Abstract
Purpose
The coronavirus 2019 (COVID-19) pandemic—caused by a new type of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)—has posed severe impacts on public health worldwide and has resulted in a total of > 6 million deaths. Notably, male patients developed more complications and had mortality rates ~ 77% higher than those of female patients. The extensive expression of the SARS-CoV-2 receptor and related proteins in the male reproductive tract and the association of serum testosterone levels with viral entry and infection have brought attention to COVID-19’s effects on male fertility.
Methods
The peer-reviewed articles and reviews were obtained by searching for the keywords SARS-CoV-2, COVID-19, endocrine, spermatogenesis, epididymis, prostate, and vaccine in the databases of PubMed, Web of Science and Google Scholar from 2020–2022.
Results
This review summarizes the effects of COVID-19 on the male reproductive system and investigates the impact of various types of SARS-CoV-2 vaccines on male reproductive health. We also present the underlying mechanisms by which SARS-CoV-2 affects male reproduction and discuss the potentially harmful effects of asymptomatic infections, as well as the long-term impact of COVID-19 on male reproductive health.
Conclusion
COVID-19 disrupted the HPG axis, which had negative impacts on spermatogenesis and the epididymis, albeit further investigations need to be performed. The development of vaccines against various SARS-CoV-2 variations is important to lower infection rates and long-term COVID risks.
Similar content being viewed by others
Introduction
The coronavirus disease 2019 (COVID-19) outbreak occurred in December 2019, and rapidly spread worldwide [1]. To date, more than 600 million COVID-19 cases have been reported, and the total death toll exceeds 6.4 million (as of September 5, 2022; https://coronavirus.jhu.edu/map.html). COVID-19 is caused by the novel single-stranded RNA virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) of the Coronaviridae family [2, 3]. More comorbidities and higher mortality rates were observed in male than in female patients [4]. Testosterone has been found to affect the SARS-CoV-2 entry and priming in male hosts, and was correlated with a weaker immune response, higher infection rates, and greater predisposition to thromboembolism [5]. A meta-analysis indicated that diabetes is a key factors associated with high mortality rates in men and women diagnosed with COVID-19 [6]. SARS-CoV-2 encodes four main proteins: a spike (S) protein that mediates virus entry into host cells, N protein that regulates nucleocapsid development, and envelope (E) and membrane (M) proteins that mediate viral assembly [7].
The potential impact and molecular mechanisms of SARS-CoV-2 on the reproductive system, particularly in male patients, have been reported in many studies [8,9,10,11,12,13], though often with conflicting results. In this review, we describe the endocrine status associated with COVID-19 infection, relationship between SARS-CoV-2 and spermatogenesis, effect of the virus on the epididymis and prostate, and potential impact of COVID-19 vaccines on male fertility.
Methods
The current review was conducted using related English literature from electronic databases, including the Web of Science, PubMed, and Google Scholar. The literature search considered publications from 2020 to 2022 with the terms COVID-19, SARS-CoV-2, endocrine, hypophysis, hypothalamus, testosterone, sperm, spermatogenesis, testis, epididymitis, prostate, fever, inflammation, diabetes, and asymptomatic. The studies were further classified by topic. The validity of each publication was assessed by reviewing the title, abstract, and conclusion. The reference lists of valid papers were checked for other relevant studies. All original research or review papers were assessed by two independent investigators and the data of each study were used to summarize their conclusions and perform a meaningful classification.
Receptors for SARS-CoV-2 invasion in the male reproductive system
To infect or enter a cell, the SARS-CoV-2 S protein binds to angiotensin I-converting enzyme 2 (ACE2) expressed on a cell-surface receptor. The host transmembrane protease serine 2 (TMPRSS2) assists in this process by further activating and cleaving the S protein [14]. ACE2 occurs in many tissues and organs, including the lungs, liver, kidneys, and testes [15, 16]. According to the GETx, FAMTOM5, and Human Protein Atlas databases, the testis is among the tissues expressing the highest RNA level of ACE2 [17]. Both ACE2 and TMPRSS2 protein levels were found to be enriched in the testes [18]. Thus, the male reproductive system is highly susceptible to SARS-CoV-2 [19, 20].
Single-cell RNA sequencing (scRNA-seq) suggested that ACE2 was enriched in various cells of the testis, including Sertoli cells, Leydig cells, spermatogonia, and somatic cells [21,22,23]. Compared to females, symptom severity and mortality rates are higher in males [24, 25]. The transcription of TMPRSS2 could be promoted by an androgen response element [26] and further activate its androgen [27]. Shastri et al. demonstrated a delay in SARS-CoV-2 clearance in male compared with female subjects, likely associated with the higher level of ACE2 present in the testes, which creates a viral reservoir and promotes viral persistence [17]. Indeed, SARS-CoV-2 antigens have been detected in fibroblasts, spermatogonia, Sertoli cells, and Leydig cells, while the viral particles reside in the cytoplasm of multiple cell types, such as Sertoli cells, Leydig cells, spermatids, endothelium cells, fibroblasts, and epithelial cells in the rete testis [28]. However, Rastrelli et al. concluded that poor prognosis and mortality were associated with lower total testosterone (T), based on the levels of free T [29]. Meta-analyses revealed that COVID-19 negatively impacted T production in the short term, which was related to an increased risk of death or admission to an intensive care unit (ICU) [11]. Although the contradictory effects of androgen on COVID-19 progression need further clarification, at least it proves that the dysregulation of T occurs after SARS-CoV-2 invasion.
Apart from ACE2, the transmembrane glycoprotein CD147 was shown to mediate SARS-CoV-2 pseudovirus entry into host cells via ADP-ribosylation factor 6 (Arf6)-regulated endocytosis [30, 31]. Our previous studies showed that CD147 was expressed at various stages of spermatogenesis, and CD147 null mutants resulted in infertility [32]. Furthermore, CD147 regulated the migration of spermatogonia and spermatocytes via metalloproteinases-2 (MMP-2) signals [33]. CD147 also repressed the extrinsic apoptosis of spermatocytes via nuclear factor κB (NF-κB) signaling [34, 35]. Moreover, CD147 played an indispensable role in sperm motility and acrosome reactions, suggesting that CD147 acts as a therapeutic target against asthenozoospermia [36]. Our previous investigations implied that SARS-CoV-2 might affect spermatogenesis via CD147 regulation. However, whether CD147 is involved in the entry of SARS-CoV-2 into the testes remains unclear.
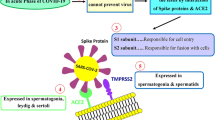
Apart from ACE2, TMPRSS2, and CD147, other recently identified factors involved in SARS-CoV-2 cell invasion may contribute to the impairment of spermatogenesis by acting as receptors (e.g., receptor tyrosine kinase [AXL], kringle containing transmembrane protein 1 [KREMEN1], and asparaginase and isoaspartyl peptidase 1 [ASGL1]), co-receptors (e.g., N-acetylneuraminate pyruvate lyase [NPL], C-type lectin domain family 4 member D [CLEC4D], neuropilin-1 [NRP1], and CD4), and cofactors (ectonucleotide pyrophosphatase/phosphodiesterase 1 [ENPP1], serine protease 1 [PRSS1], TMPRSS6, cathepsin F [CTSF], and paired basic amino acid cleaving enzyme [FURIN]; Fig. 1).
Expression profile of SARS-CoV-2 invasion-related factors in male reproductive system. A RNA expression of receptors, coreceptors, and cofactors related to SARS-CoV-2 cell entry. Gene expression data were acquired from the Human Protein Atlas [162]. ACE2 is abundant in the testis but rarely expressed in the epididymis, seminal vesicle, and prostate. B, C Differential expression of TMPRSS2 and CD147 in different tissues and organs. The expression values were clarified as normalized transcript per million (nTPM)
SARS-CoV-2 impairs male fertility by interfering with the hypothalamus–pituitary–gonadal (HPG) axis
Gonadotropins and various steroids serve as a molecular bridge between the brain and testes by regulating HPG axis activity. The activity of the HPG axis is initiated by the secretion of gonadotropin-releasing hormone (GnRH) from specialized hypothalamic neurons. Gonadotrophs, including follicle-stimulating hormone (FSH) and luteinizing hormone (LH), are secreted by GnRH-dependent adenohypophysis. FSH primarily acts on Sertoli cells to regulate spermatogenesis and keep the seminiferous tubule intact, whereas LH plays a crucial role in stimulating Leydig cells to produce T [37]. Notably, the production of androgens by steroidogenesis is restricted to Leydig cells. Androgens play a vital role in spermatogenesis and regulate gonadotropin levels via a negative feedback mechanism [38].
Male infertility is closely associated with pathological changes in HPG regulation [39, 40]. Previous studies have shown that male patients or animal models infected with SARS-CoV-2 exhibited aberrant hormone levels. Ma et al., in the first report of the effects of SARS-CoV-2 infection on male gonadal function, showed that infected males had considerably increased serum-luteinizing hormone (LH) levels and lower levels of T and FSH. The concentration of C-reactive protein (CRP, a marker of viral infection) was strongly correlated with the T:LH ratio after COVID-19 infection [41]. Coincidentally, serum T, FSH, and LH levels decreased acutely in infected patients, particularly in the case of viral pneumonia [42]. Furthermore, a series of retrospective cohort studies noted that decreased concentrations of T were mostly found in severe COVID-19 cases that required admission to the ICU and prolonged hospitalization [43,44,45]. In hospitalized men, this low T level was associated with an overactive immune response and inflammatory storm manifested by elevated levels of D-dimer, CRP, interleukin 6 (IL-6), and procalcitonin [46,47,48]. Lower levels of total T and free T were associated with poor prognosis and increased mortality rates in patients of the respiratory ICU [29]. Interestingly, estradiol levels were substantially increased in critically ill male COVID-19 patients, and their interferon γ (IFN-γ) levels were positively linked to serum estradiol levels, thereby increasing the requirement for extracorporeal membrane oxygenation (ECMO) treatment and risk of mortality [47, 49].
In a cohort of men recovering from COVID-19, a 7-month follow-up study revealed that T levels were further decreased in 10% of the patients, suggesting persistent hypogonadism, especially in cases with a higher burden of comorbid conditions. Moreover, the LH and 17β-estradiol levels in men with restored T levels decreased significantly [50]. In contrast, Apaydin et al. showed that hypogonadism persisted in 48.2% of men with lower T concentrations over a 6-month follow-up post-recovery [48]. In summary, these studies revealed that the male endocrine system was both directly and indirectly disrupted by COVID-19 infection, and reproductive health was negatively affected overall. The possible adverse effects of SARS-CoV-2 on the HPG axis are summarized in Fig. 2. The characteristics of the cited studies are listed in Table 1.
COVID-19 and spermatogenesis
Spermatogenesis can be divided into three consecutive phases. First, spermatogonia differentiate through mitosis into a B-type, which acts as the precursor of tetraploid primary spermatocytes. Second, diploid secondary spermatocytes are generated from primary spermatocytes via meiotic I division, and haploid spermatids are produced during meiosis II. Third, spermatids undergo spermiogenesis—nuclear elongation and condensation and acrosome biogenesis—to form spermatozoa. During spermatogenesis, different types of germ cells attach to Sertoli cells through specialized cell junctions to enable cell migration from the basement membrane to the abluminal compartment. Additionally, the blood–testis barrier (BTB), constituted by cell–cell junctions among Sertoli cells, limits mature sperm penetration into the circulatory system [51].
Evidence suggests that SARS-CoV-2 infection downregulates the expression of spermatogenesis-related genes [21]. The higher levels of ACE2 expression in the testes of infertile men suggests that COVID-19-mediated reproductive disorders likely depend on ACE2 activation, or that men with reproductive abnormalities may be more susceptible to viral infection [18, 22]. Indeed, SARS-CoV-2 mRNA has been detected in semen [52, 53] and COVID-19 has been associated with decreased numbers of Leydig cells in the testes [54]. Several studies have shown the effects of COVID-19 on sperm quality and spermatogenesis during infection and recovery. For instance, semen volume and total sperm number were lower in COVID-19 patients than those in control subjects (non-infected men) [55,56,57,58,59]. Sperm viability, motility, and progressive motility were decreased [56,57,58,59], and the sperm DNA fragmentation index (DFI) was positively correlated with COVID-19 [57, 59,60,61]. The deterioration of sperm function and T levels have also been associated with the dysregulation of serum FSH and LH levels [56, 59, 62, 63]. Nonetheless, a number of studies reported unaffected testis or epididymis function, but abnormal sperm parameters. Scroppo et al. showed that the levels of T, gonadotropins, and inflammatory factors were unaffected in young males with mild or moderate COVID-19 infections, in spite of abnormal seminal values [64]. Guo et al. showed that the semen of 23 COVID-19-infected male patients had no detectable SARS-CoV-2 RNA, and their sperm counts, and morphology were within normal ranges [65]. Holtmann et al. found that mild COVID-19 infection did not affect testis and epididymis function, but semen parameters were impaired in moderate infection cases [66]. Emerging evidence suggests that the detrimental impact was imposed on spermatogenesis under SARS-CoV-2 invasion, although few studies indicating no influence were reported on sperm quality, the reason for which was mostly due to the males infected with COVID-19 being asymptomatic or with very mild conditions.
Oxidative stress (OS)—induced by an imbalance between reactive oxygen species (ROS) production and clearance by antioxidants—is an important driver of male infertility and is increased under COVID-19 infection [67]. Testicular dysfunctions induced by OS include impaired sperm quality and endocrine function, with oxidative damage in sperm corresponding predominantly to increasing DFI [68]. Total antioxidant capacity was negatively correlated with COVID-19, along with a higher DFI [59]. Similarly, ROS and DFI scores were found to be markedly higher at 14 d after COVID-19 diagnosis compared to those at 120 d [57]. A case report revealed that SARS-CoV-2 invasion into male germ cells may occur prior to the onset of symptoms and disrupt spermatogenesis, as evidenced by high levels of oxidative DNA damage in sperm [61].
In addition to OS, inflammation associated with COVID-19-induced testicular lesions may adversely affect spermatogenesis. Although an unusual presentation, testicular pain can occur with COVID-19 infection, suggesting a link to orchitis [69,70,71]. Duarte-Neto et al. observed testis congestion, testicular basilemma thickening, and interstitial edema in COVID-19-infected men, which are comparable to the symptoms of interstitial orchitis [28]. Moreover, the number of Sertoli and Leydig cells decreased [28, 72, 73], and testicular blood vessels showed strong expression of vascular cell adhesion molecule (VCAM) in infected men. Fibroblasts, spermatogonia, Sertoli, and Leydig cells all tested positive for the SARS-CoV-2 antigen [28]. COVID-19-infected men also had higher levels of pro-inflammatory cytokines, such as tumor necrosis factor α (TNF-α), IFN-γ, IL-8, IL-10, IL-1β, and IL-6, in seminal plasma [74, 75]. With the upregulation of these pro-inflammatory cytokines, the expression of junctional proteins involved in the BTB, such as connexin-43, claudin-11, and occludin, is disrupted [76]. The expression of genes involved in apoptosis, such as BAX, caspase-3, caspase-8, and caspase-9, was markedly increased in testicular specimens or sperms of infected men [77]. In deceased individuals, researchers observed a marked loss of germ cells and increased levels of apoptotic cells, CD3+ (mature T lymphocytes), CD68+ (macrophage-derived immune cells), CD20+ (B cell-derived immune cells), and IgG in the testis/epididymis [18, 74]. Together, these observations suggest that SARS-CoV-2 infection disrupts reproductive microenvironments by triggering OS and inflammation, thereby inhibiting spermatogenesis (Fig. 3).
Possible mechanisms of SARS-CoV-2-mediated impairment of spermatogenesis and sperm function. Spermatogenesis was directly affected by SARS-CoV-2 infection of the testis. CD3+-, CD68+-, or CD20+-positive inflammatory cells infiltrated the seminiferous tubule upon SARS-CoV-2 infection, which elevated the levels of inflammatory factors, including TNF-α and IL-1β. Oxidative stress (OS)-mediated disruptions of male fertility occurred after virus entry, further causing germ cell apoptosis and sperm DNA fragmentation. Epididymitis was observed and mainly characterized by lymphocyte infiltration, sperm hyperaccumulation in the cauda, and a swollen lumen. SPG spermatogonia, PS primary spermatocyte, SS secondary spermatocyte, ES elongated sperm
Males with COVID-19 mostly suffered long-term repercussions and their reproductive functions were compromised even after recovery. Total sperm number at 37 d after recovery was still lower than that of age-matched viral-negative men [78]. Similarly, Guo et al. found that sperm number, concentration, and motility were lower in recovered men after 29 d than those in control subjects, while sperm parameters, including sperm morphology, improved considerably at 56 d after recovery [79]. In contrast, another report found that the concentration and total motility of sperm at 80 d after recovery remained poor [80]. Another study revealed that sperm quality only returned to normal after half a year [81]. A meta-analysis of seven studies corresponding to 934 subjects (median age of 37.34 ± 10.5 years) revealed deteriorating sperm quality and increased LH and prolactin levels during recovery. Therefore, whether deteriorating sperm quality is regulated only by the testes remains unclear [82]. Adamyan et al. investigated transcriptional alterations in semen from COVID-19-recovered men and found that genes in sperm mitochondria involved in mitochondrial oxidative phosphorylation and toll-like receptor signaling were inhibited. In fact, all protein-coding genes of the mitochondrial genome were dramatically downregulated. This could potentially explain how sperm motility is compromised in convalescent males after viral infection [83]. Moreover, semen proteomics revealed that the proteins linked to male fertility, including prosaposin and semenogelin 1, were markedly downregulated after recovery. The signaling pathways associated with reproductive functions, such as sperm motility, adhesion regulation, endopeptidase activity, oocyte–sperm recognition, and T reaction, were also repressed during recovery [84]. Gacci et al. showed that IL-8 was expressed at pathological levels in 75% of men (33 individuals) during recovery [85]. Negative correlations were found between sperm number and IL-1β and TNFα levels in semen after recovery [86]. Overall, current findings suggest that it is necessary to conduct accurate follow-ups focusing on the fertility status of patients in convalescence. Additionally, some individuals recovering from COVID-19 showed better recovery of spermatogenetic capacity than others. Gharagozloo et al. found that males with moderate COVID-19 infections along with azoospermia could recover rapidly as the infection waned [61]. Paoli et al. demonstrated that testicular function was not damaged directly, and that indirect damage was transient during recovery [10]. Hormone disorders and semen abnormalities gradually disappeared as COVID-19 was cleared [87]. The characteristics of the cited studies are listed in Table 2.
Effect of SARS-CoV-2 on the epididymis
The epididymis is a complex highly coiled duct that connects the vas deferens to the efferent ducts through four anatomically distinct parts, namely the caput, corpus, cauda, and initial segments. A highly specialized microenvironment exists along the lumen that secretes proteins, ions, and cytokines and reabsorbs rete testis fluid. Sperm generated in the testis are matured in the epididymis and acquire their full motility and fertility. The epididymis also acts as an environment to concentrate, store, and transport sperm before ejaculation [88]. Although ACE2 has limited expression in the epididymis [89], other cofactors and receptors, including NPL, NRP1, and CD147, are highly expressed in the epididymis (Fig. 1), suggesting potential for impairment by SARS-CoV-2. Furthermore, SARS-CoV-2 can attach to epididymal sperm via the spike protein [90]. Histological analysis of the epididymis in deceased patients with COVID-19 demonstrated that a large number of sperm and immature spermatocytes accumulated in the cauda [91]. In a pediatric case diagnosed concurrently with COVID-19 and orchiepididymitis, Gagliardi et al. confirmed inhomogeneous testis swelling and epididymal inflammation with reactive hydrocele [92]. Similarly, Marca et al. reported that COVID-19 induced slight swelling and gentle accentuation of vascularization in the epididymis, which were symptoms of epididymitis [69]. Epididymitis caused by COVID-19 can present as reactional hydrocele with nonuniform echo or microcyst dissemination, both of which appear as caput augmentation (> 1.2 cm) and scrotum incrassation [93]. In an extensive cohort study (142 patients hospitalized with COVID-19) conducted by Chen et al. 32 patients were diagnosed with acute orchitis, epididymitis, or orchiepididymitis. Acute scrotal infection was found to increase with age, with an incidence rate of 53.3% in men > 80 years of age. Notably, compared with mild infections, severe infections were associated with a higher risk of developing orchiepididymitis [94]. In contrast, Holtmann et al. found that mild infection had no effect on the function of the epididymis and testis [66]. Histopathological assessment of the epididymis after COVID-19 invasion indicated noticeable alterations compared with the control group. Interstitial hyperemia and edema, as well as exudation of red blood cells, were observed in the epididymis. In addition, a few T-lymphocytes appeared in the epididymal duct and infiltrated the testicular blood vessels [8]. Collectively, these results suggest that SARS-CoV-2 infection affects the epididymal microenvironment and interferes with sperm maturation. Therefore, the risk of epididymitis, especially in young aspiring parents, requires greater clinical attention. The key mechanisms associated with epididymal injury in SARS-CoV-2 are shown in Fig. 3.
Effect of SARS-CoV-2 on the prostate
The prostate, an essential accessory gland of the male reproductive system, consists of the epithelium and stroma. The epithelial compartment secretes prostatic fluid, accounting for approximately one-fifth to one-third of the ejaculate volume. A large number of factors in the prostatic fluid regulate ejaculation and mediate sperm motility, semen liquefaction, and the clotting cycle [95]. Regarding the expression of ACE2 and TMPRSS2 in prostate tissues, single-cell RNA sequencing revealed that epithelial cells expressing ACE2 and TMPRSS2 accounted for 0.32% and 18.65% of the total cells, respectively. Notably, 0.61% of the cells co-expressed ACE2 and TMPRSS2 [96]. Regarding the pathological characteristics of the prostate following SARS-CoV-2 infection, Zhang et al. demonstrated for the first time that infected males had no detectable SARS-CoV-2 RNA in the expressed prostatic secretion (EPS), despite elevated levels of CRP, erythrocyte sedimentation, and IL-6 [97]. SARS-CoV-2 RNA was also absent 80 d after complete clearance of the virus (based on RT-PCR) in mild, moderate, and severe pneumonia [80]. The hospitalized patients did not show signs of prostate inflammation, and the mean serum level of prostate-specific antigen was normal (1.13 ng/mL) [98]. The characteristics of the cited studies are listed in Table 3.
Indirect effects of COVID-19 on male reproduction
Indirect injury to the HPG axis
Salonia et al. reported that 85% of COVID-19 patients showed secondary hypogonadism with lower T levels, which predicted severe clinical outcomes [46]. A follow-up study suggested that a slower recovery rate of T levels was associated with a higher burden of comorbid conditions. Different high-dose corticosteroid formulations for treating COVID-19 have drawn widespread attention [99, 100]. It is well established that the pituitary–gonadal axis can be disturbed by hypercortisolism [101]. Decreased serum T levels, perturbed spermatogenesis, and Sertoli cell dysfunction were associated with hypercortisolism in animal models [102,103,104]. Excessive treatment with corticosteroids for congenital adrenal hyperplasia in male patients appeared to decrease sperm quality and promote hypogonadism [105]. Compared with long-term corticosteroid treatment, high-dose therapy for critical COVID-19 infections is the safer approach, given the potential risks of cortisol drugs on testosterone production and semen quality.
Effects of COVID-19-mediated fever and inflammation on male reproduction
Higher scrotal temperatures caused by fever has an adverse impact on germ cell development as well as sperm DNA integrity, quality, and viability, and can transiently induce sperm apoptosis [106]. Fever was found to occur in 80% of infected patients, and was likely to decrease sperm quality, even in the absence of SARS-CoV-2 viral particles [107, 108]. During recovery, patients presenting with fever had a decreased sperm volume, motility, concentration, and total number compared with those in patients without fever [66]. In addition, the secondary inflammatory response in the male reproductive tract is frequently associated with subfertility [109]. The “cytokine storm” caused in severe and critical SARS-CoV-2 infections presents as the dysregulation of several pro-inflammatory factors, including IL-6, IL-1β, IL-8, and TNF-α [110], which disrupts the integrity and permeability of the BTB and potentially decreases semen quality and fertilization ability. Studies have shown that, even without the expression of SARS-CoV-2 RNA in semen plasma during infection or recovery, sperm count, concentration, and motility were downregulated and frequently accompanied by alterations in the expression of oxidation and apoptosis markers [85, 111]. In general, the impact of COVID-19-mediated fever and inflammation on male fertility is unclear, especially as studies rarely regard the contribution of comorbidities and other pathologies. Further studies are needed to clarify the relationship between reproductive injury and the myriad symptoms of COVID-19.
Diabetes: a potential cofactor affecting male reproduction in COVID-19 patients
SARS-CoV-2 causes acute hyperglycemia and insulin resistance in non-diabetic patients and exacerbates diabetes in pre-diabetic individuals [112,113,114], which is likely mediated by the increased expression of ACE2 in the exocrine glands and islets of the pancreas. SARS-CoV-2 infiltration with concomitant pancreatic injury has been observed in infected patients, which may cause acute β-cell dysfunction [115]. In addition, virus-induced ROS and pro-inflammatory cytokine production promote insulin resistance [116]. Markers of insulin resistance, such as the glucose index and triglyceride content, have been related to COVID-19 severity and mortality rates [117]. Higher blood glucose levels were found to promote COVID-19 progression by facilitating cytokine production and viral replication in diabetic patients [118]. Mounting evidence indicates a relationship between diabetes and testicular injury in murine and human diabetic models [119,120,121]. Indeed, serum testosterone, FSH, LH, and antioxidant levels along with sperm number and viability were increased after diabetes resistance treatment [122]. The apelin (APLN) peptide and its receptor are overactivated in the testes of diabetic patients, which promotes the dysfunction of the BTB and spermatogenesis. ML221, an antagonist of the APLN receptor, relieved BTB damage and enhanced spermatogenic ability in cultured human testicular cells [123]. In summary, current evidence shows that diabetes exacerbates the effect of COVID-19 on male reproduction.
SARS-CoV-2 vaccine and male fertility
A vaccine can provide effective protection for susceptible populations against the more serious symptoms of a infectious disease [124]. At the end of 2020, several COVID-19 vaccines were granted Emergency Use Authorization after third-phase clinical trials, which included attenuated amplicons from China and two mRNA vaccines from Moderna and Pfizer [125, 126]. Numerous studies have investigated the safety of these vaccines in the short to medium term. Common side effects included local pain, swelling, redness at the injection site, fatigue, chills, and fever. Serious side effects have also been documented in rare cases, such as Bell’s pain, right leg paresthesia, and paroxysmal ventricular arrhythmia [127, 128]. In addition, the vaccines had a potential deteriorative effect on spermatogenesis in the recovery phase of males with persistent hypothyroidism [78], resulting in elevated ROS content [57] and an inflammation storm in the testis [8, 129], which is similar to the physiological response that could theoretically occur via inoculation of COVID-19 [130]. These side effects can promote vaccine hesitancy (20.9% in males and 79.1% in females of the USA), along with fears that fertility could be affected [131]. Common concerns have also emerged regarding sperm quality among couples receiving assisted reproductive technology (ART) treatment and sperm donators [132, 133]. A small-cohort short follow-up (1–3 months) study of healthy males indicated that there were no noticeable changes in sperm parameters after COVID-19 mRNA vaccines administration, including mRNA-1273 (Moderna) and BNT162b2 (Pfizer-BioNTech) [134,135,136,137]. A detailed analysis revealed that motility, DNA fragmentation, and the ultrastructure of sperm remained unchanged, while sperm concentration increased compared to that before three doses of mRNA vaccination [138]. A retrospective cohort study determined that there were no marked differences in sperm parameters after administration of the inactivated vaccine for SARS-CoV-2 after two doses [139]. Furthermore, vaccination with viral vectors or mRNA vaccines did not exacerbate poor fertility rates or sperm motility and concentration in patients with male-factor infertility [136, 140]. A meta-analysis showed that sperm quality was not affected by any of the mRNA COVID-19 vaccines [11]. A larger analysis involving subjects from seven countries concluded that there was no association between subfertility and COVID-19 vaccines, including BNT162b2 and mRNA-1273, in women or men [141]. mRNA vaccines also showed no relationship with the risk of developing orchitis and/or epididymitis [142]. Overall, COVID-19 vaccination has not affected sperm quality or testis and epididymis function. However, the long-term effect (> 10 years) of vaccination on male fertility requires a thorough follow-up investigation.
Conclusions and perspectives
It is clear that COVID-19 can cause dysfunction of the HPG axis [43,44,45]. Deviations in T levels reflected the pathological status of steroidogenesis in the testis, which was related to the dysregulated levels of LH and FSH in COVID-19-affected subjects. Moreover, the lowered T levels could cause erectile dysfunction and altered spermatogenesis, which promote subfertility [143]. Higher concentrations of LH and FSH reflect testicular damage and other pathological outcomes [144]. Dysregulation of the HPG axis could result in not only hypothyroidism but also neurodegenerative senescence, liver cirrhosis, and chronic kidney disease [145]. The multiple possible mechanisms by which SARS-CoV-2 affects spermatogenesis are as follows: direct damage to testicular tissue or sperm; exaggeration of immune response, OS, and apoptosis mediated by SARS-CoV-2 infection; dysregulation of hormone levels. However, SARS-CoV-2 may have longer-lasting effects on the HPG axis and spermatogenesis. First, several studies ignored the importance of simultaneously detecting hormone levels and semen quality and did not consider the existence of SARS-CoV-2 in testicular tissue. Second, it is plausible to conclude that male reproduction is disturbed during the recovery phase [83]. Therefore, greater attention should be paid to the molecular mechanisms of male reproductive disorders and the long-term impact of COVID-19 to distinguish transient and irreversible injuries to the HPG axis and spermatogenesis. Furthermore, medical interventions, such as newly developed oral medications, should be considered during recovery to improve the transient subfertility state and avoid further deterioration of the reproductive system. Third, unlike for moderate and mild diseases states, data are lacking for reproductive outcomes in critical and severe cases. In addition, the relationship between disease severity, hormone levels, and semen parameters has not yet been reported. Finally, because of the high concentration of prolactin, which inhibits the signaling of the HPG axis [146], prognosis and clinical management of COVID-19 could be improved by measuring the baseline level of prolactin in male patients.
Although epididymal damage has been verified according to the clinical pathologic status, there is no evidence that the virus is present in the epididymis, and the pathologic changes observed in biopsy and autopsy are also limited. The analysis of sperm parameters and hormone levels is recommended in the evaluation of males with COVID-19 diagnosed with epididymo-orchitis. The pathophysiological changes observed in the epididymis are likely due to an exaggerated immune response or cytokine storm in cases of prolonged illness, though this requires further consideration. Furthermore, research indicated that the prostate may not be targeted by SARS-CoV-2; however, the relationship between EPS and semen parameters remains unclear. Regarding the potential influence of SARS-CoV-2 vaccines, follow-ups should be continued to accurately determine the effective recovery of spermatogenesis and reproductive capacity. Although sperm viability has not been influenced by any of the COVID-19 vaccines [134,135,136, 138, 139], hormone levels and clinical characteristics should not be neglected in follow-up studies, especially given the known side effects. An increasing number of vaccines have been approved for clinical use, and even if the features and side effects are similar among distinct vaccines, it is unlikely that results can be generalized to other vaccines for emergency use.
To date, five primary variants of SARS-CoV-2 have been reported, including alpha (B.1.1.7), beta (B.1.351), gamma (P.1), delta (B.1.617.2), and omicron (B.1.1.529). Although these variants are associated with lower morbidity rates, they have shown higher transmission rates along with increased resistance to previous antiviral, antibody, and immune plasma treatments, which promotes the reinfection of recovered or vaccinated individuals, especially in the case of the omicron variant [147, 148]. Owing to its highly contagious nature, the omicron variant has aroused concerns of a new worldwide wave of COVID-19. In addition, findings also indicated a higher rate of asymptomatic cases associated with omicron infections, based on clinical analysis and lung computed tomography [149, 150]. A delicate balance between inflammation and antiviral action occurs in asymptomatic patients, depending on the pathogen’s capacity to clear virus-specific T cells [151]. Moreover, higher functional cellular immune responses are triggered in asymptomatic than in symptomatic individuals [151]. Higher levels of IL-2 and IFN-γ secretion were found in asymptomatic patients (including omicron-infected individuals), which were related to the increased production of IL-10, IL-6, IL-1β, and TNF-α triggered by virus-specific T cells [149, 152]. Therefore, asymptomatic individuals have a more robust cellular immune response than those with weak antiviral immunity.
In general, the innate immune response in the testicular microenvironment is counteracted by the immunosuppressive system [153]. However, severe systemic inflammation can negatively affect the male reproductive system through blood-borne transmission or secondary inflammation [109, 154]. Thus, the potential damage to the male reproductive system caused by SARS-CoV-2 cannot be neglected in asymptomatic individuals. While SARS-CoV-2 infection in young males is mostly mildly symptomatic or asymptomatic, its prevalence has been underestimated because children are often excluded from screening tests [155]. In fact, serological analysis revealed that the rate of infection among children under 18 years was 68% as compared with the reported 33% from December 2021 to February 2022 [156]. Previous studies have shown that the integrity of the BTB can be compromised by SARS-CoV-2 infestation [76], which is linked to the production of nitric oxide, IL-6, and TNF-α and increased macrophage infiltration [76]. Since the BTB is only functional at the time of puberty [157], children could be at a higher risk of long-term COVID-19 infections with direct or indirect damage caused by SARS-CoV-2-related multisystem inflammation [155]. Therefore, the potential impact of SARS-CoV-2 infection on the development of the reproductive system of preadolescent or pubertal males and their fecundity should not be overlooked.
“COVID-19 illness” is used to describe prolonged symptomatic infections or the persistence of symptoms after recovery [158]. Studies have indicated that “long COVID-19” occurs in approximately 30% of infected individuals [159]. Davis et al. characterized COVID-19 through an online international survey conducted for 7 months. The common symptoms after 6 months were cognitive impairment, fatigue, and post-exertional malaise. The mean prevalence of symptoms related to endocrine, reproductive, and genitourinary was 62.25% [160]. According to the largest cohort study, vaccination only 15% decreased the long-term risk of COVID-19 [161]. The high prevalence of long COVID symptoms associated with the male reproductive system and the limited protection offered by vaccination emphasize the need for further research on the impact of the various SARS-CoV-2 variants on male reproduction. Furthermore, more effective vaccines against various variants of SARS-CoV-2 need to be developed to reduce infection rates and long-COVID risks. Future studies should focus on the early diagnosis of reproductive abnormalities caused by COVID-19 to promote early treatment.
Data availability
All data generated or analysed during this study are included in this published article.
References
Vo HL, Doan NH, Hao N, Vu KD, Trang N (2020) Trends in Coronavirus Disease 2019 (COVID-19) research: findings from a scoping review. ResearchGate. https://doi.org/10.13140/RG.2.2.15222.34881
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL et al (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579(7798):270–273
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R et al (2020) A Novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382(8):727–733
Sharma G, Volgman AS, Michos ED (2020) Sex differences in mortality from COVID-19 pandemic: are men vulnerable and women protected? JACC Case Rep 2(9):1407–1410
Pivonello R, Auriemma RS, Pivonello C, Isidori AM, Corona G, Colao A, Millar RP (2021) Sex disparities in COVID-19 severity and outcome: are men weaker or women stronger? Neuroendocrinology 111(11):1066–1085
Corona G, Pizzocaro A, Vena W, Rastrelli G, Semeraro F, Isidori AM, Pivonello R, Salonia A, Sforza A, Maggi M (2021) Diabetes is most important cause for mortality in COVID-19 hospitalized patients: systematic review and meta-analysis. Rev Endocr Metab Disord 22(2):275–296
Ashour HM, Elkhatib WF, Rahman MM, Elshabrawy HA (2020) Insights into the recent 2019 novel coronavirus (SARS-CoV-2) in light of past human coronavirus outbreaks. Pathogens 9(3):186
Li H, Xiao X, Zhang J, Zafar MI, Wu C, Long Y, Lu W, Pan F, Meng T, Zhao K et al (2020) Impaired spermatogenesis in COVID-19 patients. E Clin Med 28:100604
Gupta P, Choudhary A, Gopal G, Kumar R, Kumar A, Tiwari P, Malhotra N. Detection of SARS-CoV2 virus using the real-time reverse transcriptase polymerase chain reaction in semen and seminal plasma from men with active COVID-19 infection–A pilot study. 2021;37(4) 331.
Paoli D, Pallotti F, Anzuini A, Bianchini S, Caponecchia L, Carraro A, Ciardi MR, Faja F, Fiori C, Gianfrilli D et al (2023) Male reproductive health after 3 months from SARS-CoV-2 infection: a multicentric study. J Endocrinol Invest 46:89–101
Corona G, Vena W, Pizzocaro A, Pallotti F, Paoli D, Rastrelli G, Baldi E, Cilloni N, Gacci M, Semeraro F et al (2022) Andrological effects of SARS-Cov-2 infection: a systematic review and meta-analysis. J Endocrinol Invest 45(12):2207–2219
Nassau DE, Best JC, Kresch E, Gonzalez DC, Khodamoradi K, Ramasamy R (2022) Impact of the SARS-CoV-2 virus on male reproductive health. BJU Int 129(2):143–150
Patel DP, Punjani N, Guo J, Alukal JP, Li PS, Hotaling JM (2021) The impact of SARS-CoV-2 and COVID-19 on male reproduction and men’s health. Fertil Steril 115(4):813–823
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(2):271–280.e8
Al NA (2020) Histopathologic and autopsy findings in patients diagnosed with coronavirus disease 2019 (COVID-19): what we know so far based on correlation with clinical, morphologic and pathobiological aspects. Adv Anat Pathol 27(6):363–370
Fu J, Zhou B, Zhang L, Balaji KS, Wei C, Liu X, Chen H, Peng J, Fu J (2020) Expressions and significances of the angiotensin-converting enzyme 2 gene, the receptor of SARS-CoV-2 for COVID-19. Mol Biol Rep 47(6):4383–4392
Shastri A, Wheat J, Agrawal S, Chaterjee N, Shastri J (2020) Delayed clearance of SARS-CoV2 in male compared to female patients: High ACE2 expression in testes suggests possible existence of gender-specific viral reservoirs. MedRxiv. https://doi.org/10.1101/2020.04.16.20060566
Ma X, Guan C, Chen R, Wang Y, Feng S, Wang R, Qu G, Zhao S, Wang F, Wang X et al (2021) Pathological and molecular examinations of postmortem testis biopsies reveal SARS-CoV-2 infection in the testis and spermatogenesis damage in COVID-19 patients. Cell Mol Immunol 18(2):487–489
Nguyen TT, Hulme J, Tran HD, Vo TK, Vo GV (2022) The potential impact of COVID-19 on male reproductive health. J Endocrinol Invest 45:1483–1495
Batiha O, Al-Deeb T, Al-Zoubi E, Alsharu E (2020) Impact of COVID-19 and other viruses on reproductive health. Andrologia 52(9):e13791
Wang Z, Xu X (2020) scRNA-seq profiling of human testes reveals the presence of the ACE2 receptor, a target for SARS-CoV-2 infection in spermatogonia, leydig and sertoli cells. Cells 9(4):920
Shen Q, Xiao X, Aierken A, Yue W, Wu X, Liao M, Hua J (2020) The ACE2 expression in Sertoli cells and germ cells may cause male reproductive disorder after SARS-CoV-2 infection. J Cell Mol Med 24(16):9472–9477
Qi J, Zhou Y, Hua J, Zhang L, Bian J, Liu B, Zhao Z, Jin S (2021) The scRNA-seq expression profiling of the receptor ACE2 and the cellular protease TMPRSS2 reveals human organs susceptible to SARS-CoV-2 infection. Int J Environ Res Public Health 18(1):284
Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P et al (2020) Factors associated with COVID-19-related death using OpenSAFELY. Nature 584(7821):430–436
Re GW (2004) Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am J Epidemiol 160(9):925–926
Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A et al (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(2):271–280
Lukassen S, Chua RL, Trefzer T, Kahn NC, Schneider MA, Muley T, Winter H, Meister M, Veith C, Boots AW et al (2020) SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J 39(10):e105114
Duarte-Neto AN, Teixeira TA, Caldini EG, Kanamura CT, Gomes-Gouvea MS, Dos Santos ABG, Monteiro RAA, Pinho JRR, Mauad T, da Silva LFF et al (2022) Testicular pathology in fatal COVID-19: a descriptive autopsy study. Andrology 10(1):13–23
Rastrelli G, Di Stasi V, Inglese F, Beccaria M, Garuti M, Di Costanzo D, Spreafico F, Greco GF, Cervi G, Pecoriello A et al (2021) Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology 9(1):88–98
Zhou YQ, Wang K, Wang XY, Cui HY, Zhao Y, Zhu P, Chen ZN (2022) SARS-CoV-2 pseudovirus enters the host cells through spike protein-CD147 in an Arf6-dependent manner. Emerg Microbes Infect 11(1):1135–1144
Wang K, Chen W, Zhang Z, Deng Y, Lian JQ, Du P, Wei D, Zhang Y, Sun XX, Gong L (2020) CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther 5(1):10
Chen H, Lam Fok K, Jiang X, Chan HC (2012) New insights into germ cell migration and survival/apoptosis in spermatogenesis: lessons from CD147. Spermatogenesis 2(4):264–272
Chen H, Fok KL, Yu S, Jiang J, Chen Z, Gui Y, Cai Z, Chan HC (2011) CD147 is required for matrix metalloproteinases-2 production and germ cell migration during spermatogenesis. Mol Hum Reprod 17(7):405–414
Chen H, Fok KL, Jiang X, Jiang J, Chen Z, Gui Y, Chan HC, Cai Z (2012) CD147 regulates apoptosis in mouse spermatocytes but not spermatogonia. Hum Reprod 27(6):1568–1576
Wang C, Fok KL, Cai Z, Chen H, Chan HC (2017) CD147 regulates extrinsic apoptosis in spermatocytes by modulating NFκB signaling pathways. Oncotarget 8(2):3132
Chen H, Shi X, Li X, Diao R, Ma Q, Jin J, Qiu Z, Li C, Yu MK, Wang C et al (2021) CD147 deficiency is associated with impairedsperm motility/acrosome reaction and offersa therapeutic target for asthenozoospermia. Mol Ther Nucleic Acids 26:1374–1386
Dwyer AA, Quinton R (2019) Anatomy and physiology of the Hypothalamic-Pituitary-Gonadal (HPG) axis. Advanced Practice in Endocrinology Nursing, pp 839–852
Selvam MKP, Sikka SC (2022) Role of endocrine disruptors in male infertility and impact of COVID-19 on male reproduction. Reproductive and developmental toxicology. Elsevier, UK, pp 1183–1194
Oyola MG, Handa RJ (2017) Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: sex differences in regulation of stress responsivity. Stress 20(5):476–494
Sengupta P, Cho C-L (2019) The Pathophysiology of male infertility. Male infertility in reproductive medicine. CRC Press, pp 1–9
Ma L, Xie W, Li D, Shi L, Mao Y, Xiong Y, Zhang Y, Zhang MJM (2020) Effect of SARS-CoV-2 infection upon male gonadal function: a single center-based study. MedRxiv. https://doi.org/10.1101/2020.03.21.20037267
Okcelik S (2021) COVID-19 pneumonia causes lower testosterone levels. Andrologia 53(1):e13909
Cayan S, Uguz M, Saylam B, Akbay E (2020) Effect of serum total testosterone and its relationship with other laboratory parameters on the prognosis of coronavirus disease 2019 (COVID-19) in SARS-CoV-2 infected male patients: a cohort study. Aging Male 23(5):1493–1503
Cinislioglu AE, Cinislioglu N, Demirdogen SO, Sam E, Akkas F, Altay MS, Utlu M, Sen IA, Yildirim F, Kartal S et al (2022) The relationship of serum testosterone levels with the clinical course and prognosis of COVID-19 disease in male patients: a prospective study. Andrology 10(1):24–33
Kadihasanoglu M, Aktas S, Yardimci E, Aral H, Kadioglu A (2021) SARS-CoV-2 pneumonia affects male reproductive hormone levels: a prospective. Cohort Study J Sex Med 18(2):256–264
Salonia A, Pontillo M, Capogrosso P, Gregori S, Tassara M, Boeri L, Carenzi C, Abbate C, Cignoli D, Ferrara AM et al (2021) Severely low testosterone in males with COVID-19: a case-control study. Andrology 9(4):1043–1052
Lanser L, Burkert FR, Thommes L, Egger A, Hoermann G, Kaser S, Pinggera GM, Anliker M, Griesmacher A, Weiss G et al (2021) Testosterone deficiency is a risk factor for severe COVID-19. Front Endocrinol (Lausanne) 12:694083
Apaydin T, Sahin B, Dashdamirova S, Dincer Yazan C, Elbasan O, Ilgin C, Bilgin H, Cam HK, Bahramzada G, Kucuk A et al (2022) The association of free testosterone levels with coronavirus disease 2019. Andrology 10(6):1038–1046
Schroeder M, Schaumburg B, Mueller Z, Parplys A, Jarczak D, Roedl K, Nierhaus A, de Heer G, Grensemann J, Schneider B et al (2021) High estradiol and low testosterone levels are associated with critical illness in male but not in female COVID-19 patients: a retrospective cohort study. Emerg Microbes Infect 10(1):1807–1818
Salonia A, Pontillo M, Capogrosso P, Gregori S, Carenzi C, Ferrara AM, Rowe I, Boeri L, Larcher A, Ramirez GA et al (2022) Testosterone in males with COVID-19: a 7-month cohort study. Andrology 10(1):34–41
Nishimura H, L’Hernault SW (2017) Spermatogenesis. Curr Biol 27(18):R988–R994
Li D, Jin M, Bao P, Zhao W, Zhang S (2020) Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw Open 3(5):e208292
Machado B, Barcelos Barra G, Scherzer N, Massey J, Santos Luz H, Henrique Jacomo R, Santa Rita TH, Davis R (2021) Presence of SARS-CoV-2 RNA in semen-cohort study in the United States COVID-19 positive patients. Infect Dis Rep 13(1):96–101
Yang M, Chen S, Huang B, Zhong JM, Su H, Chen YJ, Cao Q, Ma L, He J, Li XF et al (2020) Pathological findings in the testes of COVID-19 patients: clinical implications. Eur Urol Focus 6(5):1124–1129
Muttar AAJ. 2022. Assessment of the sexual level of patients with COVID-19 thorough evaluation and analysis of sperm and reproductive hormones. NVEO. 1008–1015.
Koc E, Keseroglu BB (2021) Does COVID-19 worsen the semen parameters? early results of a tertiary healthcare center. Urol Int 105(9–10):743–748
Falahieh FM, Zarabadipour M, Mirani M, Abdiyan M, Dinparvar M, Alizadeh H, Paktinat S, Hosseinirad H (2021) Effects of moderate COVID-19 infection on semen oxidative status and parameters 14 and 120 days after diagnosis. Reprod Fertil Dev 33(12):683–690
Erbay G, Sanli A, Turel H, Yavuz U, Erdogan A, Karabakan M, Yaris M, Gultekin MH (2021) Short-term effects of COVID-19 on semen parameters: a multicenter study of 69 cases. Andrology 9(4):1060–1065
Piroozmanesh H, Cheraghi E, Naserpoor L, Aghashahi M, Jannatifar R (2021) The effect of COVID-19 infection on sperm quality and male fertility. Jentashapir J Cell Molec Biol 12(2):e115390
Abdelhamid MHM, Fellah AA, Elmarghani AJRS (2023) An assessment of men semen alterations in SARS-CoV-2: is fever the principal concern? 30:72–80
Gharagozloo P, Cartagena S, Moazamian A, Drevet JR, Somkuti S, Aitken RJ (2022) Rapid impact of COVID-19 infection on semen quality: a case report. Transl Androl Urol 11(1):110–115
Temiz MZ, Dincer MM, Hacibey I, Yazar RO, Celik C, Kucuk SH, Alkurt G, Doganay L, Yuruk E, Muslumanoglu AY (2021) Investigation of SARS-CoV-2 in semen samples and the effects of COVID-19 on male sexual health by using semen analysis and serum male hormone profile: a cross-sectional, pilot study. Andrologia 53(2):e13912
Ma L, Xie W, Li D, Shi L, Ye G, Mao Y, Xiong Y, Sun H, Zheng F, Chen Z et al (2021) Evaluation of sex-related hormones and semen characteristics in reproductive-aged male COVID-19 patients. J Med Virol 93(1):456–462
Scroppo FI, Costantini E, Zucchi A, Illiano E, Trama F, Brancorsini S, Crocetto F, Gismondo MR, Dehò F, Mercuriali A et al (2022) COVID-19 disease in clinical setting: impact on gonadal function, transmission risk, and sperm quality in young males. J Basic Clin Physiol Pharmacol 33(1):97–102
Guo L, Zhao S, Li W, Wang Y, Li L, Jiang S, Ren W, Yuan Q, Zhang F, Kong F et al (2021) Absence of SARS-CoV-2 in semen of a COVID-19 patient cohort. Andrology 9(1):42–47
Holtmann N, Edimiris P, Andree M, Doehmen C, Baston-Buest D, Adams O, Kruessel JS, Bielfeld AP (2020) Assessment of SARS-CoV-2 in human semen-a cohort study. Fertil Steril 114(2):233–238
Halliwell B, Cross CE (1994) Oxygen-derived species: their relation to human disease and environmental stress. Environ Health Perspect 102(Suppl 10):5–12
Dutta S, Majzoub A, Agarwal A (2019) Oxidative stress and sperm function: a systematic review on evaluation and management. Arab J Urol 17(2):87–97
La Marca A, Busani S, Donno V, Guaraldi G, Ligabue G, Girardis M (2020) Testicular pain as an unusual presentation of COVID-19: a brief review of SARS-CoV-2 and the testis. Reprod Biomed Online 41(5):903–906
Ediz C, Tavukcu HH, Akan S, Kizilkan YE, Alcin A, Oz K, Yilmaz O (2021) Is there any association of COVID-19 with testicular pain and epididymo-orchitis? Int J Clin Pract 75(3):e13753
Bridwell RE, Merrill DR, Griffith SA, Wray J, Oliver JJ (2021) A coronavirus disease 2019 (COVID-19) patient with bilateral orchitis. Am J Emerg Med 42:260-e263
Cai Y, Wu X, Zhang Y, Xia J, Zhan Q (2020) Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) contamination in air and environment in temporary COVID-19 ICU Wards. ResearchSquare. https://doi.org/10.21203/rs.3.rs-21384/v1
Nie X, Qian L, Sun R, Huang B, Dong X, Xiao Q, Zhang Q, Lu T, Yue L, Chen S et al (2021) Multi-organ proteomic landscape of COVID-19 autopsies. Cell 184(3):775–791
Zafar MI, Li H (2020) COVID-19 and impairment of spermatogenesis: implications drawn from pathological alterations in testicles and seminal parameters. EClinicalMedicine 29:100671
Kunnumakkara AB, Rana V, Parama D, Banik K, Girisa S, Henamayee S, Thakur KK, Dutta U, Garodia P, Gupta SC et al (2021) COVID-19, cytokines, inflammation, and spices: how are they related? Life Sci 284:119201
Peirouvi T, Aliaghaei A, Eslami Farsani B, Ziaeipour S, Ebrahimi V, Forozesh M, Ghadipasha M, Mahmoudiasl GR, Aryan A, Moghimi N et al (2021) COVID-19 disrupts the blood-testis barrier through the induction of inflammatory cytokines and disruption of junctional proteins. Inflamm Res 70(10–12):1165–1175
Moghimi N, Eslami Farsani B, Ghadipasha M, Mahmoudiasl GR, Piryaei A, Aliaghaei A, Abdi S, Abbaszadeh HA, Abdollahifar MA, Forozesh M (2021) COVID-19 disrupts spermatogenesis through the oxidative stress pathway following induction of apoptosis. Apoptosis 26(7–8):415–430
Best JC, Kuchakulla M, Khodamoradi K, Lima TFN, Frech FS, Achua J, Rosete O, Mora B, Arora H, Ibrahim E et al (2021) Evaluation of SARS-CoV-2 in human semen and effect on total sperm number: a prospective observational study. World J Mens Health 39(3):489–495
Guo TH, Sang MY, Bai S, Ma H, Wan YY, Jiang XH, Zhang YW, Xu B, Chen H, Zheng XY et al (2021) Semen parameters in men recovered from COVID-19. Asian J Androl 23(5):479–483
Ruan Y, Hu B, Liu Z, Liu K, Jiang H, Li H, Li R, Luan Y, Liu X, Yu G et al (2021) No detection of SARS-CoV-2 from urine, expressed prostatic secretions, and semen in 74 recovered COVID-19 male patients: a perspective and urogenital evaluation. Andrology 9(1):99–106
Hu B, Liu K, Ruan Y, Wei X, Wu Y, Feng H, Deng Z, Liu J, Wang T (2022) Evaluation of mid- and long-term impact of COVID-19 on male fertility through evaluating semen parameters. Transl Androl Urol 11(2):159–167
Tiwari S, Kc N, Thapa S, Ghimire A, Bijukchhe S, Sah GS, Isnuwardana R (2021) Semen parameters in men recovered from COVID-19: a systematic review and meta-analysis. Middle East Fertil Soc J 26(1):44
Adamyan L, Elagin V, Vechorko V, Stepanian A, Dashko A, Doroshenko D, Aznaurova Y, Sorokin M, Suntsova M, Drobyshev A et al (2021) COVID-19-associated inhibition of energy accumulation pathways in human semen samples. F S Sci 2(4):355–364
Ghosh S, Parikh S, Nissa MU, Acharjee A, Singh A, Patwa D, Makwana P, Athalye A, Barpanda A, Laloraya M et al (2022) Semen proteomics of COVID-19 convalescent men reveals disruption of key biological pathways relevant to male reproductive function. ACS Omega 7(10):8601–8612
Gacci M, Coppi M, Baldi E, Sebastianelli A, Zaccaro C, Morselli S, Pecoraro A, Manera A, Nicoletti R, Liaci A et al (2021) Semen impairment and occurrence of SARS-CoV-2 virus in semen after recovery from COVID-19. Hum Reprod 36(6):1520–1529
Morselli S, Sebastianelli A, Liaci A, Zaccaro C, Pecoraro A, Nicoletti R, Manera A, Bisegna C, Campi R, Pollini S et al (2022) Male reproductive system inflammation after healing from coronavirus disease 2019. Andrology 10(6):1030–1037
Bao J, Guo Z, He J, Leng T, Wei Z, Wang C, Chen F (2022) Semen parameters and sex hormones as affected by SARS-CoV-2 infection: a systematic review. Prog Urol 32(16):1431–1439
Robaire B, Hinton BT, Orgebin-Crist M-C (2006) The epididymis. Knobil and Neill’s physiology of reproduction. Elsevier, pp 1071–1148
Wang Y, Wang Y, Luo W, Huang L, Xiao J, Li F, Qin S, Song X, Wu Y, Zeng Q et al (2020) A comprehensive investigation of the mRNA and protein level of ACE2, the putative receptor of SARS-CoV-2, in human tissues and blood cells. Int J Med Sci 17(11):1522–1531
Yao XH, Luo T, Shi Y, He ZC, Tang R, Zhang PP, Cai J, Zhou XD, Jiang DP, Fei XC et al (2021) A cohort autopsy study defines COVID-19 systemic pathogenesis. Cell Res 31(8):836–846
Davis GG, Williamson AK (2021) Testicular changes associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Arch Pathol Lab Med 145(1):7b–78
Gagliardi L, Bertacca C, Centenari C, Merusi I, Parolo E, Ragazzo V, Tarabella V (2020) Orchiepididymitis in a boy with COVID-19. Pediatr Infect Dis J 39(8):e200–e202
Carneiro F, Teixeira TA, Bernardes FS, Pereira MS, Milani G, Duarte-Neto AN, Kallas EG, Saldiva PHN, Chammas MC, Hallak J (2021) Radiological patterns of incidental epididymitis in mild-to-moderate COVID-19 patients revealed by colour Doppler ultrasound. Andrologia 53(4):e13973
Chen L, Huang X, Yi Z, Deng Q, Jiang N, Feng C, Zhou Q, Sun B, Chen W, Guo R (2021) Ultrasound imaging findings of acute testicular infection in patients with coronavirus disease 2019: a single-center-based study in Wuhan. China J Ultrasound Med 40(9):1787–1794
Verze P, Cai T, Lorenzetti S (2016) The role of the prostate in male fertility, health and disease. Nat Rev Urol 13(7):379–386
Reyfman PA, Walter JM, Joshi N, Anekalla KR, McQuattie-Pimentel AC, Chiu S, Fernandez R, Akbarpour M, Chen CI, Ren Z et al (2019) Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am J Respir Crit Care Med 199(12):1517–1536
Zhang S, Wang X, Zhang H, Xu A, Fei G, Jiang X, Tu J, Qu G, Xu X, Li Y (2020) The absence of coronavirus in expressed prostatic secretion in COVID-19 patients in Wuhan city. Reprod Toxicol 96:90–94
Di Vincenzo A, Busetto L, Vettor R, Rossato M (2021) Prostate specific antigen in COVID-19 patients. Andrology 9(4):1042
van Paassen J, Vos JS, Hoekstra EM, Neumann KMI, Boot PC, Arbous SM (2020) Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Crit Care 24(1):696
Singh AK, Majumdar S, Singh R, Misra A (2020) Role of corticosteroid in the management of COVID-19: a systemic review and a clinician’s perspective. Diabetes Metab Syndr 14(5):971–978
Castinetti F, Brue T (2022) Impact of Cushing’s syndrome on fertility and pregnancy. Ann Endocrinol (Paris) 83(3):188–190
Orazizadeh M, Khorsandi LS, Hashemitabar M (2010) Toxic effects of dexamethasone on mouse testicular germ cells. Andrologia 42(4):247–253
Zou P, Wang X, Yang W, Liu C, Chen Q, Yang H, Zhou N, Zeng Y, Chen H, Zhang G et al (2019) Mechanisms of stress-induced spermatogenesis impairment in male rats following unpredictable Chronic Mild Stress (uCMS). Int J Mol Sci 20(18):4470
Ren L, Zhang Y, Xin Y, Chen G, Sun X, Chen Y, He B (2021) Dysfunction in Sertoli cells participates in glucocorticoid-induced impairment of spermatogenesis. Mol Reprod Dev 88(6):405–415
Boregowda K, Joels L, Stephens JW, Price DE (2011) Persistent primary hypogonadism associated with anabolic steroid abuse. Fertil Steril 96(1):e7-8
Ilkhani S, Moradi A, Aliaghaei A, Norouzian M, Abdi S, Rojhani E, Ebadinejad A, Sajadi E, Abdollahifar MA (2020) Spatial arrangement of testicular cells disrupted by transient scrotal hyperthermia and subsequent impairment of spermatogenesis. Andrologia 52(9):e13664
Bendayan M, Boitrelle F (2021) What could cause the long-term effects of COVID-19 on sperm parameters and male fertility? QJM 114(4):287
Hajizadeh Maleki B, Tartibian B (2021) COVID-19 and male reproductive function: a prospective, longitudinal cohort study. Reproduction 161(3):319–331
Schuppe HC, Meinhardt A (2005) Immune privilege and inflammation of the testis. Chem Immunol Allergy 88:1–14
Gao YD, Ding M, Dong X, Zhang JJ, Kursat Azkur A, Azkur D, Gan H, Sun YL, Fu W, Li W et al (2021) Risk factors for severe and critically ill COVID-19 patients: a review. Allergy 76(2):428–455
Donders GGG, Bosmans E, Reumers J, Donders F, Jonckheere J, Salembier G, Stern N, Jacquemyn Y, Ombelet W, Depuydt CE (2022) Sperm quality and absence of SARS-CoV-2 RNA in semen after COVID-19 infection: a prospective, observational study and validation of the SpermCOVID test. Fertil Steril 117(2):287–296
Mallapaty S (2020) Mounting clues suggest the coronavirus might trigger diabetes. Nature 583(7814):16–17
Rubino F, Amiel SA, Zimmet P, Alberti G, Bornstein S, Eckel RH, Mingrone G, Boehm B, Cooper ME, Chai Z (2020) New-onset diabetes in COVID-19. N Engl J Med 383(8):789–790
Singh AK, Singh R (2020) Hyperglycemia without diabetes and new-onset diabetes are both associated with poorer outcomes in COVID-19. Diabetes Res Clin Pract 167:108382
Liu F, Long X, Zhang B, Zhang W, Chen X, Zhang Z (2020) ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin Gastroenterol Hepatol 18(9):2128–2130
Laforge M, Elbim C, Frere C, Hemadi M, Massaad C, Nuss P, Benoliel JJ, Becker C (2020) Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat Rev Immunol 20(9):515–516
Ren H, Yang Y, Wang F, Yan Y, Shi X, Dong K, Yu X, Zhang S (2020) Association of the insulin resistance marker TyG index with the severity and mortality of COVID-19. Cardiovasc Diabetol 19(1):58
Codo AC, Davanzo GG, Monteiro LB, de Souza GF, Muraro SP, Virgilio-da-Silva JV, Prodonoff JS, Carregari VC, de Biagi Junior CAO, Crunfli F et al (2020) Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1alpha/glycolysis-dependent axis. Cell Metab 32(3):437–446
Arikawe AP, Oyerinde A, Olatunji B II, Obika LF (2012) Streptozotocin diabetes and insulin resistance impairment of spermatogenesis in adult rat testis: central vs. local mechanism. Niger J Physiol Sci 27(2):171–179
Han XX, Jiang YP, Liu N, Wu J, Yang JM, Li YX, Sun M, Sun T, Zheng P, Jian-Qiang Y (2019) Protective effects of Astragalin on spermatogenesis in streptozotocin-induced diabetes in male mice by improving antioxidant activity and inhibiting inflammation. Biomed Pharmacother 110:561–570
Arikawe AP, Daramola AO, Odofin AO, Obika LF (2006) Alloxan-induced and insulin-resistant diabetes mellitus affect semen parameters and impair spermatogenesis in male rats. Afr J Reprod Health 10(3):106–113
Khaki A, Khaki AA, Hajhosseini L, Golzar FS, Ainehchi N (2014) The anti-oxidant effects of ginger and cinnamon on spermatogenesis dys-function of diabetes rats. Afr J Tradit Complement Altern Med 11(4):1–8
Song K, Yang X, An G, Xia X, Zhao J, Xu X, Wan C, Liu T, Zheng Y, Ren S et al (2022) Targeting APLN/APJ restores blood-testis barrier and improves spermatogenesis in murine and human diabetic models. Nat Commun 13(1):7335
Sharma O, Sultan AA, Ding H, Triggle CR (2020) A review of the progress and challenges of developing a vaccine for COVID-19. Front Immunol 11:585354
Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, McClung N, Campos-Outcalt D, Morgan RL, Mbaeyi S et al (2020) The advisory committee on immunization practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep 69(50):1922–1924
Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, McClung N, Campos-Outcalt D, Morgan RL, Mbaeyi S et al (2021) The advisory committee on immunization practices’ interim recommendation for use of moderna COVID-19 vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep 69(5152):1653–1656
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Perez Marc G, Moreira ED, Zerbini C et al (2020) Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 383(27):2603–2615
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB et al (2021) Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 384(5):403–416
Navarra A, Albani E, Castellano S, Arruzzolo L, Levi-Setti PE (2020) Coronavirus disease-19 infection: implications on male fertility and reproduction. Front Physiol 11:574761
Kumar V, Kaur M (2021) COVID-19 vaccine and male fertility. Urol J 6897–6899
Diaz P, Zizzo J, Balaji NC, Reddy R, Khodamoradi K, Ory J, Ramasamy R (2022) Fear about adverse effect on fertility is a major cause of COVID-19 vaccine hesitancy in the United States. Andrologia 54(4):e14361
Lo SP, Hsieh TC, Pastuszak AW, Hotaling JM, Patel DP (2022) Effects of SARS CoV-2, COVID-19, and its vaccines on male sexual health and reproduction: where do we stand? Int J Impot Res 34(2):138–144
Lawson AK, McQueen DB, Swanson AC, Confino R, Feinberg EC, Pavone ME (2021) Psychological distress and postponed fertility care during the COVID-19 pandemic. J Assist Reprod Genet 38(2):333–341
Gonzalez DC, Nassau DE, Khodamoradi K, Ibrahim E, Blachman-Braun R, Ory J, Ramasamy R (2021) Sperm parameters before and after COVID-19 mRNA vaccination. JAMA 326(3):273–274
Lifshitz D, Haas J, Lebovitz O, Raviv G, Orvieto R, Aizer A (2022) Does mRNA SARS-CoV-2 vaccine detrimentally affect male fertility, as reflected by semen analysis? Reprod Biomed Online 44(1):145–149
Gonzalez D, Nassau DE, Khodamoradi K, Ibrahim E, Blachman-Braun R, Dubin JM, Ory J, Ramasamy RJF (2021) Effect of COVID-19 mRNA vaccines on sperm quality. Fertil Steril 116(3):297
Olana S, Mazzilli R, Salerno G, Zamponi V, Tarsitano MG, Simmaco M, Paoli D, Faggiano A (2022) 4BNT162b2 mRNA COVID-19 vaccine and semen: what do we know? Andrology 10(6):1023–1029
Chatzimeletiou K, Fleva A, Sioga A, Georgiou I, Nikolopoulos TT, Markopoulou M, Petrogiannis N, Anifandis G, Patrikiou A, Kolibianakis E et al (2022) Effects of different drug therapies and COVID-19 mRNA vaccination on semen quality in a man with ankylosing spondylitis: a case report. Medicina (Kaunas) 58(2):173
Zhu H, Wang X, Zhang F, Zhu Y, Du MR, Tao ZW, Sun C, Ma HT, Li YD, Liang GQ et al (2022) Evaluation of inactivated COVID-19 vaccine on semen parameters in reproductive-age males: a retrospective cohort study. Asian J Androl 24(5):441–444
Reschini M, Pagliardini L, Boeri L, Piazzini F, Bandini V, Fornelli G, Dolci C, Cermisoni GC, Vigano P, Somigliana E et al (2022) COVID-19 vaccination does not affect reproductive health parameters in men. Front Public Health 10:839967
Zace D, La Gatta E, Petrella L, Di Pietro ML (2022) The impact of COVID-19 vaccines on fertility-a systematic review and meta-analysis. Vaccine 40(42):6023–6034
Carto C, Nackeeran S, Ramasamy R (2022) COVID-19 vaccination is associated with a decreased risk of orchitis and/or epididymitis in men. Andrologia 54(2):e14281
Patel AS, Leong JY, Ramos L, Ramasamy R (2019) Testosterone is a contraceptive and should not be used in men who desire fertility. World J Mens Health 37(1):45–54
Brennemann W, Kohler W, Zierz S, Klingmuller D (1997) Testicular dysfunction in adrenomyeloneuropathy. Eur J Endocrinol 137(1):34–39
Selvaraj K, Ravichandran S, Krishnan S, Radhakrishnan RK, Manickam N, Kandasamy M (2021) Testicular atrophy and hypothalamic pathology in COVID-19: possibility of the incidence of male infertility and HPG axis abnormalities. Reprod Sci 28(10):2735–2742
Nargund VH (2015) Effects of psychological stress on male fertility. Nat Rev Urol 12(7):373–382
Yang Z, Zhang S, Tang YP, Zhang S, Xu DQ, Yue SJ, Liu QL (2022) Clinical characteristics, transmissibility, pathogenicity, susceptible populations, and re-infectivity of prominent COVID-19 variants. Aging Dis 13(2):402–422
McLean G, Kamil J, Lee B, Moore P, Schulz TF, Muik A, Sahin U, Tureci O, Pather S (2022) The impact of evolving SARS-CoV-2 mutations and variants on COVID-19 vaccines. MBio 13(2):e0297921
Garrett N, Tapley A, Andriesen J, Seocharan I, Fisher LH, Bunts L, Espy N, Wallis CL, Randhawa AK, Ketter N, et al. 2022. High rate of asymptomatic carriage associated with variant strain omicron. Medrxiv.
Mouliou DS, Gourgoulianis KI (2022) COVID-19 “asymptomatic” patients: an old wives’ tale. Expert Rev Respir Med 16(4):399–407
Le Bert N, Clapham HE, Tan AT, Chia WN, Tham CYL, Lim JM, Kunasegaran K, Tan LWL, Dutertre CA, Shankar N et al (2021) Highly functional virus-specific cellular immune response inasymptomatic SARS-CoV-2 infection. J Exp Med 218(5):e20202617
Zhao XN, You Y, Wang GL, Gao HX, Ma MJ (2020) Longitudinal single-cell immune profiling revealed distinct innate immune response in asymptomatic COVID-19 patients. BioRxiv. https://doi.org/10.1101/2020.09.02.276865
Dutta S, Sandhu N, Sengupta P, Alves MG, Henkel R (2021) Agarwal a somatic-immune cells crosstalk in-the-making of testicular immune privilege. Reprod Sci 29:2707–2718
Dutta S, Sengupta P, Chhikara BS (2020) Reproductive inflammatory mediators and male infertility. Chem Biol Lett 7(2):73–74
Nikolopoulou GB (2022) Maltezou HC COVID-19 in children: where do we stand? Arch Med Res 53(1):1–8
Mallapaty S (2022) Most US kids have caught the coronavirus, antibody survey finds. Nature 605(7909):207–207
Stanton PG (2016) Regulation of the blood-testis barrier. Semin Cell Dev Biol 59:166–173
Mahase E (2020) COVID-19: what do we know about “long COVID”? BMJ 370:2815
Yoo SM, Liu TC, Motwani Y, Sim MS, Viswanathan N, Samras N, Hsu F (2022) Wenger NS factors associated with Post-acute sequelae of SARS-CoV-2 (PASC) after diagnosis of symptomatic COVID-19 in the inpatient and outpatient setting in a diverse cohort. J Gen Intern Med 37(8):1988–1995
Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re’em Y, Redfield S, Austin JP, Akrami A (2021) Characterizing long COVID in an international cohort 7 months of symptoms and their impact. Clin Med 38:101019
Al-Aly Z, Bowe B, Xie Y (2022) Long COVID after breakthrough SARS-CoV-2 infection. Nat Med 28:1461–1467
Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A et al (2015) Proteomics. Tissue-based map of the human proteome. Science 347(6220):1260419
Funding
Funding was provided by the National Key R&D Program of China (2018YFC1003602 to H.C.), Nantong Project of Science and Technology (MS12022027 to H.C.), National Natural Science Foundation of China (81871202 to H.C.), Jiangsu Innovation and Entrepreneurship Talent Plan to H.C, GRF/RGC grant (14127316 to K.L.F.), Lo Kwee Seong Start-up fund to K.L.F., Food and Health Bureau of Hong Kong (06170476 to K.L.F.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
The present article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
The formal consent is not required by this type of study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dai, P., Qiao, F., Chen, Y. et al. SARS-CoV-2 and male infertility: from short- to long-term impacts. J Endocrinol Invest 46, 1491–1507 (2023). https://doi.org/10.1007/s40618-023-02055-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-023-02055-x