinck Keizer-Schrama SM, Jaddoe VW, Visser WE et al (2014) Women with high early pregnancy urinary iodine levels have an increased risk of hyperthyroid newborns: the population-based generation R study. Clin Endocrinol (Oxf) 80(4):598–606
Ghassabian A, El Marroun H, Peeters RP, Jaddoe VW, Hofman A, Verhulst FC et al (2014) Downstream effects of maternal hypothyroxinemia in early pregnancy: nonverbal IQ and brain morphology in school-age children. J Clin Endocrinol Metab 99(7):2383–2390
Noten AM, Loomans EM, Vrijkotte TG, van de Ven PM, van Trotsenburg AS, Rotteveel J et al (2015) Maternal hypothyroxinaemia in early pregnancy and school performance in 5-year-old offspring. Eur J Endocrinol 173(5):563–571
Modesto T, Tiemeier H, Peeters RP, Jaddoe VWV, Hofman A, Verhulst FC et al (2015) Maternal mild thyroid hormone insufficiency in early pregnancy and attention-deficit/hyperactivity disorder symptoms in children. JAMA Pediatr 169(9):838–845
Oostenbroek MHW, Kersten RHJ, Tros B, Kunst AE, Vrijkotte TGM, Finken MJJ (2017) Maternal hypothyroxinaemia in early pregnancy and problem behavior in 5-year-old offspring. Psychoneuroendocrinology 81:29–35
Li Y, Shan Z, Teng W, Yu X, Li Y, Fan C et al (2010) Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25–30 months. Clin Endocrinol (Oxf) 72(6):825–829
Chen L, Yang H, Ye E, Lin Z, Peng M, Lin H et al (2020) Insignificant effect of isolated hypothyroxinemia on pregnancy outcomes during the first and second trimester of pregnancy. Front Endocrinol (Lausanne) 11:528146
Zhu YD, Han Y, Huang K, Zhu BB, Yan SQ, Ge X et al (2018) The impact of isolated maternal hypothyroxinaemia on the incidence of large-for-gestational-age infants: the Ma’anshan Birth Cohort study. BJOG 125(9):1118–1125
Liu Y, Guo F, Zhou Y, Yang X, Zhang Y, Fan J (2021) The Interactive effect of prepregnancy overweight/obesity and isolated maternal hypothyroxinemia on macrosomia. J Clin Endocrinol Metab 106(7):e2639–e2646
Medici M, Korevaar TIM, Schalekamp-Timmermans S, Gaillard R, de Rijke YB, Visser WE et al (2014) Maternal early-pregnancy thyroid function is associated with subsequent hypertensive disorders of pregnancy: the generation R study. J Clin Endocrinol Metab 99(12):E2591–E2598
Casey BM, Thom EA, Peaceman AM, Varner MW, Sorokin Y, Hirtz DG et al (2017) Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med 376(9):815–825
Varner MW, Mele L, Casey BM, Peaceman AM, Sorokin Y, Reddy UM et al (2018) Thyroid function in neonates of women with subclinical hypothyroidism or hypothyroxinemia. J Perinatol 38(11):1490–1495
Lazarus JH, Bestwick JP, Channon S, Paradice R, Maina A, Rees R et al (2012) Antenatal thyroid screening and childhood cognitive function. N Engl J Med 366(6):493–501
Maldonado-Araque C, Valdés S, Lago-Sampedro A, Lillo-Muñoz JA, Garcia-Fuentes E, Perez-Valero V et al (2018) Iron deficiency is associated with hypothyroxinemia and hypotriiodothyroninemia in the Spanish general adult population: Di@bet.es study. Sci Rep 8(1):6571
Teng X, Shan Z, Li C, Yu X, Mao J, Wang W et al (2018) Iron deficiency may predict greater risk for hypothyroxinemia: a retrospective cohort study of pregnant women in China. Thyroid: Off J Am Thyroid Assoc 28(8):968–975
Zimmermann MB (2006) The influence of iron status on iodine utilization and thyroid function. Annu Rev Nutr 26:367–389
Li S, Gao X, Wei Y, Zhu G, Yang C (2016) The relationship between iron deficiency and thyroid function in Chinese women during early pregnancy. J Nutr Sci Vitaminol (Tokyo) 62(6):397–401
Moreno-Reyes R, Corvilain B, Daelemans C, Wolff F, Fuentes Peña C, Vandevijvere S (2021) Iron deficiency is a risk factor for thyroid dysfunction during pregnancy: a population-based study in Belgium. Thyroid: Off J Am Thyroid Assoc 31(12):1868–1877
Zhao Y, Cao Z, Li H, Su X, Yang Y, Liu C et al (2019) Air pollution exposure in association with maternal thyroid function during early pregnancy. J Hazard Mater 367:188–193
Laurberg P, Knudsen N, Andersen S, Carlé A, Pedersen IB, Karmisholt J (2012) Thyroid function and obesity. Eur Thyroid J 1(3):159–167
Moog NK, Entringer S, Heim C, Wadhwa PD, Kathmann N, Buss C (2017) Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience 342:68–100
Pop VJ, Biondi B, Wijnen HA, Kuppens SM, Vader LH (2013) Maternal thyroid parameters, body mass index and subsequent weight gain during pregnancy in healthy euthyroid women. Clin Endocrinol 79(4):577–583
Haddow JE, Craig WY, Palomaki GE, Neveux LM, Lambert-Messerlian G, Canick JA et al (2013) Impact of adjusting for the reciprocal relationship between maternal weight and free thyroxine during early pregnancy. Thyroid 23(2):225–230
Lin Y, Sun Z (2011) Thyroid hormone potentiates insulin signaling and attenuates hyperglycemia and insulin resistance in a mouse model of type 2 diabetes. Br J Pharmacol 162(3):597–610
Lewis RM, Desoye G (2017) Placental lipid and fatty acid transfer in maternal overnutrition. Ann Nutr Metab 70(3):228–231
Drover SSM, Villanger GD, Aase H, Skogheim TS, Longnecker MP, Zoeller RT et al (2019) Maternal thyroid function during pregnancy or neonatal thyroid function and attention deficit hyperactivity disorder: a systematic review. Epidemiology 30(1):130–144
Getahun D, Fassett MJ, Jacobsen SJ, Xiang AH, Takhar HS, Wing DA et al (2021) Autism spectrum disorders in children exposed in utero to hyperemesis gravidarum. Am J Perinatol 38(3):265–272
Cleary-Goldman J, Malone FD, Lambert-Messerlian G, Sullivan L, Canick J, Porter TF et al (2008) Maternal thyroid hypofunction and pregnancy outcome. Obstet Gynecol 112(1):85–92
Runkle I, de Miguel MP, Barabash A, Cuesta M, Diaz Á, Duran A et al (2021) Early levothyroxine treatment for subclinical hypothyroidism or hypothyroxinemia in pregnancy: the st carlos gestational and thyroid protocol. Front Endocrinol 12:743057
Batistuzzo A, Ribeiro MO (2020) Clinical and subclinical maternal hypothyroidism and their effects on neurodevelopment, behavior and cognition. Arch Endocrinol Metab 64(1):89–95
Wang P, Gao J, Zhao S, Guo Y, Wang Z, Qi F (2016) Maternal thyroxine levels during pregnancy and outcomes of cognitive development in children. Mol Neurobiol 53(4):2241–2248
Casey BM, Dashe JS, Spong CY, McIntire DD, Leveno KJ, Cunningham GF (2007) Perinatal significance of isolated maternal hypothyroxinemia identified in the first half of pregnancy. Obstet Gynecol 109(5):1129–1135
Hamm MP, Cherry NM, Martin JW, Bamforth F, Burstyn I (2009) The Impact of isolated maternal hypothyroxinemia on perinatal morbidity. J Obstet Gynecol Can 31(11):1015-1021
Männistö T, Vääräsmäki M, Pouta A, Hartikainen AL, Ruokonen A, Surcel HM et al (2010) Thyroid dysfunction and autoantibodies during pregnancy as predictive factors of pregnancy complications and maternal morbidity in later life. J Clin Endocrinol Metabol 95(3):1084-1094
Breathnach FM, Donnelly J, Cooley SM, Geary M, Malone FD (2013) Subclinical hypothyroidism as a risk factor for placental abruption: evidence from a low-risk primigravid population. Australian New Zealand J Obstet Gynecol 53(6):553-560
Ong GS, Hadlow NC, Brown SJ, Lim EM, Walsh JP (2014) Does the thyroid-stimulating hormone measured concurrently with first trimester biochemical screening tests predict adverse pregnancy outcomes occurring after 20 weeks gestation?. J Clin Endocrinol Metabol 99(12):E2668-E2672
León G, Murcia M, Rebagliato M, Álvarez-Pedrerol M, Castilla AM, Basterrechea M et al (2015) Maternal thyroid dysfunction during gestation preterm delivery and birthweight. The infancia y medio ambiente cohort Spain. Paediatr Perinat Epidemiol 29(2):113-122
Grau G, Aguayo A, Vela A, Aniel-Quiroga A, Espada M, Miranda G et al (2015) Normal intellectual development in children born from women with hypothyroxinemia during their pregnancy. J Trace Elements Med Biol 31:18-24
Avramovska M, Karanfilski B, Dimitrov G, Dzikova E, Daneva Markova A, Hadzi Lega M et al (2021) Isolated maternal hypothyroxinemia and its perinatal outcome in North Macedonia. Acta Clinica Croatica 60(2):246-253
Karbownik-Lewińska M, Stępniak J, Lewiński A (2021) Potential risk factors for isolated hypothyroxinemia in women of childbearing age—results from retrospective analysis. J Clin Med 10(22):5384
Funding
This study was funded by the National Natural Science Foundation (81922016, 81870607), Peking University Medicine Fund of Fostering Young Scholars’ Scientific & Technological Innovation (BMU2022PYB035), Fundamental Research Funds for the Central University, Key Clinical Projects of Peking University Third Hospital (BYSYZD2021030), and Shandong Provincial Natural Science Foundation (ZR2019JQ25, ZR2020ZD14) of China.
Author information
Authors and Affiliations
Contributions
Y Song and S Wang designed the study and take responsibility for the paper. L Zhuo, Z Wang, Y Yang and Z Liu conducted the literature search, data collection and data entry. L Zhuo and Z Wang contributed to data quality assessment and data analysis. Y Song and S Wang contributed to interpreting the results, draft reviewing, and finalizing the paper. All authors approved the paper for publication. Thanks to all authors above.
Corresponding authors
Ethics declarations
Conflict of interest
Song receives grants from the National Natural Science Foundation and Shandong Provincial Natural Science Foundation. Zhuo receives grants from Peking University Medicine Fund of Fostering Young Scholars’ Scientific & Technological Innovation and Fundamental Research Funds for the Central University, Key Clinical Projects of Peking University Third Hospital. The other authors have nothing to disclose.
Research involving human participants and/or animals
The study was based on publicly available literatures and carried out in accordance with the protocol and with principles enunciated in the current version of the Declaration of Helsinki, and related law and regulatory authority's requirements.
Informed consent
There was no public or patient involvement in the conception of the research question or the design or implementation of the study
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhuo, L., Wang, Z., Yang, Y. et al. Obstetric and offspring outcomes in isolated maternal hypothyroxinaemia: a systematic review and meta-analysis. J Endocrinol Invest 46, 1087–1101 (2023). https://doi.org/10.1007/s40618-022-01967-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-022-01967-4