Abstract
Purpose
Adrenal cortical adenomas (ACAs) represent one of the most common endocrine neoplasms. Recently, a genetic syndrome, characterized by tumor-suppressor ARMC5-gene mutations and causing primary macronodular bilateral adrenal hyperplasia with concomitant meningiomas of the central nervous system, has been described. Apart from this rare disorder and despite the well-known influence of steroid hormones on meningiomas, no data are available about the association between ACAs and meningiomas.
Methods
We investigated the prevalence of ACAs in a group of patients with cerebral meningioma undergoing unenhanced chest CT scans before attending surgical treatment. Patients with meningioma were age- and sex-matched in a 1:3 ratio with hospitalized patients for COVID-19.
Results
Fifty-six patients with meningioma were included and matched with 168 control patients with COVID-19. One-hundred forty-four (66.1%) were female and the median age was 63 years. Twenty ACAs were detected in the overall population (8.9% of the subjects): 10 in patients with meningioma (18%) and the remaining 10 (6%) in the control group (p = 0.007). Multivariate analysis showed that age and presence of meningioma were statistically associated with the presence of ACAs (p = 0.01, p = 0.008).
Conclusion
We report, for the first time, a higher prevalence of ACAs in patients with meningioma as compared to age- and sex-matched controls. Larger studies are needed to confirm our data and to clarify the characteristics of the ACAs in patients with meningioma. Whether the detection of ACAs should prompt a neuroimaging evaluation to exclude the presence of meningiomas needs also to be considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adrenal cortical adenomas (ACAs) represent one of the most common endocrine neoplasms, affecting 2–4% of the general population and up to 7% of subjects older than 70 years [1,2,3]. ACAs represent a large proportion of the so called incidentally discovered adrenal masses (adrenal incidentalomas), characterized by a wide spectrum of benign and malignant lesions, whose incidence and prevalence have markedly increased in the last decades due to the growing availability of powerful diagnostic tools and the progressive aging of the general population [4,5,6,7,8].
The genetic and biomolecular mechanisms underlying the generation of ACAs remain poorly understood and have been identified only in small subgroups of patients [9,10,11,12,13].
Germline and somatic loss of function mutations in Armadillo repeat-containing protein gene (ARMC5), a putative tumor-suppressor gene, have been identified in 27% and 55% of patients with an adrenocorticotropic hormone (ACTH)-independent Cushing’s syndrome due to apparently sporadic and familial forms of primary macronodular bilateral adrenal hyperplasia (PMAH), respectively [14,15,16,17]. ARMC5 loss of function mutations have also been found to play a pathogenetic role in the development of central nervous system meningiomas [18,19,20,21,22], although the actual prevalence of somatic ARMC5 mutations is currently unknown in meningiomas. Besides the small subgroup of patients with ARMC5 mutation, there are no data in the literature about the association or increased risk of occurrence of ACAs in patients affected by meningioma and vice versa in the general population.
The aim of the present study was to investigate the prevalence of ACAs in patients affected by meningioma as compared to sex- and age-matched control subjects.
Material and methods
Study design and patients
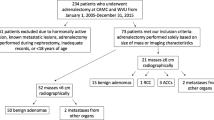
This was an observational and retrospective study performed at IRCCS Ospedale San Raffaele, a tertiary health-care hospital in Milan, Italy. The study protocol complies with the Declaration of Helsinki and was approved by the Hospital Ethics Committee (protocol no. 34/int/2020). A signed informed consent was obtained from all the patients participating in this study. We included all consecutive adult patients with a neuroradiological diagnosis of meningioma attending the Neurosurgery Unit for surgical removal of the tumor between March 2020 and March 2021. During this time interval, special measures were implemented in the neurosurgical department to reduce the risk of performing elective major surgical procedures in patients affected by COVID-19 infection. Besides a thorough clinical history and a negative molecular swab, a routine preoperative unenhanced chest CT scan was performed the day before the surgical procedure. Patients with meningioma were matched for age and sex in a 1:3 ratio with control patients hospitalized in the same period in a medical ward of our Hospital for COVID-19 and, who underwent an unenhanced chest CT scan as part of the diagnostic workup. Patients with a history of malignancy were excluded from the study.
CT scan and adrenal evaluation
CT scans were retrospectively and carefully re-evaluated by two experienced radiologists (S.L.M., R.M.), blinded to the underlying pathology. Only chest CT scans that allowed a high-quality adrenal assessment were used for the analysis. Chest CT scans that did not include or that did not allow a correct evaluation of both adrenal glands, because of pneumonia opacities or low-quality images, were excluded from the study.
Chest CT scans were performed on two different scanners: LightSpeed VCT (64sl)-GE Medical System and Incisive (64sl)-Philips, in supine position. CT scan parameters were as follows: 120 kV tube voltage, automatic tube current modulation ([149–549] mA), slice thickness 1–1.25 mm, matrix 512 × 512. The raw data were reconstructed using smooth kernels with filtered back projection as well as adaptive statistical iterative reconstruction and viewed with mediastinal window (width/level: 300/10 HU).
Unenhanced CT scan evaluations with 3–5 mm cuts are the cornerstone of the adrenal imaging used in side detection, characterization, size and density measurements of the lesions [23, 24]. The normal adrenal glands appear symmetric and homogeneous in appearance with a density approximately equal to that of the kidney. The radiologic criteria to define ACAs on CT scans were the detection of well-defined round or oval lesions, homogeneous attenuation, CT density < 10 HU, and no radiological features of malignancy [23, 24].
In the meningioma group a hormonal assessment was available only in patients with pre-surgical evidence of an adrenal mass (see “Results”). In a large proportion of control patients, high-dose cortisone therapy for the control of COVID-19 prevented from any hormonal evaluation.
Statistical analyses
Descriptive statistics were obtained for all study variables. Categorical variables were summarized as counts and percentages. Kolmogorov–Smirnov normality test was performed (p < 0.05) and continuous variables were expressed as medians and interquartile range (IQR) [25th–75th percentile]. Fisher exact test or χ2 test and the Wilcoxon signed-rank test or the Kruskal–Wallis test were employed to determine the statistical significance of differences in proportions and medians, respectively. Univariate and multivariate logistic regression analyses were used to estimate the odds ratios (ORs) of ACAs presence with 95% confidence intervals (CIs). All statistical tests were two-sided. A p value of < 0.05 was considered statistically significant. Statistical analysis was conducted using IBM SPSS Statistics (IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.).
Results
Fifty-six patients affected by meningioma and with an unenhanced CT scan available for analysis of both adrenals were included into the study and were matched for age and sex in a 1:3 ratio with 168 control patients affected by COVID-19. Two hundred and twenty-four patients were finally considered for the analyses.
One-hundred and forty-four patients (66.1%) were female and 76 (33.9%) were male. Median age was 63 [52–71] years. Median age of the patients affected by meningioma was 62 years [52–72] and 19 (33.9%) were male. Median age of control patients was 63.5 years [52–71] and 57 (33.9%) were male. No statistical differences were observed regarding age and sex distribution between the meningioma and the control group (p = 0.99, p = 1; respectively; Table 1).
After a careful retrospective re-evaluation of CT scan examinations, globally 20 ACAs (8.9%) were detected overall. Ten ACAs were detected in patients with meningioma (18%) and other ten ACAs (6%) in the control group (p = 0.007) (Table 1).
Ten ACAs were in male patients (13%) and ten in female (6.7%) patients (p = 0.1). Patients with evidence of ACA were significantly older as compared to those with no ACA (70 [65–74] vs 62 [52–71] years; p = 0.005). Twelve (60%) were located in the left adrenal gland and 8 (40%) in the right. Median maximum diameter of the lesions was 16 mm [14,15,16,17,18,19,20,21,22].
At univariate analysis, variables significantly associated with the presence of ACAs included age [OR 1.06, 95% CI (1.01–1.1), p = 0.01] and diagnosis of meningioma [OR 3.43, 95% CI (1.35–8.76), p = 0.01]. Sex was not associated with the presence of ACAs [OR 2.1, 95% CI (0.83–5.3), p = 0.12] (Table 2). These findings were confirmed at multivariate analysis, where age and diagnosis of meningioma were still and independently associated with ACAs detection (p = 0.01 and p = 0.008; respectively) (Table 2).
Four out of ten ACAs (40%) in patients with meningioma were originally described in the radiologic report and prompted a complete pituitary-adrenal axis hormonal evaluation including basal aldosterone, renin, dehydroepiandrosterone sulfate (DHEAS), and 17-hydroxyprogesterone levels, 24-h urinary free cortisol concentration, and an overnight low-dose dexamethasone suppression, performed in these patients. The results of hormonal evaluation excluded an autonomous hormonal secretion in all four patients. Sodium and potassium levels were normal in the ten patients with meningioma and ACAs. Two adenomas (20%) in control patients were described in the original CT scan radiological report, but a hormonal evaluation was not performed due to the concomitant acute illness.
No significant differences were found regarding age, sex, ACAs size, and ACAs left or right location between meningioma and control patients (Table 1).
Discussion
To our knowledge, this is the first study assessing and comparing the prevalence of ACAs in patients affected by meningioma and in an age- and sex-matched control group. We found a statistically significant higher prevalence of ACAs in patients with meningioma as compared to controls (18 vs. 6%). The prevalence of ACAs in the control cohort was in keeping with previously published autoptic and radiologic series reporting a mean prevalence ranging 1.05% to 8.7% and 1 to 7.1%, respectively [1, 25, 26]. Moreover, multivariate analysis confirmed that the diagnosis of meningioma was an independent predictor for ACAs detection.
Coexistence of meningiomas and ACAs has been reported in patients with ACTH-independent Cushing’s syndrome due to PMAH and ARMC5 mutations [18,19,20,21,22]. In these patients, a common pathophysiological mechanism underlying ACA and meningioma occurrence was speculated. A monoallelic germline alteration of ARMC5 may cause PMAH via a subsequent somatic alteration on the other allele as the second hit, with inactivation of a tumor-suppressor gene confirmed by the analysis on ACA tumoral tissue. The same ARMC5 somatic mutation has recently been reported in the meningiomatous tissue of patients affected by both PMAH and intracranial meningioma [16, 19].
The role of ARMC5 in the pathogenesis of sporadic unilateral ACAs has been, to date, poorly investigated. Recently, one study evaluated germline allelic variants of the ARMC5 gene in 59 patients with unilateral adrenal incidentalomas, identifying the presence of non-pathogenetic allelic variants in 69.5% of cases [27] and a significant number of variants of uncertain significance (VUS).
Meningiomas are one of the most common primary brain tumors [28], with an annual incidence ranging 6 to 8:100 000, a peak incidence in middle-aged patients, a female to-male ratio of 2 to 3.5:1 depending on the age group, and a risk of occurrence increasing with age in both sexes [29,30,31]. Autopsy and imaging studies estimate a prevalence of asymptomatic meningioma in up to 2.8% of women [32, 33]. Over the past decade, the overall incidence of meningiomas has increased, probably due to an incidental diagnosis related to the growing use and improved accuracy of neuroimaging [34]. Most of these tumors are benign, 15% to 20% are atypical, while malignant forms are rare (1 to 2% of cases) [35]. Hormonal influence on the development, progression, and recurrence of meningiomas has been recognized since the late 1920s, when Cushing and Eisenhardt first described a case of a rapid progression of meningioma during pregnancy [31, 36, 37]. The expression of sex hormones and somatostatin receptors in meningiomas is well established in the literature [36,37,38,39,40,41,42,43]. In particular, the expression of progesterone receptors (PRs) has been reported in more than 90% of meningiomas and has been associated with a more favorable prognosis and a lower risk of recurrence. On the other hand, the expression of estrogens receptors (ERs) described in up to 30% of meningiomas has been associated, although not consistently, with an unfavorable prognosis, while the expression of androgens receptors (ARs) reaches a prevalence of 88% in a recent study [36, 38, 39]. Moreover, meningiomas enriched with PIK3CA mutations were found in patients treated with progestins [40] and similarly, PIK3CA-mutated skull base meningiomas were observed in patients receiving cyproterone acetate [41, 42].
We can speculate that, besides the small percentage of cases with a genetically-determined coexistence of ACAs and meningiomas, an abnormal circulating steroidal milieu due to the adrenal mass may promote the development, the recurrence and the dimensional growing of a meningioma. In this setting, a relevant issue is represented by ACAs showing a subtle, apparently subclinical autonomous function with potentially negative clinical implications [44, 45], for instance those associated with low DHEAS and/or ACTH levels or a partial cortisol inhibition after overnight dexamethasone [46,47,48,49].
Limitations of our study include its retrospective nature that prevented us from obtaining a full assessment of the pituitary–adrenal axis of the patients with ACA and the small number of patients with meningioma.
Apart from these limitations, we report for the first time, a higher prevalence of ACAs in patients affected by meningioma as compared to age- and sex-matched controls.
Larger prospective studies are needed to consistently clarify whether the detection of a meningioma might unveil the presence of a concomitant ACA, and in which case the identification of an ACA should prompt the search for the presence of a meningioma.
References
Lloyd RV, Osamura RY, Klöppel G, Rosai J. WHO classification of tumours of endocrine organs. International Agency for Research on Cancer, January 2017. 978–9–28–324493–6
Sherlock M, Scarsbrook A, Abbas A et al (2020) Adrenal Incidentaloma. Endocr Rev 41(6):775–820. https://doi.org/10.1210/endrev/bnaa008
Kebebew E (2021) Adrenal Incidentaloma. N Engl J Med 384(16):1542–1551. https://doi.org/10.1056/NEJMcp2031112
Papanicolas I, Woskie LR, Jha AK. Health Care Spending in the United States and Other High-Income Countries [published correction appears in JAMA. 2018 May 1;319(17 ):1824]. JAMA. 2018;319(10):1024–1039. https://doi.org/10.1001/jama.2018.1150
Kebebew E. Management of adrenal masses in children and adults. Springer, Cham. 2017. https://doi.org/10.1007/978-3-319-44136-8
Young WF Jr. Clinical practice. The incidentally discovered adrenal mass. N Engl J Med. 2007;356(6):601–610. https://doi.org/10.1056/NEJMcp065470
Incampo G, Di Filippo L, Grossrubatscher EM et al (2022) Adrenal schwannoma: why should endocrinologists be aware of this uncommon tumour? Endocrine 75(3):684–697. https://doi.org/10.1007/s12020-022-02997-x
Mínguez Ojeda C, Gómez Dos Santos V, Álvaro Lorca J, et al. Tumour size in adrenal tumours: its importance in the indication of adrenalectomy and in surgical outcomes-a single-centre experience [published online ahead of print, 2022 Jun 24]. J Endocrinol Invest. 2022;https://doi.org/10.1007/s40618-022-01836-0. https://doi.org/10.1007/s40618-022-01836-0
Choi M, Scholl UI, Yue P et al (2011) K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science 331(6018):768–772. https://doi.org/10.1126/science.1198785
Azizan EA, Poulsen H, Tuluc P et al (2013) Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet 45(9):1055–1060. https://doi.org/10.1038/ng.2716
Beuschlein F, Fassnacht M, Assié G et al (2014) Constitutive activation of PKA catalytic subunit in adrenal Cushing’s syndrome. N Engl J Med 370(11):1019–1028. https://doi.org/10.1056/NEJMoa1310359
Cao Y, He M, Gao Z et al (2014) Activating hotspot L205R mutation in PRKACA and adrenal Cushing’s syndrome. Science 344(6186):913–917. https://doi.org/10.1126/science.1249480
Bonnet S, Gaujoux S, Launay P et al (2011) Wnt/β-catenin pathway activation in adrenocortical adenomas is frequently due to somatic CTNNB1-activating mutations, which are associated with larger and nonsecreting tumors: a study in cortisol-secreting and -nonsecreting tumors. J Clin Endocrinol Metab 96(2):E419–E426. https://doi.org/10.1210/jc.2010-1885
Assié G, Libé R, Espiard S et al (2013) ARMC5 mutations in macronodular adrenal hyperplasia with Cushing’s syndrome. N Engl J Med 369(22):2105–2114. https://doi.org/10.1056/NEJMoa1304603
Gagliardi L, Schreiber AW, Hahn CN et al (2014) ARMC5 mutations are common in familial bilateral macronodular adrenal hyperplasia. J Clin Endocrinol Metab 99(9):E1784–E1792. https://doi.org/10.1210/jc.2014-1265
Stratakis CA, Berthon A (2019) Molecular mechanisms of ARMC5 mutations in adrenal pathophysiology. Curr Opin Endocr Metab Res 8:104–111. https://doi.org/10.1016/j.coemr.2019.07.010
Elbelt U, Trovato A, Kloth M et al (2015) Molecular and clinical evidence for an ARMC5 tumor syndrome: concurrent inactivating germline and somatic mutations are associated with both primary macronodular adrenal hyperplasia and meningioma. J Clin Endocrinol Metab 100(1):E119–E128. https://doi.org/10.1210/jc.2014-2648
Alencar GA, Lerario AM, Nishi MY et al (2014) ARMC5 mutations are a frequent cause of primary macronodular adrenal Hyperplasia. J Clin Endocrinol Metab 99(8):E1501–E1509. https://doi.org/10.1210/jc.2013-4237
Correa R, Zilbermint M, Berthon A et al (2015) The ARMC5 gene shows extensive genetic variance in primary macronodular adrenocortical hyperplasia. Eur J Endocrinol 173(4):435–440. https://doi.org/10.1530/EJE-15-0205
Jojima T, Kogai T, Iijima T et al (2020) Genetic alteration of ARMC5 in a patient diagnosed with meningioma and primary macronodular adrenal hyperplasia: a case report. Eur J Endocrinol 183(6):K7–K12. https://doi.org/10.1530/EJE-20-0014
Ferreira MJ, Pedro J, Salazar D, et al. ARMC5 Primary Bilateral Macronodular Adrenal Hyperplasia Associated with a Meningioma: A Family Report. Case Rep Endocrinol. 2020;2020:8848151. Published 2020 Sep 2. https://doi.org/10.1155/2020/8848151
Berthon A, Faucz F, Bertherat J, Stratakis CA (2017) Analysis of ARMC5 expression in human tissues. Mol Cell Endocrinol 441:140–145. https://doi.org/10.1016/j.mce.2016.08.018
Ilias I, Alesci S, Pacak K (2004) Current views on imaging of adrenal tumors. Horm Metab Res 36(6):430–435. https://doi.org/10.1055/s-2004-814582
Lockhart ME, Smith JK, Kenney PJ (2002) Imaging of adrenal masses. Eur J Radiol 41(2):95–112. https://doi.org/10.1016/s0720-048x(01)00444-2
Ceccato F, Barbot M, Scaroni C, Boscaro M (2021) Frequently asked questions and answers (if any) in patients with adrenal incidentaloma. J Endocrinol Invest 44(12):2749–2763. https://doi.org/10.1007/s40618-021-01615-3
Bovio S, Cataldi A, Reimondo G et al (2006) Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest 29(4):298–302
Mariani BMP, Nishi MY, Wanichi IQ, et al. Allelic Variants of ARMC5 in Patients With Adrenal Incidentalomas and in Patients With Cushing's Syndrome Associated With Bilateral Adrenal Nodules. Front Endocrinol (Lausanne). 2020;11:36. Published 2020 Feb 7. doi:https://doi.org/10.3389/fendo.2020.00036
Rogers L, Barani I, Chamberlain M et al (2015) Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review J Neurosurg 122(1):4–23. https://doi.org/10.3171/2014.7.JNS131644
Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011–2015 [published correction appears in Neuro Oncol. 2018 Nov 17;:null]. Neuro Oncol. 2018;20(suppl_4):iv1-iv86. https://doi.org/10.1093/neuonc/noy131
Baldi I, Gruber A, Alioum A et al (2011) Descriptive epidemiology of CNS tumors in France: results from the gironde registry for the period 2000–2007. Neuro Oncol 13(12):1370–1378. https://doi.org/10.1093/neuonc/nor120
Wiemels J, Wrensch M, Claus EB (2010) Epidemiology and etiology of meningioma. J Neurooncol 99(3):307–314. https://doi.org/10.1007/s11060-010-0386-3
Krampla W, Newrkla S, Pfisterer W et al (2004) Frequency and risk factors for meningioma in clinically healthy 75-year-old patients: results of the Transdanube Ageing Study (VITA). Cancer 100(6):1208–1212. https://doi.org/10.1002/cncr.20088
Vernooij MW, Ikram MA, Tanghe HL et al (2007) Incidental findings on brain MRI in the general population. N Engl J Med 357(18):1821–1828. https://doi.org/10.1056/NEJMoa070972
Buerki RA, Horbinski CM, Kruser T, Horowitz PM, James CD, Lukas RV (2018) An overview of meningiomas. Future Oncol 14(21):2161–2177. https://doi.org/10.2217/fon-2018-0006
WHO Classification of Tumours Editorial Board. Central Nervous System Tumours. International Agency for Research on Cancer, January 2021. 978–9–28–324508–7
Hage M, Plesa O, Lemaire I, Raffin Sanson ML. Estrogen and Progesterone Therapy and Meningiomas. Endocrinology. 2022;163(2):bqab259. https://doi.org/10.1210/endocr/bqab259
Cushing H, Eisenhardt L (1929) Meningiomas arising from the tuberculum sellae: with the syndrome of primary optic atrophy and bitemporal field defects combined with a normal sella turcica in a middle-aged person. Acta Neuropathol 1(2):168–206
Donnell MS, Meyer GA, Donegan WL (1979) Estrogen-receptor protein in intracranial meningiomas. J Neurosurg 50(4):499–502. https://doi.org/10.3171/jns.1979.50.4.0499
Poisson M, Magdelenat H, Foncin JF et al (1980) Estrogen and progestin receptors in meningiomas: a study in 22 cases [author’s transl]. Rev Neurol (Paris) 136(3):193–203
Du Z, Santagata S (2018) Uncovering the links between systemic hormones and oncogenic signaling in the pathogenesis of meningioma. Ann Oncol 29(3):537–540. https://doi.org/10.1093/annonc/mdy010
Peyre M, Gaillard S, de Marcellus C et al (2018) Progestin-associated shift of meningioma mutational landscape. Ann Oncol 29(3):681–686. https://doi.org/10.1093/annonc/mdx763
Portet S, Naoufal R, Tachon G, et al. Histomolecular characterization of intracranial meningiomas developed in patients exposed to high-dose cyproterone acetate: an antiandrogen treatment. Neurooncol Adv. 2019;1(1):vdz003. Published 2019 May 28. https://doi.org/10.1093/noajnl/vdz003
De Menis E, Tulipano G, Villa S et al (2003) Development of a meningioma in a patient with acromegaly during octreotide treatment: are there any causal relationships? J Endocrinol Invest 26(4):359–363. https://doi.org/10.1007/BF03345185
Frara S, Allora A, di Filippo L et al (2021) Osteopathy in mild adrenal Cushing’s syndrome and Cushing disease. Best Pract Res Clin Endocrinol Metab 35(2):101515. https://doi.org/10.1016/j.beem.2021.101515
Rodrigues MO, Moraes AB, de Paula MP, Pereira VA, Leão ATT, Vieira NL (2021) Adrenal incidentaloma as a novel independent predictive factor for periodontitis. J Endocrinol Invest 44(11):2455–2463. https://doi.org/10.1007/s40618-021-01557-w
Yener S, Yilmaz H, Demir T, Secil M, Comlekci A (2015) DHEAS for the prediction of subclinical Cushing’s syndrome: perplexing or advantageous? Endocrine 48(2):669–676. https://doi.org/10.1007/s12020-014-0387-7
Dennedy MC, Annamalai AK, Prankerd-Smith O et al (2017) Low DHEAS: a sensitive and specific test for the detection of subclinical hypercortisolism in adrenal incidentalomas. J Clin Endocrinol Metab 102(3):786–792. https://doi.org/10.1210/jc.2016-2718
Winzinger EP, Jandikova H, Haase M et al (2021) DHEAS and differential blood counts as indirect signs of glucocorticoid excess in adrenal non-producing adenomas. Horm Metab Res 53(8):512–519. https://doi.org/10.1055/a-1539-6442
Berke K, Constantinescu G, Masjkur J et al (2022) Plasma steroid profiling in patients with adrenal incidentaloma. J Clin Endocrinol Metab 107(3):e1181–e1192. https://doi.org/10.1210/clinem/dgab751
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector. The work submitted for publication is original and has not been published in any language or format and has not been submitted elsewhere for print or electronic publication consideration.
Author information
Authors and Affiliations
Contributions
All authors contributed equally.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest. The authors have nothing to disclose.
Human participants and Animals
The study protocol complies with the 1964 Declaration of Helsinki and was approved by the Hospital Ethics Committee (protocol no. 34/int/2020).
Informed consent
A signed informed consent was obtained from all the patients participating in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
di Filippo, L., La Marca, S., Losa, M. et al. High prevalence of adrenal cortical adenomas in patients with cerebral meningiomas. J Endocrinol Invest 46, 763–768 (2023). https://doi.org/10.1007/s40618-022-01935-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-022-01935-y